Abstract
Obesity and metabolic disturbances are prevalent in ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF); however, the involvement of dyslipidemia (DL) in OPLL/OLF remains uncertain. We investigated the association between dyslipidemia and OPLL/OLF using a dataset of 458 individuals receiving health screening tests, including computed tomography. Subjects were grouped according to the presence or location of OPLL/OLF: controls (no OPLL/OLF, n = 230), OLF (n = 167), cervical OPLL (n = 28), and thoracic OPLL (n = 33). They were also grouped according to the presence of dyslipidemia (DL[+], n = 215; DL[−], n = 243). The proportion of dyslipidemia in the OLF and OPLL groups was 1.6–2.2 times higher than that in the control group. The proportion of OLF and OPLL in the DL(+) group was significantly higher than that in the DL(−) group (OLF, 43% vs. 29%; cervical OPLL, 14.4% vs. 3.2%; thoracic OPLL, 11.1% vs. 3.7%). Multivariate logistic regression analysis showed an association between all ossification types and dyslipidemia. This study demonstrated an association of dyslipidemia with OPLL/OLF; further investigation on the causal relationship between dyslipidemia and ectopic spinal ligament ossification is warranted to develop a therapeutic intervention for OPLL/OLF.
Similar content being viewed by others
Introduction
Ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF) are the major causes of myelopathy in the East Asian population 1,2,3,4,5,6,7,8, and are often coexistent 9,10,11. Recent epidemiological studies have reported that obesity is a common aggravating factor in patients with diffuse types of OPLL and OLF, in which the entire spinal ligament tends to ossify 10,11,12,13,14,15. This suggests that lifestyle-related diseases and visceral obesity may be involved in the development and progression of these conditions 11,12,13.
Dyslipidemia is caused by malnutrition, obesity, sarcopenic obesity, and genetic predisposition and is closely related to metabolic disturbances16,17,18. If dyslipidemia is left untreated, atherosclerosis will develop. This may cause ischemic heart disease and stroke, and increase the risk of fatty liver and other diseases16,17,18,19. Lipid metabolism also regulates osteoblasts via the Wnt signaling pathway and is closely related to calcification mechanisms and bone metabolism20,21,22. In recent years, the association of OPLL/OLF with obesity, malnutrition, genetic factors, and metabolic dysfunction–associated fatty liver disease (MAFLD) has become clear10,11,12,13,14,15,23,24,25,26. This suggests that metabolic disturbances related to spinal ligament ossification could affect systemic bone metabolism and trigger ectopic ossification. Despite the common features of dyslipidemia and spinal ligament ossification, no study has investigated their relationship in detail.
To date, there is no effective treatment for OPLL/OLF, and this motivated us to identify the underlying causes associated with the onset and exacerbation of this disease. Hence, we aimed to investigate the association between dyslipidemia and OPLL/OLF using a large dataset of subjects who underwent health screening tests, including computed tomography (CT).
Methods
Study design
A retrospective cross-sectional study was conducted in accordance with the Declaration of Helsinki (1964), including subjects between April 2020 and May 2021. The study was approved by ethical review board of the Hakodate Central Hospital and Hokkaido University Hospital, and the need for obtaining patients’ informed consent was waived owing to the retrospective nature of the study and the deidentified data used.
Patients
A database of 12,740 Japanese patients from a single institution was used. All subjects, with or without symptoms, underwent routine health examinations once per year or once every few years. Most of the subjects were community residents and facility staff, including physicians, nurses, nursing assistants, therapists, and clerks; the majority underwent blood tests at their discretion. The subjects underwent CT of the trunk at their discretion; they also selected the scan region (neck to chest, abdomen to pelvis, or neck to pelvis). Among the 1,002 subjects who underwent CT, 525 were selected for whom CT allowed assessment of the cervical spine to pelvis region and for whom blood test data were available. Finally, a total of 458 subjects (251 men, 207 women), aged 30 to 78 years, with OLF and/or OPLL and without spinal ligament ossification were included in this study (Fig. 1).
Demographics and comorbidities
Demographic data were obtained from all subjects using a questionnaire assessing height, weight, smoking history, the presence of comorbidities (hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, stroke, and renal disease), and the current and past use of medication for the comorbidities. The questionnaire also assessed the presence of weight gain from the age of 20 years and exercise routine of more than 30 min per day.
