Abstract
Maternal childhood trauma (MCT) is an important factor affecting offspring size at birth. Whether the effect of MCT persists during the subsequent development remains unclear. We present the results of a semi-longitudinal investigation examining the physical growth of infants born to mothers with high (HCT) and low (LCT) childhood trauma during the first year of life. One hundred healthy mother-infant dyads were included based on following criteria: exclusive breastfeeding, birth on term with appropriate weight for gestational age. MCT was assessed using the Early Life Stress Questionnaire. The weight, length, and head circumference of the infant were taken at birth, 5 and 12 months postpartum. Separate MANCOVA models were run for infant size at each age. We found an association between MCT and infant size at 5 and 12 months. The children of mothers with HCT had higher weight and greater head circumference than the children of mothers with LCT. These results suggest that MCT might contribute to developmental programming of offspring growth during the first year of life. From an evolutionary perspective, the larger size of HCT mother's offspring might represent an adaptation to potentially harsh environmental conditions. This effect might be mediated by epigenetic changes to DNA and altered breast milk composition.
Similar content being viewed by others
Introduction
The prenatal period is a crucial stage of individual development with long-term consequences for subsequent well-being and health1,2,3. This period is characterized by increased vulnerability and sensitivity to environmental disruptors that, if persistent, can disrupt prenatal development and result in fetal growth retardation4. One of such factors that influence pre- and postnatal development is maternal stress5.
Studies have shown that the placental mechanisms can protect the fetus against high maternal stress to some extent; however, these mechanisms are insufficient to completely diminish the effect of increased stress6. For example, the placental enzyme 11β-hydroxysteroid dehydrogenase, which converts cortisol to its less physiologically active form cortisone7,8, only does so to a certain extent; therefore, maternal and fetal cortisol levels during high stress periods correlate with each other8. The observed effects of maternal stress on the development of offspring include increased reactivity of the hypothalamus–pituitary–adrenal (HPA) axis 9,10, changes in body size and composition at birth and during postnatal life11,12. More specifically, a high level of maternal stress during pregnancy has been found to be associated with a lower weight, length, and head circumference at birth in several studies11,12,13,14, however, some authors have shown a positive or nonsignificant effect15,16,17.
Interestingly, a new body of evidence suggests that some maternal stressors experienced even before pregnancy might influence the growth and well-being of an offspring18. In particular, extreme traumatic stress during childhood can affect the sensitivity of the HPA axis and, consequently, lead to its long-term dysregulation19,20. For example, pregnant women who experience traumatic stress during childhood have a higher awaking cortisol response21,22. Recent studies investigating the effects of maternal childhood trauma (MCT) suggest that these developmental effects may be transgenerational (transmitted to the offspring). Although most studies have focused on the behavioral and emotional consequences of MCT, some of them suggest that the biological development of the offspring might be also affected23,24,25. For example, MCT, including violence, is associated with preterm birth26, lower birth weight24,27 (although this effect is not universal across all studies28), lower infant intracranial volume25, and a higher cephalization index23.
Our preliminary results showed that body weight and head circumference are positively associated with increased maternal childhood trauma among exclusively breastfed infants29. Simultaneously, the study also suggested that neither catch-up growth nor breast milk energy density contributed to the observed growth effects29. While MCT-associated changes in infant body size have been detected in the very early stages of development–at birth and early postpartum, it remains unclear whether these changes persist in later stages of development. To address this, we studied body size in relation to the MCT level in the same breastfed infants during the first year of life. We hypothesized that the differences in the growth pattern between infants of mothers with high childhood trauma (HCT) and low childhood trauma (LCT) observed during the initial months would persist further in their development.
Materials and methods
Study group and protocol
The study group consisted of 100 mother-infant dyads from Poland. The recruitment of dyads took place when the babies were about 5 months old and was based on the following inclusion criteria: (a) for mothers: age older than 18 years old; not taking steroid medication, smoking, or drinking alcohol during pregnancy or lactation; without metabolic or congenital diseases (b) for infants: being born from a single and uncomplicated pregnancy; at least 37 weeks of gestation with birth weight at least 2600 g and exclusively breastfed for at least 5 months. The above criteria where established based on the literature indicating that children born prematurely or small for gestational age have a different developmental pattern30,31,32, especially during the first year of life. Most of them experience catch-up growth, but some remain consequently smaller33 during this period. Thus, including prematurely born infants in the study group could potentially confound the results.
