Abstract
Bone metastasis (BM) is rare in patients with pancreatic cancer (PC), but often neglected at the initial diagnosis and treatment. Bone metastasis is associated with a worse prognosis. This study was aimed to perform a large data analysis to determine the predictors and prognostic factors of BM in PC patients and to develop two nomograms to quantify the risks of BM and the prognosis of PC patients with BM. In the present study, we reviewed and collected the data of patients who were diagnosed as PC from 2010 to 2015 in the Surveillance, Epidemiology, and End Results (SEER) database. Univariate and multivariate logistic regression analyses were used together to screen and validate the risk factors for BM in PC patients. The independent prognostic factors for PC patients with BM were identified by Cox regression analysis. Finally, two nomograms were established via calibration curves, receiver operating characteristic (ROC) curve, and decision curve analysis (DCA). This study included 16,474 PC patients from the SEER database, and 226 of them were diagnosed with BM. The risk factors of BM for PC patients covered age, grade, T stage, N stage, tumor size, and primary site. The independent prognostic factors for PC patients with BM included age, race, grade, surgery, and lung metastasis. The AUC of the diagnostic nomogram was 0.728 in the training set and 0.690 in the testing set. In the prognostic nomogram, the AUC values of 6/12/18 month were 0.781/0.833/0.849 in the training set and 0.738/0.781/0.772 in the testing set. The calibration curve and DCA furtherly indicated the satisfactory clinical consistency of the nomograms. These nomograms could be accurate and personalized tools to predict the incidence of BM in PC patients and the prognosis of PC patients with BM. The nomograms can help clinicians make more personalized and effective treatment choices.
Similar content being viewed by others
Introduction
Pancreatic cancer (PC) is a malignancy with an extremely poor prognosis with the 5-year survival as low as 6%, ranking 7th in cancer mortality in England and Wales1,2. Due to the lack of effective and precise early diagnostic methods and non-specific symptoms, most patients are diagnosed in the late phase of the course. Only 10% of patients are capable to accept standard surgical excision which is still the only hope to treatPC patients at present1,3. The effect of distant metastases on prognosis is catastrophic. Distant metastases are responsible for nearly 90% of PC death4. In previous studies, 49.2% of patients had regional or distant metastases at initial diagnosis5. Distant metastases frequently occur in liver or peritoneum but rarely in the bone6. Bone metastasis (BM) is often an underappreciated site of PC metastasis, which has been reported that the incidence account 5% to 20% from all metastatic sites7,8.
Although the mechanism of BM in PC remains unclear, BM can lead to a series of complications such as hypercalcemia and pathological fracture, which further deterioratethe prognosis. Bone examinations, like X-rays, CT scans, magnetic resonance imaging, and positron emission topography (PET) scans, have been used to detect the presence of BM in PC. However, none of them have a superior detection rate9. It is reported that the association of BM and PC is higher in patients who have a primary tumor in the tail of the pancreas10. What’s more, there were many studies suggesting that some cytokines might play a non-negligible role in the invasion of pancreatic cancer in bone, like type I collagen (1CTP)11, interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and parathyroid hormone-related protein (PTHrP)10.
Nomogram has been accepted as a visual predictive tool based on the statistical regression models, which could help clinicians to make accurate decisions and promote the development of precision medicine12. The were some nomograms developed to evaluate the outcome and metastases of PC13,14,15,16. To our knowledge, there is no study focus on the predictive models for forecasting the BM in PC patients and the prognosis of PC patients with BM.
In the present study, we aimed to develop two nomograms that can predict the BM in PC patients and the overall survival (OS) of PC patients with BM based on the data of the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Study population
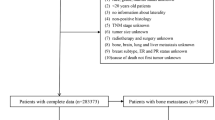
In the present study, we searched and obtained the clinical data from the Surveillance, Epidemiology, and End Results (SEER) database (Version 8.3.6). The inclusion criteria were as follows: (1) Patients were diagnosed histologically as PC between 2010 and 2015; (2) The primary tumor site was pancreas; (3) The information of clinical and demographic features needed for the study were intact and accessible. In addition, the patients diagnosed with autopsies or death certificates were excluded from the study. Finally, there were 16,474 patients included in the cohort to study the risk factors of BM in PC patients and to establish a predictive nomogram. As for the exploration of prognostic factors for PC patients with BM, a total of 226 patients were enrolled in the cohort. As this study did not involve human subjects or personal privacy, the information consent from patients and ethical approval were not required.