Serological assessment parameters
All serological assessments were performed using fasting blood samples. The assessed parameters included total cholesterol (T-Cho), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin A1c (HbA1c), and blood glucose. The LDL-C/HDL-C ratio (L/H ratio) was calculated and used as one of the parameters to indicate the severity of dyslipidemia. The rationale is based on the suggestion that the L/H ratio is a better predictor of atherosclerosis progression and ischemic cardiovascular disease than LDL-C and HDL-C separately; an L/H ratio ≤ 2.0 significantly inhibits the progression of coronary artery plaque, while a ratio ≤ 1.5 further strengthens this effect27.
Diagnostic criteria for dyslipidemia by the Japan Atherosclerosis Society
For adults, the 2012 guidelines of the Japan Atherosclerosis Society define lipid abnormality as a TG concentration ≥ 150 mg/dL, LDL-C ≥ 140 mg/dL, and/or HDL-C < 40 mg/dL28.
In the present study, subjects with dyslipidemia were defined as those who met any of the above criteria or who were taking therapeutic drugs for dyslipidemia.
Assessment of the presence of OLF and OPLL
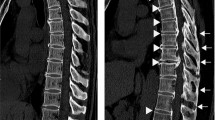
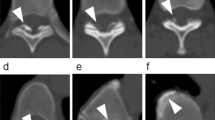
Axial CT images were used to evaluate the distribution of OLF and OPLL (cervical [C], thoracic [T], and/or lumbar [L]). CT was performed using an Aquilion One™/Genesis Edition system (Canon Medical Systems Inc., Tochigi, Japan). The presence of OLF and OPLL was determined according to previous reports (Fig. 2); OLF was essentially defined as ossification of the ligamentum flavum with a thickness of 3 mm or more. Ossification of less than 3 mm, which was clear on axial images, was also considered OLF3,11, as was mushroom-shaped ossification localized in the center of the lamina3,11. OPLL was essentially defined as ossification of the posterior longitudinal ligament with a thickness of 2 mm or more on axial images11,29. All CT scans were assessed by two board-certified spine surgeons; disagreements were resolved by consensus.
Criteria for the identification of OLF and OPLL on computed tomography. (a) OLF that is clearly visible but less than 3 mm in thickness (white arrowheads). (b) OLF with an apparent thickness of 3 mm or more (black arrowheads). (c) A mushroom-shaped OLF that is localized at the center of the laminae (white arrow). (d,e) OPLL with an apparent thickness of 2 mm or more (black arrow). (f) OPLL that is small but with a thickness of 2 mm or more (bold white arrow). OLF, ossification of the ligamentum flavum; OPLL, ossification of the posterior longitudinal ligament.
Grouping of subjects
All subjects were divided into the following four groups according to the type of concomitant spinal ligament ossification present: no ligament ossification (control; n = 230), OLF (n = 167), cervical OPLL (C-OPLL; n = 28), thoracic OPLL (T-OPLL; n = 33). Subjects with OPLL in the cervical spine exclusively—with or without concomitant OLF—were classified as the C-OPLL group, and subjects with OPLL in the thoracic spine—with or without concomitant cervical OPLL, lumbar OPLL (L-OPLL), or OLF—were classified as the T-OPLL group. Subjects with OLF without concomitant C-OPLL, T-OPLL, or L-OPLL were classified as the OLF group. This classification was based on the following rationale: (1) C-OPLL has a comparatively high probability of occurring concomitantly with OLF9; (2) compared to C-OPLL, T-OPLL has distinct features, such as morbid obesity, early onset of symptoms, and diffuse ligament ossification of the entire spine, including OLF, C-OPLL, and L-OPLL12,15,24; and (3) OLF occurs mostly in the thoracic spine and rarely in the cervical or lumbar spine29.
All subjects were also divided into the following two groups according to the presence or absence of concomitant dyslipidemia: DL(+) (n = 215), and DL(−) (n = 243).
Statistical analysis
The data were analyzed using BellCurve for Excel (version 3.10; Social Survey Research Information Co., Ltd., Tokyo, Japan). Results are presented as mean ± standard deviation. Four-group comparisons were evaluated using the Kruskal–Wallis and Fisher’s exact tests. Statistical significance was set at P < 0.05; however, in the four-group comparisons, it was set at P < 0.012 (0.05/4) with a Bonferroni correction considering multiple testing.