The study protocol included two meetings with mothers and infants. The first meeting occurred when the babies were approximately 5 months old. During this meeting, we collected maternal and infant measurements, information on maternal socioeconomic status and life satisfaction, birth outcomes, and postpartum depression. At the second meeting that took place when the children were about 12 months, maternal and infant measurements were collected again. At this point, mothers were also asked about their traumatic experience during childhood. This research protocol was approved by the Bioethical Committee of Lower Silesian Medical Chamber in Wroclaw (protocol code 1/NT/2016 from 10.02.2016).
Each participant in the study received information about the course and purpose of the study, giving informed, written consent to participate in the study in accordance with the tenets of the Declaration of Helsinki. Respondents were allowed to opt out of the study at any stage of the study, and there were no legal or financial consequences for opting out.
Maternal childhood trauma, postpartum depression, and socioeconomic status
The MCT was evaluated using the Polish version of the Early Life Stress Questionnaire (ELSQ)34, which is an international psychological tool constructed based on the Child Abuse and Trauma Scale35. Women were asked to indicate their childhood traumatic events up to the age of 12 years. The questionnaire included 19 events such as peer bullying, domestic violence, sexual harassment, long-term illness, or natural disasters. Each event scored 1 point.
Experiencing symptoms of maternal postnatal depression (maternal PD risk) was evaluated using the Polish version of the Edinburgh Postpartum Depression Scale (EPDS). This 4-point and 10-item questionnaire is a widely used tool in clinical and nonclinical settings28,36. Following other studies, including those conducted on Polish samples, the cut-off point for higher risk of postpartum depression was defined by a score value of at least 1436. Additionally, the participants were asked to assess their financial satisfaction on a 7-point Likert scale (1- very unsatisfied; 7- very satisfied) and declare their educational status (higher education with at least Bachelor's or nonhigher education).
Anthropometric measurements
Anthropometric measurements of the dyads were taken twice. First, when the infants were approximately five months old and second, at twelve months. Maternal body weight was measured with a Tanita SC-240 MA scale (accuracy of 0.1 kg) and height with a stadiometer (accuracy of 0.1 cm). The mothers also reported their pre-pregnancy body weight. The measurements were used to calculate body mass index (BMI; BMI = body weight [kg]/body height [cm]^2). For infants, measurements included body length using a Seca measuring board (model 417; accuracy of 0.1 cm), weight using an analog hospital scale (with an accuracy of 0.1 kg), and head circumference using a measuring tape (with the accuracy of 0.1 cm). Birth outcomes (gestational age, weight, length, and head circumference) were taken from the child's health record.
Statistical methods
Mothers were divided into LCT or HCT groups according to the median value (Me = 2) of the ELSQ score. Differences in mean values of main study variables between LCT and HCT mothers were tested using t-test. Differences in the number of mothers according to increased depression risk and infant sex between LCT and HCT group was tested using chi2 test. The association between infant weight, length, head circumference, and maternal childhood trauma was tested using General Linear Models. The separate multivariate analyses of covariance (MANCOVA) models were built for growth parameters at each infant age (at birth, 5 and 12 months) with all size parameters as dependent variables, the level of MCT (low–high), infant sex (boy-girl) and risk of maternal postpartum depression (low–high) as categorical predictors, and maternal BMI and infant age as covariates. Following the MANCOVA models, we also ran separate univariate analyses of covariance (ANCOVA) to test which of the dependent variables were statistically significant. In addition, Cohens’ d values (group comparisons with different sample size)37 was calculated to quantify the effect of MCT on infant body size parameters.
The Henze-Zirkler test indicated multivariate normality of the dependent variables, and the Box M test confirmed the homogeneity of the variance–covariance matrices. Since the deviations from normality were relatively minor and our sample size was sufficient to obtain robust results (n > 30 in both groups), we followed the standard parametric procedure for the univariate analysis. Statistical analysis was performed using StatSoft STATISTICA (data analysis software system), version 12 (www.statsoft.com), and the R statistical environment (version 3.6.0). The statistical significance level was established at p < 0.05, however, we also reported a marginally significant effect at 0.06 > p > 0.05.