Data collection
In the present study, seven variables were used to identify the risk factors of BM in PC, including sex, age, race, primary site, grade, T stage (AJCC 7th), N stage (AJCC 7th), and tumor size. For the study about the prognostic factors for PC patients with BM, the information of treatment variables and metastasis date were also added to the cohort, including surgery, chemotherapy, radiotherapy, liver metastasis, lung metastasis, and brain metastasis. In the prognostic study, overall survival (OS) was identified as the primary outcome, which was defined as the survival time from diagnosis to death due to any cause.
Statistical analysis
All statistic analyses in the present study were performed with SPSS 25.0, R software (version 4.0.1), and X-tile. The univariate and multivariate analyses were used to identify the independent risk factors of BM and prognostic factors of PC patients with BM. In the present study, a P-value < 0.05 was considered as significant difference. The predictive and prognostic nomograms were established by the R packages “rms” and “regplot”.
In the study of the risk factor of BM from PC, univariate and multivariate logistic were applied to identify the risk factor of BM. Besides, we also performed the receiver operating characteristic (ROC) and area under the curve (AUC) to estimate the discrimination of the model. The calibration curve and decision curve analysis (DCA) were developed to further estimate the performance of the models. As for the prognosis of PC patients with BM, the time-dependent ROC, calibration, and DCA were also caculated. Furthermore, according to the cut-off value of the total nomogram points, patients were divided into two risk levels and the Kaplan–Meier (K–M) survival curve with a log-rank test was generated to verify the prognostic value of the nomogram. In addition, ROC curves or time-dependent ROC curves of all independent variables were also generated to compare the AUCs of the nomogram with all independent variables.
Results
The characteristics of the population in the diagnostic cohort
In the present study, a total of 16,474 patients were included in the cohort, and 226 (1.40%) of them were diagnosed as BM at diagnosis. Meanwhile, 11,530 (70%) patients were divided into the training set and others 4944 (30%) were into the validation set. The baseline of the 16,474 patients was shown in Table 1. In the training set, the majority patients were White in race distribution (80.16%) and had lesion in pancres head (65.72%). There were no difference in clinical characteristics, like age, race, grade, T/N stage, sites, tumor size, and bone mastasis, between two cohorts (P > 0.05).
Risk factors of BM in PC patients
The univariate logistic analyses were performed to identify the risk factors of BM in PC patients. As shown in Table 2, the results showed that age, primary site, tumor size, grade, T stage, and N stage were related to BM. After that, variables were incorporated into the multivariate logistic analysis, all the six factors were independent risk factors of BM in newly diagnosed PC patients.
Development and validation of the diagnostic nomogram
Based on the six independent risk factors, a diagnostic nomogram was established (Fig. 1A). The ROC curves of the training set and validation set were generated, and the corresponding AUC values were 0.728 and 0.690 in the training set and validation set, respectively (Fig. 1B,C). Furthermore, ROC curves comparisons against all other risk factors were also generated (Fig. 2A,B). The results showed that the AUC of the nomogram was higher than any other single factors, both in the training set and validation set. More importantly, the calibration curves of both sets showed high consistency between the observed and predicted results (Fig. 2C,D). Finally, the DCA indicated the nomogram could be a more effective tool than other single factors in clinical practice (Fig. 3A,B).
Prognostic factors for PC patients with BM
A total of 226 were eventually enrolled in the prognostic analyses. Similarly, 159 (70%) patients were randomly assigned into to the training set and others 67 (40%) were assigned into the validation set. The demographic and clinical information of the 226 patients was shown in Table 3. There were no difference in clinical characteristics between two cohorts, like age, race, grade, T/N stage, sites, tumor size, surgery, radiotherapy, and chemotherapy (P > 0.05).
To identify the independent prognostic factors for PC patients with BM, the univariate and multivariate Cox analyses were performed. As shown in Table 4, the OS-related factors included age, race, grade, surgery, lung metastasis, and tumor size. Finally, the multivariate Cox analysis revealed that age, race, grade, surgery, and lung metastasis were independent prognostic risk factors of PC patients with BM.
Development and validation of the prognostic nomogram
A prognostic nomogram was established based on the five independent prognostic risk factors (Fig. 4A). The AUC values for the nomogram predicting 6-, 12-, and 18-month OS were 0.781/0.833/0.849 in the training set and 0.738/0.781/0.772 in the validation set, respectively (Fig. 4B,C). In the comparison with other single factors, the nomogram had higher AUCs at 6-, 12-, and 18-month than all other single factors for each set (Fig. 5A–F). Moreover, the calibration curves showed that the nomogram-predicted OS were in satisfactory agreement with actual OS at 6-, 12-, 18-month in both sets (Fig. 6A–F). Eventually, the DCA curves of both sets indicated that the nomogram had better predictive performance than single factors in predicting OS of PC patients with BM (Fig. 7A–F). As a whole, the nomogram can be served as a reliable tool for predicting the OS for PC patients with BM and help clinicians make more personal medical decisions.