Univariate logistic regression analysis was performed for age, body mass index (BMI), sex, comorbidities, the presence of weight gain since the age of 20 years, T-Cho (mg/dL), TG (mg/dL), HDL-C (mg/dL), LDL-C (mg/dL), and the L/H ratio as risk factors for the prevalence of OLF, C-OPLL, and T-OPLL. For variables determined to be significant (P < 0.05) in the univariate logistic regression analysis, the relationship between factors affecting the presence of the three types of spinal ligament ossification (i.e., OLF, C-OPLL, and T-OPLL) was evaluated using multivariate logistic regression analysis. Diabetes mellitus was analyzed as a candidate variable regardless of its significance in the univariate logistic regression analysis because it has been mentioned as a risk factor for C-OPLL in the literature. Statistical significance was set at P < 0.016 (0.05/3) with a Bonferroni correction considering multiple testing of subjects with multiple concomitant types of ossification.
Cut-off values of the parameters of lipid metabolism for the presence of OLF, C-OPLL, and T-OPLL were calculated from receiver operating characteristic (ROC) curves. In this analysis, only subjects who were not receiving medication were included, considering the effect of medications on serum parameter levels.
Results
Baseline patient characteristics
The characteristics of the subjects with OLF and/or OPLL, and the controls without ligament ossification, are shown in Table 1. A total of 49.7% (228/458) had OLF or OPLL; the proportion of subjects with OLF was 36.4% (167/458); C-OPLL, 6.1% (28/458); and T-OPLL, 7.2% (33/458).
Association between the ossification types and the proportion of concomitant dyslipidemia
We first examined the comorbidity and severity of dyslipidemia in subjects with each type of spinal ligament ossification. Data on the characteristics of each group are shown in Table 2. The mean BMI of the OLF, C-OPLL, and T-OPLL groups was significantly higher than that of the control group (P < 0.01). The mean BMI of the T-OPLL group was the highest among all the groups.
The comorbidity of dyslipidemia in the OLF, C-OPLL, and T-OPLL groups was significantly higher than that in the control group (P < 0.01). Moreover, the comorbidity of dyslipidemia in the C-OPLL and T-OPLL groups exceeded 70% (control, 33.0%; OLF, 56.2%; C-OPLL, 73.0%, and T-OPLL, 72.7%) (Fig. 3). The mean TG concentration in the OLF, C-OPLL, and T-OPLL groups was significantly higher (P < 0.01), while the mean HDL-C concentration was significantly lower (P < 0.01), than that of the control group. We also assessed the L/H ratio, one of the indicators of the severity of dyslipidemia. The proportion of subjects with an L/H ratio ≤ 1.5 in the T-OPLL group was significantly lower than that in the control group (P < 0.01). The proportion of subjects with an L/H ratio ≥ 3.0 in the OLF, C-OPLL, and T-OPLL groups was higher than that in the control group, while the proportion in the T-OPLL group was the highest; however, the differences were not significant.
Comparison of the prevalence of dyslipidemia among the ossification types. (a) Prevalence of dyslipidemia among the ossification types. (b) Rate of the subjects using medication for dyslipidemia among the ossification types. *P < 0.01. OLF, ossification of the ligamentum flavum; OPLL, ossification of the posterior longitudinal ligament; C, cervical; T, thoracic.
Rate of subjects with concomitant spinal ligament ossification according to the presence of dyslipidemia
Baseline clinical information and the results of lipid-related parameters based on blood sampling are shown in Table 3. The mean age, BMI, male ratio, and proportion of concomitant hypertension and diabetes mellitus in the DL(+) group were significantly higher than those in the DL(−) group. Furthermore, the proportion of subjects without ligament ossification in the DL(+) group was significantly lower than that in the DL(−) group (P < 0.001), whereas the comorbidity of OLF, C-OPLL, and T-OPLL in the DL(+) group was significantly higher than that in the DL(−) group (P < 0.01).