Results
The ELSQ score in the study group ranged from 0 to 11 traumatic events during childhood. 46% (n = 46) of the participants suffered from more than 2 traumatic events up to 12 years of age, while only 14% (n = 14) of the women in the study group did not experience any traumatic events. Therefore, mothers who experienced more than 2 traumas were included in the HCT group. Out of all participating mothers, 14 (14%) had an increased risk of postpartum depression according to the defined cut-off point when their babies were five months old. The results of the chi2 test indicated that there was no association between the MCT and the risk of postpartum depression. The number of women with an increased risk of postpartum depression did not differ between the LCT and HCT groups (chi2 < 0.01, p = 0.972). Furthermore, neither maternal economic satisfaction nor education was associated with MCT (Table 1).
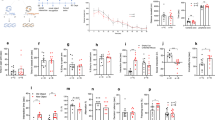
Significant differences between groups in the infant size characteristics (body weight, and head circumference) were found at the age of 5 and 12 months (Table 1). In particular infants of HCT mothers were significantly heavier (t = −2.71 p = 0.008; t = −2.91, p = 0.005 at 5 and 12 months respectively) and had larger head circumference than infants of LCT mothers (t = −2.04, p = 0.044; t = −2.27, p = 0.028 for 5 and 12 months, respectively). No significant differences were found in infant body size at birth and the gestational age. Maternal characteristics also did not differ between the HCT and LCT groups, excluding maternal age when the children were 5 months old (Table 1). This accidental but significant difference in age had no effect on any of the infant size parameters.
The MANCOVA models show, that the differences between LCT and HCT in infant growth characteristics (Wilks λ = 0.90, F (3,92) = 3.57, p = 0.0 17) remained significant at the age of 5 months and marginally significant at the age of 12 months (Wilks λ = 0.92, F (3,87) = 2.61, p = 0.056) after controlling for maternal BMI and risk of postpartum depression as well as infant sex, and age (Table 2). The effect of MCT on growth parameters at birth was not statistically significant (Wilks λ = 0.98, F (3,87) = 0.64, p = 0.592).
The results of the univariate analysis did not show a significant effect of maternal trauma at birth (Table3). In contrast, a significant association between the level of MCT and infant weight (F (1,94) = 8.06, p = 0.006, Cohen’s d = 0.53) as well as head circumference (F (1,94) = 6.17, p = 0.015, Cohen’s d = 0.42) was found at 5 months (Table 4 and Fig. 1). Similar effects were observed for both also at 12 months (F (1,89) = 7.17, p = 0.009, d = 0.60; F (1,89) = 5.06, p = 0.027, d = 0.45 for weight and head circumference, respectively) (Table 5 and Fig. 1). The effect of MCT on body length at 5 and 12 months was not significant.
Discussion
The current semi-longitudinal study for the first time demonstrates that although maternal childhood trauma does not affect infant size at birth, it is significantly and positively associated with infant size during the first year of life. Mothers with HCT had infants with almost 10% higher weight and 2% greater head circumference than mothers with LCT at the age of 5 and 12 months.
The presented results corroborate the results of an experimental study in hens showing that traumatic stress experienced during early life and puberty is related to earlier hatching of offspring and their increased body weight at the age of one month38. In contrast, a human study by Choi et al. 28 has found a negative but indirect association between maternal childhood trauma and infant body size (weight and length) via maternal postpartum depression. Almost 30% of the participants in this study suffered from postpartum depression, which in turn predicted a negative infant development outcome at the age of 12 months28. The low prevalence of postpartum depression observed in our study (about 14% of the participants) could be related to a common practice of universal and exclusive breastfeeding among the study participants, which has been previously shown to have beneficial effects on maternal well-being39 and infant development40. Contrasting findings also came from the study by Smith et al.24, who reported a lower birth weight in infants born to mothers who experienced HCT than those with LCT. The discrepancies in the findings between this and our study could be due to the fact that the former did not control for preterm birth and/or low gestational age, both associated with decreased weight at birth24.
The higher weight in babies born to HCT mothers observed in our study might be associated with impaired glucose metabolism41,42 which in the future might result in an increased risk of glucose intolerance and metabolic syndrome. Such effects usually co-occur with overweight and obesity2,43 and have been previously reported in adult descendants of parents with higher trauma44. In addition, higher body weight during infancy is related to the risk of being overweight and obesity during adulthood45. Thus, the results of our study suggest that the tendency towards an increased weight among children born to HCT mothers might be long-lasting, and probably result in a higher risk of metabolic conditions in adulthood.