Comparison of the values of area under the curve between nomogram and single independent factors. 6-month in the training set (A); 12-month survival in the training set (C); 18-month survival in the training set (E); 6-month survival in the validation set (B); 12-month survival in the validation set (D); 18-month survival in the validation set (F).
Comparison of decision curve analysis between the prognostic nomogram and single independent factors. 6-month survival in the training set (A); 12-month survival in the training set (C); 18-years survival in the training set (E); 6-month survival in the validation set (B); 12-month survival in the validation set (D); 18-years survival in the validation set (F).
Stratification of risk groups
According to the cut-off value of the risk score in training set via the X-tile program, patients were divided into two groups, low-risk group (score ≤ 208) and high-risk group (score > 209). The K–M survival curves with a log-rank test were established and it was not difficult to find that the prognoses among the two groups were significantly different (P < 0.0001, Fig. 8A). The same cut-off values were also used in the validation set. The K–M survival curves of validation set showed that the high-risk group had a worse prognosis than low-risk groups (P = 0.0052, Fig. 8B). In addition, to show the difference between the groups more intuitively, two scatter diagrams were plotted (Fig. 8C,D). It was clear to find that the patients’ survival time gets shorter and shorter as the risk score increases.
Ethics approval and consent to participate
We received permission to access the research data file in the SEER program from the National Cancer Institute, US (reference number 15260-Nov2018). Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified.
Discussion
PC remains one of the most deadly disease of all cancers. Autopsy series have shown that nearly 90% of cases of PC were complicated with distant metastases17. According to the previous studies, patients with distant metastases have a lower 5-year survival rate compared with patients without metastases18. Bone metastases in PC are considered to be relatively rare compared to liver or peritoneal metastases8. However, with the development of PC incidence and detection techniques, more and more patients with BM will be found, so attention should be paid to these patients. In the present study, tumor size, grade, T stage, N stage, age, and primary site were independent diagnostic factors of BM for PC patients. In addition, surgery performed, grade, lung metastases, race, and age were identified as independent prognostic factors for PC patients with BM. Based on these risk factors, we established two nomograms to predict the risk of BM for PC patients and the 6-, 12-, 18-month OS of PC patients with BM, respectively. Both nomograms showed good consistency between the predictive results and the actual result. The use of the diagnostic and prognostic nomograms can benefit both the clinicians and individual patients.
There is no doubt that improving the clinical skills and means to detect early-stage of PC and metastases plays a vital role in prolonging the survivals of PC patients. However, despite considerable efforts, the pathogenesis and specific molecular mechanism of BM in PC remain unclear. Therefore, genetic screening and tumor biomarker screening are still difficult to be effectively applied in clinic at present. Although some previous studies have focused on the risk factors of PC, to our knowledge, there is no study focusing on the risk factors associated with BM in PC. In our results, higher T stage, N stage, and larger tumor size were associated with a higher risk of BM, similar to other kinds of tumors19,20,21. Notably, younger patients (≤ 66 years) were more likely to develop BM than older patients. This may be due to younger patients with a family history of PC more probably. Previous studies have suggested that PC patients with a family history may have an earlier onset22,23. Wang et al. also reported that the relatives of patients with PC had a higher risk of dying from cancers at other sites24. In addition, our results showed that patients with T2 stage had a higher risk of BM than patients with T3 stage. The phenomenon may be because that patients with T3 stage have more obvious clinical symptoms due to the invasion of surrounding organs and tissues so that patients are more proactive in the disease examination25.