Risk factors for the prevalence of OLF, C-OPLL, and T-OPLL
After characterizing the comorbid ossification types associated with dyslipidemia, we conducted a multivariate logistic regression analysis to identify risk factors for an increased prevalence of OLF and OPLL. Risk factors for the presence of OLF, C-OPLL, and T-OPLL, respectively, were compared to subjects without spinal ligament ossification. (i.e., a maximum of three duplicate analyses were conducted for subjects who had all three types of concomitant ossification). The regression coefficients (β) and standardized β-values are shown in Table 4 and Fig. 4. Dyslipidemia was found to be associated with the prevalence of OLF, C-OPLL, and T-OPLL. Comparing the β-values among the ossification types, C-OPLL was the most strongly associated with dyslipidemia (β, 1.48; 95% CI 0.35–2.60). On analysis of risk factors by sex, T-OPLL was the most strongly associated with dyslipidemia in men (β, 2.24; 95% CI 0.65–3.84), whereas C-OPLL was the most strongly associated with dyslipidemia in women (β, 3.64; 95% CI 0.31–6.97). Furthermore, age was associated with the prevalence of T-OPLL, being male with the prevalence of OLF and C-OPLL, and BMI with the prevalence of OLF and T-OPLL. Diabetes mellitus and the L/H ratio was not associated with the prevalence of any ossification type.
Comparison of the correlation of dyslipidemia with OLF, C-OPLL, and T-OPLL. Results shown are β-values with 95% CIs (error bars). (a) All subjects. (b) Men. (c) Women. OLF, ossification of the ligamentum flavum; OPLL, ossification of the posterior longitudinal ligament; β, regression coefficient; CI, confidence interval; C, cervical; T, thoracic.
To determine the relative contribution of risk factors to ossification, we compared the standardized β-values for each type of spinal ligament ossification. For both OLF and C-OPLL, the standardized β-value for dyslipidemia was higher than that for age, BMI, being male, the L/H ratio, and diabetes mellitus. For T-OPLL, the standardized β-value for BMI was higher than that for dyslipidemia.
Finally, we calculated the cut-off values of the parameters of lipid metabolism for the presence of OLF, C-OPLL, and T-OPLL by the ROC curves. For C-OPLL, TG was 104 mg/dL (area under the ROC curve [AUC], 0.71; sensitivity, 0.80; specificity, 0.71) and HDL-C was 57 mg/dL (AUC, 0.70; sensitivity, 0.66; specificity, 0.65) (Supplemental Table 1).
Discussion
The present study showed that the prevalence of dyslipidemia in subjects with OLF and OPLL was higher than we expected (56–73%), and was 1.6–2.2 times higher than that of subjects without ligament ossification (33%). The clinical significance of this finding is that dyslipidemia in OLF and OPLL was identified using a large dataset, which has not been previously focused on. Considering that this study included a large number of healthy community residents and institutional staff, the possible reason for the high prevalence of dyslipidemia in these subjects may involve metabolic disturbances related to visceral fat—such as obesity and MAFLD—rather than a sedentary lifestyle, physical inactivity, and sarcopenia, which are related symptoms of myelopathy. Because OLF and OPLL often coexist9,10,11,12, risk factors may have been confounded in the past; however, we distinguished between ossification types and successfully analyzed the association with dyslipidemia.
Our results suggest that dyslipidemia is associated with the development of spinal ligament ossification. This was reinforced by the (1) increased proportion of subjects with higher L/H ratios in OLF and OPLL; (2) higher proportion of these conditions in the DL(+) than the DL(−) group; and (3) results of the multivariate logistic regression analysis that correlated the prevalence of these conditions with dyslipidemia. An important finding was that dyslipidemia was more strongly associated with the prevalence of OLF and C-OPLL than with other risk factors when compared using standardized β-values for the relative risk of each ossification type. We also determined the relative strength of the association of dyslipidemia with the prevalence of OLF, C-OPLL, and T-OPLL by comparing β-values. Interestingly, dyslipidemia was most strongly associated with the prevalence of C-OPLL in women. These results may reflect the fact that some patients with symptomatic OLF and those with C-OPLL have a BMI exceeding the average BMI of the general Japanese population, and that patients with T-OPLL tend to have severe obesity (BMI ≥ 35 kg/m2). Recently, it has been reported that the severity of ossification of the entire spine in patients with symptomatic OPLL is associated with the severity of MAFLD rather than being associated with BMI24. The fact that patients with OPLL have a high frequency of comorbid lifestyle-related diseases also suggests that ossified lesions may be exacerbated by abnormal lipid metabolism related to visceral fat obesity, rather than by mere local instability or mechanical stimulation30,31,32.
The mechanisms by which abnormal lipid metabolism causes ectopic ossification of spinal ligaments are speculative. (1) Visceral fat accumulation and fatty liver promote the production of LDL-C in the liver17,18,19. (2) The oxidative stress environment caused by hyper-LDLemia activates Wnt3 secretion from vascular endothelial cells and Wnt signaling via LDL receptor-related protein 5 (LRP5) or LRP6, which induce atherosclerotic calcification20,21,22. (3) In bone, LRP5/Wnt/Frizzled forms a complex21,22. This signaling activity results in the accumulation of beta-catenin, which regulates the expression of many genes, including Cbfa-1, which is essential for osteoblast differentiation20,21,22. The details of the relationship between abnormal lipid metabolism and ossification of spinal ligaments should be studied in the future.