Our analysis also showed a larger head circumference among infants of HCT mothers compared to the infant of LCT mothers. This result is in line with a study by Appleton et al.23, who found that infants born to women with HCT had a higher cephalization index. An increase in the value of this index is usually associated with an increase in head circumference.
Overall, these results suggest that MCT experience may induce intergenerational changes in physical development even without trauma present in the next generation46. These effects are hypothesized to be mediated by epigenetic effects on germline and somatic cells, including DNA methylation and histone and RNA modifications46. Breast milk, which contains noncoding RNAs, such as microRNAs, serves as epigenetic vectors in molecular communication between mother and offspring and constitutes the first vital gate allowing these developmental effects46. Furthermore, HCT may program HPA axis reactivity to produce an increased level of stress hormones, including cortisol, many years after exposition47. Thus, cortisol transmitted from serum to breast milk might serve as a second gate48. Glucocorticoid levels during the perinatal period demonstrate a long-term programming effect on growth and health during later life48,49. Recent literature has shown, that higher values of head circumference and body weight are related to altered levels of fatty acids50,51 and glucocorticoids52,53,54 in breast milk. Additionally, our previous research showed that maternal stress reactivity is positively associated with the level of polyunsaturated fatty acids in milk55, which are crucial for brain growth and development56. It is important to note that the infants in our study group were breastfed exclusively for at least 5 months. Thus, it is possible that maternal HCT was reflected in a modified level of polyunsaturated fatty acids and higher cortisol in milk and, as a result, a faster increase in body mass and head circumference.
From the evolutionary perspective, a larger size of offspring born to HCT mothers might result from a faster life pace, as posited by the Life History Theory. The term Life History was introduced by Stearns in 1992 and emphasizes that environmental conditions can push individuals into two types of life strategies: fast and slow57. Individuals that exist under harsh environmental conditions, higher levels of stress and increased risk of mortality must adapt, so such conditions would result in accelerated sexual maturation (e.g., early rapid fat gain) and earlier successful reproduction58. On the other hand, it would be associated with significant costs to health and longevity58,59. For example, early life adiposity predisposes to obesity and other metabolic disorders in adulthood, while earlier and faster reproduction generates considerable metabolic cost to the individual60. According to this theory we postulate that the MCT can be, to some extent, treated as an indicator of a stressful environment and harsh condition within the meaning of the Life History Theory. It is also possible that the observed effect may represent a mismatch between maternal and offspring developmental environments, where the maternal adaptive response to a harsh environment might induce long-term changes in offspring development, even in the absence of ecological obstacles.
One of the limitations of our study might be the fact that participants childhood traumatic events retrospectively. However, this limitation is difficult to avoid due to the extended period between maternal childhood and pregnancy. We also did not know how often and for how long women had been exposed to the reported experiences. To minimize the recall bias, we assessed MCT using a standardized psychological questionnaire, which was successfully applied in several other studies34,61,62,63. Furthermore, we did not control for post-traumatic stress disorder (PTSD) and resilience as the additional factors in the analysis. Several studies have underlined the effect of childhood trauma on the prevalence of PTSD64. The latter has been shown to have a long-lasting effect on maternal metabolism and as a consequence, on the development and health of the offspring65. Whereas resilience is postulated as a protective factor with the potential to decrease the negative effect of childhood trauma on the development of offspring and future health66. Finally, the information about birth outcomes was collected from child health records instead of being measured, so the measurement protocol could not have been entirely consistent between different hospitals.
Our study demonstrates that MCT is significantly associated with the size of the offspring during the first year of life even after adjusting for other significant factors that influenced body size such as maternal BMI and postnatal depression, infant age, sex, and age. Children of mothers with HCT had higher weight and larger head circumference than peers born to mothers with LCT, and this effect was independent of body size at birth. These results suggest that MCT might contribute to alterations in the maternal physiology of the HPA axis, which in turn program the development of offspring in the long-term perspective. We propose that this effect may arise from the faster pace of life syndrome mediated by epigenetic changes to DNA and the altered composition of breast milk.
Data availability
The data set analyzed during the current study is available from the corresponding author on request.
References
Kitsiou-Tzeli, S. & Tzetis, M. Maternal epigenetics and fetal and neonatal growth. Curr. Opin. Endocrinol. Diabetes Obes. 24, 43–46 (2017).