Early-stage PC is usually clinically silent, and most patients who developed symptoms may have missed the best time for treatment. In our study, surgery performed remains an important prognostic factor. With the development of surgical techniques, such as laparoscopy, more and more patients who could not tolerate surgery in the past are able to receive surgical treatment. However, due to the complexity and serious complications of the surgery, the prognosis of patients who received surgery in hospitals at different ranks have a big difference26. A previous population-based study indicated an approximately 50% reduction in the risk of postoperative mortality in the high-volume hospital versus low-volume hospitals between 2009 and 201427. In addition, the nihilistic and skeptical attitude among patients and the concerns about the safety and efficacy of surgery of clinicians may also contribute to the low resection rates3. In our results, the difference in prognosis caused by racial difference is also noteworthy. Some past studies have suggested that the black patients had a worse prognosis due to the lower overall economic status or worse lifestyles habits such as a higher rate of smoking, alcohol consumption, and diabetes28,29. However, Nipp et al. indicated that even adjusting the potential confounding sociodemographic and clinical factors, the black PC patients also have a worse survival outcome30. More detailed prospective studies and genetic related studies will further explain these phenomena in the future. Moreover, older age also means worse prognosis, which is consistent with some previous studies31,32,33. Although chemotherapy is routinely used for the treatment of metastatic PC, it was not an independent prognostic factor in our results. This may be because the improvement of the prognosis of BM of PC patients by existing chemotherapy plans is not obvious. The lack of detailed chemotherapy information in the SEER database may also contribute to this phenomenon. Some new chemotherapy plans in recent years may further expand the impact of chemotherapy on the prognosis of PC patients with BM34,35. Neoadjuvant chemotherapy and immunotherapy also provide a new insight for the treatment of patients36,37. Interestingly, we found that the presence or absence of lung metastases also influenced the prognosis of PC patients with BM. This may be because some biomarkers such as IF-6 may be expressed at the same high level in lung metastasis and BM, so that lung metastasis can reflect the severity of BM to some extent and affect the prognosis10,38,39. Further research may provide a direction for future research on blocking drugs.
Limitations
To our knowledge, this study is the first diagnostic and prognostic model to predict the risks and the prognosis of BM in PC based on a large population. However, there were still some limitations in the present study. First, the limited number of patients may make conclusions less conclusive. Second, the inevitable selection bias associated with retrospective studies. Third, due to the limited factors included in the SEER database, some possible risk factors were not included in the study, such as dietary habits, diabetes, and specific treatment plans. Finally, the more advanced version 8th AJCC staging could not be used due to the data logging limitations and the inability to efficiently convert. Despite these limitations, the present study offers the possibility of predicting the risk of BM in PC and the prognosis of PC patients with BM.
Conclusion
The present showed that tumor size, grade, T stage, N stage, age, and primary site were independent diagnostic factors of BM for PC patients. As for PC patients with BM, surgery performed, grade, lung metastases, race, and age were independent prognostic factors. Two nomograms were established and proved to have satisfactory performance. These convenient and visual tools can be used in risk assessment and prognostic prediction for BM in PC.
Data availability
The datasets generated and/or analyzed during the current study are available in the SEER database (https://seer.cancer.gov/).
Abbreviations
- BM:
-
Bone metastasis
- PC:
-
Pancreatic cancer
- SEER:
-
Surveillance, Epidemiology, and End Results
- ROC:
-
Receiver operating characteristic
- DCA:
-
Decision curve analysis
- 1CTP:
-
Type I collagen
- IL-6:
-
Interleukin-6
- VEGF:
-
Vascular endothelial growth factor
- PTHrP:
-
Parathyroid hormone-related protein
- OS:
-
Overall survival
- AUC:
-
Area under the curve
References
Gillen, S., Schuster, T., Meyer ZumBüschenfelde, C., Friess, H. & Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 7(4), e1000267 (2010).
Quaresma, M., Coleman, M. P. & Rachet, B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: A population-based study. Lancet (London, England). 385(9974), 1206–1218 (2015).
Strobel, O., Neoptolemos, J., Jäger, D. & Büchler, M. W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 16(1), 11–26 (2019).
DiMagno, E. P., Reber, H. A. & Tempero, M. A. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology 117(6), 1464–1484 (1999).
Luo, J., Xiao, L., Wu, C., Zheng, Y. & Zhao, N. The incidence and survival rate of population-based pancreatic cancer patients: Shanghai Cancer Registry 2004–2009. PLoS One 8(10), e76052 (2013).
Guan, Z., Lan, H., Sun, D., Wang, X. & Jin, K. A potential novel therapy for FGFR1-amplified pancreatic cancer with bone metastasis, screened by next-generation sequencing and a patient-derived xenograft model. Oncol. Lett. 17(2), 2303–2307 (2019).
Hatfield, D. R., DeLand, F. H. & Maruyama, Y. Skeletal metastases in pancreatic carcinoma: Study by isotopic bone scanning. Oncology 33(1), 44–47 (1976).
Borad, M. J. et al. Skeletal metastases in pancreatic cancer: A retrospective study and review of the literature. Yale J. Biol. Med. 82(1), 1–6 (2009).
Tamm, E. & Charnsangavej, C. Pancreatic cancer: Current concepts in imaging for diagnosis and staging. Cancer J. 7(4), 298–311 (2001).
Iguchi, H., Yasuda, M., Matsuo, T., Sumii, T. & Funakoshi, A. Clinical features and management of pancreatic cancer with bone metastases. Nihon Shokakibyo Gakkai Zasshi. 101(8), 872–878 (2004).