By means of multivariate logistic regression analysis, we identified further independent risk factors for spinal ligament ossification: BMI for OPLL and T-OPLL; being male for OLF and C-OPLL; and age for T-OPLL. Obesity is more common in symptomatic OLF and T-OPLL, with a strong ossification tendency of the entire spinal ligament12,13. In addition, OLF and C-OPLL are more likely to occur in men6,29, whereas T-OPLL is more likely to occur in women5. Our results confirm the previous findings; however, we would like to emphasize that our results are not necessarily synonymous with the risk of worsening ossified lesions, because the present study was based on a risk analysis of an increased prevalence of OLF and OPLL, and included many asymptomatic subjects with relatively small ossified lesions.
The prevalence of OLF (36.4%), C-OPLL (6.1%), and T-OPLL (7.2%) should be interpreted with caution because subjects with only ossification of the anterior longitudinal ligament (OALL) were not included in this study. In our previous study with a similar approach, asymptomatic subjects with only OALL were about 14%11. In previous large CT-based studies of Japanese subjects, the prevalence of OLF was 36.3%, C-OPLL was 6.3%, and T-OPLL was 1.6–1.9%3,5,29; the prevalence of OLF and C-OPLL was comparable to our results. The reason why the prevalence of T-OPLL was different from previous studies was probably due to the different definition of T-OPLL in this study compared to previous ones.
There are several limitations to the present study. First, it has a cross-sectional design and it is not possible to conclude whether dyslipidemia is a causative factor or a consequence of OLF or OPLL. Second, we used a large single-center database, but the sample size of the C-OPLL and T-OPLL groups was small. Given that the prevalence of OPLL in the general Japanese population is low5,6,7, a multicenter study is needed to validate our results. Third, the present study is based on data from the Japanese population, which may not necessarily be applicable to other populations. Forth, all subjects underwent neck-to-pelvis CT by their own choice, although the majority of the 12,740 subjects who underwent a physical examination did not undergo CT, or underwent either neck-to-chest or abdomen-to-pelvis CT. Thus, the risk of selection bias cannot be completely excluded. Finally, we did not compare the size of OPLL/OLF with specific values of lipid abnormalities.
In summary, this study found that dyslipidemia is a risk factor for an increased prevalence of OLF, C-OPLL, and T-OPLL in Japanese individuals. Dyslipidemia was more strongly associated with spinal ligament ossification than other risk factors, especially in OLF and C-OPLL. Our results suggest that dyslipidemia is not only notably associated with the development of spinal ligament ossification, but that it also bears equal, if not higher, importance to other previously noted lifestyle-related diseases and obesity. Further studies are needed to clarify whether dyslipidemia is a causative factor for spinal ligament ossification.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the nature of this research, participants of this study did not agree for their data to be shared publicly, but are available from the corresponding author on reasonable request.
References
Guo, J. J., Luk, K. D., Karppinen, J., Yang, H. & Cheung, K. M. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: A population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine (Phila Pa 1976) 35, 51–56 (2010).
Lang, N. et al. Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur. Spine J. 22, 857–862 (2013).
Mori, K. et al. Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese: Results of CT-based cross-sectional study. Spine (Phila Pa 1976) 38, E1216–E1222 (2013).
Moon, B. J. et al. Prevalence, distribution, and significance of incidental thoracic ossification of the ligamentum flavum in Korean patients with back or leg pain: MR-based cross sectional study. J. Korean Neurosurg. Soc. 58, 112–118 (2015).
Mori, K. et al. Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: results of CT-based cross-sectional study. Spine (Phila Pa 1976) 39, 394–399 (2014).
Yoshimura, N. et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: A 3-year follow-up of the ROAD study. Osteoporos. Int. 25, 1089–1098 (2014).
Matsunaga, S. & Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976) 37, E309–E314 (2012).
Kim, T. J. et al. Prevalence of ossification of the posterior longitudinal ligament of the cervical spine. Joint Bone Spine 75, 471–474 (2008).