Rodgers, A. & Sferruzzi-Perri, A. N. Developmental programming of offspring adipose tissue biology and obesity risk. Int. J. Obes. 45, 1170–1192 (2021).
Cattane, N. et al. Depression, obesity and their comorbidity during pregnancy: Effects on the offspring’s mental and physical health. Mol. Psychiatry 26, 462–481 (2021).
Gordijn, S. J. et al. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 48, 333–339 (2016).
Graignic-Philippe, R., Dayan, J., Chokron, S., Jacquet, A.-Y. & Tordjman, S. Effects of prenatal stress on fetal and child development: A critical literature review. Neurosci. Biobehav. Rev. 43, 137–162 (2014).
Benediktsson, R., Calder, A. A., Edwards, C. R. & Seckl, J. R. Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46, 161–166 (1997).
Murphy, B. E., Clark, S. J., Donald, I. R., Pinsky, M. & Vedady, D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am. J. Obstet. Gynecol. 118, 538–541 (1974).
Gitau, R., Cameron, A., Fisk, N. M. & Glover, V. Fetal exposure to maternal cortisol. The Lancet 352, 707–708 (1998).
Entringer, S., Kumsta, R., Hellhammer, D. H., Wadhwa, P. D. & Wüst, S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm. Behav. 55, 292–298 (2009).
Glover, V., O’Connor, T. G. & O’Donnell, K. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 35, 17–22 (2010).
Lima, S. A. M. et al. Is the risk of low birth weight or preterm labor greater when maternal stress is experienced during pregnancy? A systematic review and meta-analysis of cohort studies. PLoS ONE 13, e0200594 (2018).
Matvienko-Sikar, K. et al. Maternal stress in the first 1000 days and risk of childhood obesity: A systematic review. J. Reprod. Infant Psychol. 39, 180–204 (2021).
Ae-Ngibise, K. A. et al. Prenatal maternal stress and birth outcomes in rural Ghana: Sex-specific associations. BMC Pregnancy Childbirth 19, 391 (2019).
Lederman, S. A. et al. The effects of the world trade center event on birth outcomes among term deliveries at three lower manhattan hospitals. Environ. Health Perspect. 112, 1772–1778 (2004).
Endara, S. M. et al. Does acute maternal stress in pregnancy affect infant health outcomes? Examination of a large cohort of infants born after the terrorist attacks of September 11, 2001. BMC Public Health 9, 252 (2009).
Liczbińska, G. & Králík, M. Body size at birth in babies born during World War II: The evidence from Poland. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 32, e23421 (2020).
Torche, F. & Villarreal, A. Prenatal exposure to violence and birth weight in Mexico: Selectivity, exposure, and behavioral responses. Am. Sociol. Rev. 79, 966–992 (2014).
Scorza, P. et al. Research review: Intergenerational transmission of disadvantage: Epigenetics and parents’ childhoods as the first exposure. J. Child Psychol. Psychiatry 60, 119–132 (2019).
Kalmakis, K. A., Meyer, J. S., Chiodo, L. & Leung, K. Adverse childhood experiences and chronic hypothalamic-pituitary-adrenal activity. Stress Amst. Neth. 18, 446–450 (2015).
Juruena, M. F., Eror, F., Cleare, A. J. & Young, A. H. The role of early life stress in HPA axis and anxiety. In Anxiety Disorders: Rethinking and Understanding Recent Discoveries (ed. Kim, Y.-K.) 141–153 (Springer, 2020). https://doi.org/10.1007/978-981-32-9705-0_9.
Bublitz, M. H. & Stroud, L. R. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology 37, 1425–1430 (2012).
Bublitz, M. H. & Stroud, L. R. Maternal history of child abuse moderates the association between daily stress and diurnal cortisol in pregnancy: A pilot study. Stress Amst. Neth. 16, 706–710 (2013).
Appleton, A. A., Kiley, K., Holdsworth, E. A. & Schell, L. M. Social support during pregnancy modifies the association between maternal adverse childhood experiences and infant birth size. Matern. Child Health J. 23, 408–415 (2019).
Smith, M. V., Gotman, N. & Yonkers, K. A. Early childhood adversity and pregnancy outcomes. Matern. Child Health J. 20, 790–798 (2016).
Moog, N. K. et al. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol. Psychiatry 83, 120–127 (2018).