Nanashima, A. et al. Measurement of serum marker for bone metastasis (1-CTP) in hepatobiliary and pancreas malignancies. Hepatogastroenterology. 57(101), 694–697 (2010).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 16(4), e173-180 (2015).
Goldstein, D. et al. Nomogram for estimating overall survival in patients with metastatic pancreatic cancer. Pancreas 49(6), 744–750 (2020).
Chen, S. et al. Development and validation of a novel nomogram for pretreatment prediction of liver metastasis in pancreatic cancer. Cancer Med. 9(9), 2971–2980 (2020).
Ding, J. et al. Prognostic Nomogram for pancreatic cancer with lung metastasis: A SEER database-based study. J. Pancreatol. 4(1), 28–36 (2021).
Hua, J. et al. Development and multicenter validation of a nomogram for preoperative prediction of lymph node positivity in pancreatic cancer (NeoPangram). Hepatobiliary Pancreat. Dis. Int. HBPD INT. 20(2), 163–172 (2021).
Kamisawa, T., Isawa, T., Koike, M., Tsuruta, K. & Okamoto, A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 11(4), 345–349 (1995).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66(1), 7–30 (2016).
Hu, C. et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer 20(1), 494–494 (2020).
Huang, Z. et al. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: A population-based study. BMC Cancer 20(1), 1145 (2020).
Yan, B. et al. Characteristics and risk differences of different tumor sizes on distant metastases of hepatocellular carcinoma: A retrospective cohort study in the SEER database. Int. J. Surg. (London, England). 80, 94–100 (2020).
Petersen, G. M. et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cospons. Am. Soc. Prev. Oncol. 15(4), 704–710 (2006).
Brune, K. A. et al. Importance of age of onset in pancreatic cancer kindreds. J. Natl. Cancer Inst. 102(2), 119–126 (2010).
Wang, L. et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cospons. Am. Soc. Prev. Oncol. 18(11), 2829–2834 (2009).
van Roessel, S. et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 153(12), e183617 (2018).
Ghaferi, A. A., Birkmeyer, J. D. & Dimick, J. B. Variation in hospital mortality associated with inpatient surgery. N. Engl. J. Med. 361(14), 1368–1375 (2009).
Krautz, C., Nimptsch, U., Weber, G. F., Mansky, T. & Grützmann, R. Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann. Surg. 267(3), 411–417 (2018).
Silverman, D. T. et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans?. Epidemiology 14(1), 45–54 (2003).
Singal, V., Singal, A. K. & Kuo, Y. F. Racial disparities in treatment for pancreatic cancer and impact on survival: A population-based analysis. J. Cancer Res. Clin. Oncol. 138(4), 715–722 (2012).
Nipp, R. et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 7(2), 525–535 (2018).
Enzinger, P. C. & Mayer, R. J. Gastrointestinal cancer in older patients. Semin. Oncol. 31(2), 206–219 (2004).
Yancik, R. Population aging and cancer: A cross-national concern. Cancer J. (Sudbury, Mass). 11(6), 437–441 (2005).
Egawa, S. et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 41(7), 985–992 (2012).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369(18), 1691–1703 (2013).
Peixoto, R. D. et al. Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am. J. Clin. Oncol. 40(5), 507–511 (2017).
Heinrich, S. & Lang, H. Neoadjuvant therapy of pancreatic cancer: Definitions and benefits. Int. J. Mol. Sci. 18(8), 1622 (2017).
Morrison, A. H., Byrne, K. T. & Vonderheide, R. H. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 4(6), 418–428 (2018).
van Duijneveldt, G., Griffin, M. D. W. & Putoczki, T. L. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin. Sci. (London, England: 1979) 134(16), 2091–2115 (2020).
Farren, M. R. et al. Systemic immune activity predicts overall survival in treatment-naïve patients with metastatic pancreatic cancer. Clin. Cancer Res. 22(10), 2565–2574 (2016).
Funding
This research was supported by the Jilin Provincial Science and Technology Development Program (No.20200404036YY).
Author information
Authors and Affiliations
Contributions
Z.L. and D.W. contributed to the conception and design of the study; Z.L. and H.L. performed the experiments, collected and analyzed data; Z.L. wrote the manuscript; all authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Liu, H. & Wang, D. Establishment of the diagnostic and prognostic nomograms for pancreatic cancer with bone metastasis. Sci Rep 12, 18085 (2022). https://doi.org/10.1038/s41598-022-21899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21899-6
This article is cited by
-
Machine learning was used to predict risk factors for distant metastasis of pancreatic cancer and prognosis analysis
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.