Kawaguchi, Y. et al. Characteristics of ossification of the spinal ligament; incidence of ossification of the ligamentum flavum in patients with cervical ossification of the posterior longitudinal ligament—Analysis of the whole spine using multidetector CT. J. Orthop. Sci. 21, 439–445 (2016).
Chaput, C. D., Siddiqui, M. & Rahm, M. D. Obesity and calcification of the ligaments of the spine: A comprehensive CT analysis of the entire spine in a random trauma population. Spine J. 19, 1346–1353 (2019).
Endo, T. et al. Association between obesity and ossification of spinal ligaments in 622 asymptomatic subjects: A cross-sectional study. J. Bone Miner. Metab. 40, 337–347 (2022).
Endo, T., Takahata, M., Koike, Y. & Iwasaki, N. Clinical characteristics of patients with thoracic myelopathy caused by ossification of the posterior longitudinal ligament. J. Bone Miner. Metab. 38, 63–69 (2020).
Endo, T. et al. Aggravation of ossified ligamentum flavum lesion is associated with the degree of obesity. Glob. Spine J. https://doi.org/10.1177/21925682211031514 (2021).
Kobashi, G. et al. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine (Phila Pa 1976) 29, 1006–1010 (2004).
Hisada, Y. et al. Distinct progression pattern of ossification of the posterior longitudinal ligament of the thoracic spine versus the cervical spine: A longitudinal whole-spine CT study. J. Neurosurg. Spine https://doi.org/10.3171/2022.1.SPINE211010 (2022).
Brown, E. E. et al. Genetic testing in dyslipidemia: A scientific statement from the National Lipid Association. J. Clin. Lipidol. 14, 398–413 (2020).
Vekic, J., Zeljkovic, A., Stefanovic, A., Jelic-Ivanovic, Z. & Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 92, 71–81 (2019).
Kopin, L. & Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 167, ITC81–ITC96 (2017).
Katsiki, N., Mikhailidis, D. P. & Mantzoros, C. S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 65, 1109–1123 (2016).
Albanese, I., Khan, K., Barratt, B., Al-Kindi, H. & Schwertani, A. Atherosclerotic calcification: Wnt is the hint. J. Am. Heart Assoc. 7, e007356 (2018).
Karner, C. M. & Long, F. Wnt signaling and cellular metabolism in osteoblasts. Cell. Mol. Life Sci. 74, 1649–1657 (2017).
Alekos, N. S., Moorer, M. C. & Riddle, R. C. Dual effects of lipid metabolism on osteoblast function. Front. Endocrinol. (Lausanne) 11, 578194 (2020).
Endo, T. et al. Association between vitamin A intake and disease severity in early-onset heterotopic ossification of the posterior longitudinal ligament of the spine. Glob. Spine J. https://doi.org/10.1177/2192568221989300 (2021).
Endo, T. et al. Close association between non-alcoholic fatty liver disease and ossification of the posterior longitudinal ligament of the spine. Sci. Rep. 11, 17412 (2021).
Okamoto, K. et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); Findings from a case-control study in Japan. J. Bone Miner. Metab. 22, 612–617 (2004).
Nakajima, M. et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 46, 1012–1016 (2014).
Nicholls, S. J. et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 297, 499–508 (2007).
Teramoto, T. et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J. Atheroscler. Thromb. 20, 517–523 (2013).
Fujimori, T. et al. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: Results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976) 41, 1668–1676 (2016).
Maigne, J. Y., Ayral, X. & Guérin-Surville, H. Frequency and size of ossifications in the caudal attachments of the ligamentum flavum of the thoracic spine. Role of rotatory strains in their development. An anatomic study of 121 spines. Surg. Radiol. Anat. 14, 119–124 (1992).
Nakamura, T. et al. Degeneration and ossification of the yellow ligament in unstable spine. J. Spinal Disord. 3, 288–292 (1990).
Fan, D. et al. Osterix is a key target for mechanical signals in human thoracic ligament flavum cells. J. Cell. Physiol. 211, 577–584 (2007).
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
T.E. designed the project and provided overall project management. T.E. and M.T. drafted the main manuscript. T.E., M.T., R.F. and Y.K. contributed to analysis and interpretation of data. All other authors have contributed to data collection and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Endo, T., Takahata, M., Fujita, R. et al. Strong relationship between dyslipidemia and the ectopic ossification of the spinal ligaments. Sci Rep 12, 22617 (2022). https://doi.org/10.1038/s41598-022-27136-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27136-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.