Christiaens, I., Hegadoren, K. & Olson, D. M. Adverse childhood experiences are associated with spontaneous preterm birth: A case–control study. BMC Med. 13, 124 (2015).
Cederbaum, J. A., Putnam-Hornstein, E., King, B., Gilbert, K. & Needell, B. Infant birth weight and maltreatment of adolescent mothers. Am. J. Prev. Med. 45, 197–201 (2013).
Choi, K. W. et al. Maternal childhood trauma, postpartum depression, and infant outcomes: Avoidant affective processing as a potential mechanism. J. Affect. Disord. 211, 107–115 (2017).
Apanasewicz, A. et al. Traumatized women’s infants are bigger than children of mothers without traumas. Anthropol. Anz. Ber. Uber Biol.-Anthropol Lit. 77, 359–374 (2020).
Riddle, W. R., DonLevy, S. C., Qi, X. F., Giuse, D. A. & Rosenbloom, S. T. Equations to support predictive automated postnatal growth curves for premature infants. J. Perinatol. 26, 354–358 (2006).
Cho, W. K. & Suh, B.-K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J. Pediatr. 59, 1–7 (2016).
Latal-Hajnal, B., von Siebenthal, K., Kovari, H., Bucher, H. U. & Largo, R. H. Postnatal growth in VLBW infants: Significant association with neurodevelopmental outcome. J. Pediatr. 143, 163–170 (2003).
Albertsson-Wikland, K. & Karlberg, J. Natural growth in children born small for gestational age with and without catch-up growth. Acta Paediatr. 83, 64–70 (1994).
Sokołowski, A. & Dragan, W. Ł. New empirical evidence on the validity and the reliability of the early life stress questionnaire in a polish sample. Front. Psychol. 8, 365 (2017).
Sanders, B. & Becker-Lausen, E. The measurement of psychological maltreatment: Early data on the child abuse and trauma scale. Child Abuse Negl. 19, 315–323 (1995).
Gibson, J., McKenzie-McHarg, K., Shakespeare, J., Price, J. & Gray, R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 119, 350–364 (2009).
Lenhard, W. & Lenhard, A. Computation of Effect Sizes. Retrieved from: https://www.psychometrica.de/effect_size.html. Psychometrica. https://doi.org/10.13140/RG.2.2.17823.92329 (2016).
Ericsson, M. et al. Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus). PLoS ONE 11, e0153879 (2016).
Figueiredo, B., Canário, C. & Field, T. Breastfeeding is negatively affected by prenatal depression and reduces postpartum depression. Psychol. Med. 44, 927–936 (2014).
Petherick, A. Development: Mother’s milk: A rich opportunity. Nature 468, S5-7 (2010).
Tosato, S. et al. Childhood and adulthood severe stressful experiences and biomarkers related to glucose metabolism: A Possible Association? Front. Psychiatry 12, (2021).
Tosato, S. et al. Childhood trauma and glucose metabolism in patients with first-episode psychosis. Psychoneuroendocrinology 113, 104536 (2020).
Krentz, A. J. Fortnightly review: Insulin resistance. BMJ 313, 1385–1389 (1996).
Stoica, V. et al. Transgenerational effects of traumatic historical events on the incidence of metabolic syndrome/nonalcoholic fatty liver disease in the romanian population. J. Med. Life 13, 475–483 (2020).
Field, A. E., Cook, N. R. & Gillman, M. W. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood**. Obes. Res. 13, 163–169 (2005).
Jawaid, A., Roszkowski, M. & Mansuy, I. M. Chapter twelve–transgenerational epigenetics of traumatic stress. In Progress in Molecular Biology and Translational Science (ed. Rutten, B. P. F.) vol. 158 273–298 (Academic Press, 2018).
Miller, G. E., Chen, E. & Zhou, E. S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 133, 25–45 (2007).
Hollanders, J. J., Heijboer, A. C., van der Voorn, B., Rotteveel, J. & Finken, M. J. J. Nutritional programming by glucocorticoids in breast milk: Targets, mechanisms and possible implications. Best Pract. Res. Clin. Endocrinol. Metab. 31, 397–408 (2017).
Entringer, S., Buss, C. & Wadhwa, P. D. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 17, 507–516 (2010).
