Abstract
Nuclear receptors (NR) are ligand-modulated transcription factors that regulate multiple cell functions and thus represent excellent drug targets. However, due to a considerable NR structural homology, NR ligands often interact with multiple receptors. Here, we describe a multiplex reporter assay (the FACTORIAL NR) that enables parallel assessment of NR ligand activity across all 48 human NRs. The assay comprises one-hybrid GAL4-NR reporter modules transiently transfected into test cells. To evaluate the reporter activity, we assessed their RNA transcripts. We used a homogeneous RNA detection approach that afforded equal detection efficacy and permitted the multiplex detection in a single-well format. For validation, we examined a panel of selective NR ligands and polypharmacological agonists and antagonists of the progestin, estrogen, PPAR, ERR, and ROR receptors. The assay produced highly reproducible NR activity profiles (r > 0.96) permitting quantitative assessment of individual NR responses. The inferred EC50 values agreed with the published data. The assay showed excellent quality (<Z’> = 0.73) and low variability (<CV> = 7.2%). Furthermore, the assay permitted distinguishing direct and non-direct NR responses to ligands. Therefore, the FACTORIAL NR enables comprehensive evaluation of NR ligand polypharmacology.
Similar content being viewed by others
Introduction
The human NR superfamily comprises forty-eight ligand-modulated transcription factors that regulate transcriptional responses to endocrine stimuli1,2. Individual NRs recognize specific hormonal and metabolic ligands and regulate different metabolic functions1,2,3. Each NR has a ligand-binding domain (LBD) that regulates the transcriptional activity and a DNA-binding domain (DBD) that interacts with the regulated genes. The NRs are readily druggable targets, and thus NR ligands comprise a large class of drugs for multiple diseases and conditions, including inflammation, contraception, diabetes, and cancer4,5.
A major challenge for drug development efforts stems from a considerable structural homology of NR proteins5. Because of that, NR drugs often interact with multiple receptors. The polypharmacological effects can compromise drug safety, but, on the other hand, they can drastically increase drug efficacy6,7. The proper polypharmacology evaluation requires assessing NR ligand effects on all human NRs, but tools for such comprehensive analysis are missing.
Most existing NR assays belong to two classes: the ligand-binding and the reporter gene assays8. The former permits evaluating ligand binding across multiple receptors but lacks information about NR activity changes. The main limitation of reporter gene assays is that they usually enable assessing only a single NR response8,9,10. The throughput can be increased using reporter cell lines with stably integrated NR reporter constructs9,10, but this approach is hindered by the gradual inactivation of reporter transgene expression11 and by unpredictable effects of surrounding chromatin.
Here, we describe a multiplex reporter assay (the FACTORIAL NR) that enables profiling NR ligands’ activity across all human NRs. The assay makes use of one-hybrid GAL4-NR reporter modules transiently transfected into test cells. The principal difference of the FACTORIAL NR from existing one-hybrid reporter assays is that we evaluate NR reporters’ activity by assessing their transcription. For that, we use a homogeneous detection approach12 that affords assessing multiple reporters in a single well of cells with equal detection efficacy. In our previous publications, we demonstrated the feasibility of this multiplex assay and showed its applications for screening for the endocrine disrupting activity of chemicals and water samples13,14. However, the prototypical (trans-FACTORIAL) assay of those studies covered only a fraction of the human NRome.
Here, we describe the comprehensive FACTORIAL NR encompassing all human NRs. To validate the assay, we assessed NR activity profiles for selective NR ligands and polypharmacological agonists and antagonists13. We show that these profiles permitted unequivocal identification of NR targets, and the inferred EC50 values were in agreement with the literature data. Furthermore, we have characterized the quantitative assay parameters, including the specificity, variability, quality, and repeatability. We also explored the assay's utility for dissecting direct and indirect effects of NR ligands.