Hahn-Holbrook, J., Le, T. B., Chung, A., Davis, E. P. & Glynn, L. M. Cortisol in human milk predicts child BMI. Obesity 24, 2471–2474 (2016).
Meyer, D. M. et al. Associations between long-chain PUFAs in maternal blood, cord blood, and breast milk and offspring body composition up to 5 years: Follow-up from the INFAT study. Eur. J. Clin. Nutr. 73, 458–464 (2019).
Scholtens, S. et al. Long-chain polyunsaturated fatty acids in breast milk and early weight gain in breast-fed infants. Br. J. Nutr. 101, 116–121 (2009).
Pedersen, L., Lauritzen, L., Brasholt, M., Buhl, T. & Bisgaard, H. Polyunsaturated fatty acid content of mother’s milk is associated with childhood body composition. Pediatr. Res. 72, 631–636 (2012).
Pundir, S. et al. Human milk glucocorticoid levels are associated with infant adiposity and head circumference over the first year of life. Front. Nutr. 7, 166 (2020).
Painter, R. C., Roseboom, T. J. & de Rooij, S. R. Long-term effects of prenatal stress and glucocorticoid exposure. Birth Defects Res. Part C Embryo Today Rev. 96, 315–324 (2012).
Koletzko, B. & Rodriguez-Palmero, M. Polyunsaturated fatty acids in human milk and their role in early infant development. J. Mammary Gland Biol. Neoplasia 4, 269–284 (1999).
Stearns, S. C., Allal, N. & Mace, R. Life history theory and human development. In Foundations of evolutionary psychology 47–69 (Taylor & Francis Group/Lawrence Erlbaum Associates, 2008).
Lehmann, A., Eccard, J. A., Scheffler, C., Kurvers, R. H. & Dammhahn, M. Under pressure: Human adolescents express a pace-of-life syndrome. Behav. Ecol. Sociobiol. 72, 57–57 (2018).
Sýkorová, K. & Flegr, J. Faster life history strategy manifests itself by lower age at menarche, higher sexual desire, and earlier reproduction in people with worse health. Sci. Rep. 11, 11254 (2021).
Jasienska, G. Costs of reproduction and ageing in the human female. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190615 (2020).
Sokołowski, A., Folkierska-Żukowska, M., Jednoróg, K., Wypych, M. & Dragan, W. Ł. It is not (always) the mismatch that beats you–on the relationship between interaction of early and recent life stress and emotion regulation, an fMRI Study. Brain Topogr. 35, 219–231 (2022).
Sokołowski, A., Folkierska-Żukowska, M., Jednoróg, K., Moodie, C. A. & Dragan, W. Ł. The relationship between early and recent life stress and emotional expression processing: A functional connectivity study. Cogn. Affect. Behav. Neurosci. 20, 588–603 (2020).
McFarlane, A. et al. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J. Integr. Neurosci. 4, 27–40 (2005).
Oh, W. et al. Comorbid trajectories of postpartum depression and PTSD among mothers with childhood trauma history: Course, predictors, processes and child adjustment. J. Affect. Disord. 200, 133–141 (2016).
Yehuda, R. et al. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 171, 872–880 (2014).
Sciaraffa, M. A., Zeanah, P. D. & Zeanah, C. H. Understanding and promoting resilience in the context of adverse childhood experiences. Early Child. Educ. J. 46, 343–353 (2018).
Acknowledgements
We thank Marieke Tollenaar for inspiring discussion about the consequences of maternal childhood trauma. The study was funded by a Polish National Science Center grant (project ID 2015/17/B/NZ8/02436) to Anna Ziomkiewicz. The study was approved by the Ethics Committee of Lower Silesian Medical Chamber in Wroclaw (protocol code 1/NT/2016 from 10.02.2016).
Author information
Authors and Affiliations
Contributions
A.A. contributed to the conception and design of the study, collected data, conducted the statistical analysis of data, and drafted the manuscript, D.D. conducted the statistical analysis of data and commented on the manuscript, M.P. and P.W. collected data and commented on the manuscript, M.B.-A. contributed to design of the study and commented on the manuscript, A.Z. contributed to design of the study, supervision, managed the data collection, funding beneficiary, and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Apanasewicz, A., Danel, D., Piosek, M. et al. Maternal childhood trauma is associated with offspring body size during the first year of life. Sci Rep 12, 19619 (2022). https://doi.org/10.1038/s41598-022-23740-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23740-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.