Methods
Reagents
17β-estradiol (CAT# 10006315, CAS: 50-28-2, purity: ≥ 98%), 4-Hydroxytamoxifen (CAT# 17308, CAS: 68392-35-8, purity: ≥ 98%), Bexarotene (CAT# 11571, CAS: 153559-49-0, purity: ≥ 98%), Chenodeoxycholic acid (CAT# 10011286, CAS: 474-25-9, purity: ≥ 95%), Dexamethasone (CAT# 11015, CAS: 50-02-2, purity: ≥ 98%), Docosahexaenoic acid (DHA) (CAT# 90310, CAS: 6217-54-5, purity: ≥ 98%), DY131 (CAT# 17999, CAS: 95167-41-2, purity: ≥ 98%), Eicosapentaenoic acid (EPA) (CAT# 90110, CAS: 10417-94-4, purity: ≥ 98%), Fexaramine (CAT# 17369, CAS: 574013-66-4, purity: ≥ 98%), GSK805 (CAT# 9002444, CAS: 1426802-50-7, purity: ≥ 98%), GW0742 (CAT# 10006798, CAS: 317318-84-6, purity: ≥ 95%), GW4064 (CAT# 10006611, CAS: 278779-30-9, purity: ≥ 95%), GW590735 (CAT# 10009880, CAS: 622402-22-6, purity: ≥ 98%), GW7647 (CAT# 10008613, CAS: 265129-71-3, purity: ≥ 98%), Levonorgestrel (CAT# 10006318, CAS: 797-63-7, purity: ≥ 95%), Pioglitazone (CAT# 71745, CAS: 111025-46-8, purity: ≥ 98%), PK 11195 (CAT# 10525, CAS: 85532-75-8, purity: ≥ 98%), Progesterone (CAT# 15876, CAS: 57-83-0, purity: ≥ 98%), T0901317 (CAT# 71810, CAS: 293754-55-9, purity: ≥ 98%), Troglitazone (CAT# 71750, CAS: 97322-87-7, purity: ≥ 98%), XCT790 (CAT# 16035, CAS: 725247-18-7, purity: ≥ 98%) were purchased from Cayman Chemical (Ann Arbor, Michigan 48108 USA).
5α-Dihydrotestosterone (CAT# D-073, CAS: 521-18-6 , purity: ≥ 98%), 9-cis-Retinoic acid (CAT# R4643, CAS: 5300-03-8, purity: ≥ 98%), Aldosterone (CAT# A9477, CAS: 52-39-1, purity: ≥ 95%), Azocyclotin (CAT# 45335, CAS: 41083-11-8, purity: analytical standard), Ciglitazone (CAT# C3974, CAS: 74772-77-3 , purity: ≥ 98%), Cyhexatin (CAT# 45411, CAS: 13121-70-5, purity: analytical standard), Ethynodiol diacetate (CAT# E7263, CAS: 297-76-7, purity: ≥ 98%), Etonogestrel (CAT# SML0356, CAS: 54048-10-1, purity: ≥ 98%), Gestodene (CAT# SML0292, CAS: 60282-87-3, purity: ≥ 98%), GW501516 (CAT# SML 1491, CAS: 317318-70-0, purity: ≥ 98%), Medroxyprogesterone Acetate (CAT# PHR1589, CAS: 71-58-9, purity: analytical standard), Norgestimate (CAT# 94497, CAS: 35189-28-7, purity: analytical standard), Retinoic acid (CAT# R2625, CAS: 302-79-4, purity: ≥ 98%), Rifampicin (CAT# R3501, CAS: 13292-46-1, purity: ≥ 97%), Rosiglitazone (CAT# R2408, CAS: 122320-73-4, purity: ≥ 98%), Tributyltin chloride (CAT# T50202, CAS: 1461-22-9, purity: ≥ 96%), 3,3′,5-Triiodo-L-thyronine (CAT# T2877, CAS: 6893-02-3, purity: ≥ 95%), Triphenyltin chloride (CAT# 245712, CAS: 639-58-7, purity: ≥ 95%), 1α,25-Dihydroxyvitamin D3 (CAT# D1530, CAS: 32222-06-3, purity: ≥ 99%) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). 6α-Fluorotestosterone (cat# BML-S250-0005; CAS Number: 1597-68-8; Purity: ≥ 99.0%) was from Enzo Life Sciences (Farmingdale, NY, USA). All chemicals were dissolved in dimethyl sulfoxide (DMSO), with the final concentration of 0.2% DMSO in the cell growth media.
Cells
As in our previous studies, the FACTORIAL NR assay was conducted in the HG19 clone (Attagene, NC, USA) of human hepatocellular carcinoma HepG2 cell line (ATCC # HB-8065). The HG19 clone had an elevated xenobiotic metabolizing activity12,13,14. Cells were propagated in a Dulbecco-modified essential culture medium (DMEM) Gibco (Waltham, MA, USA) supplemented with a 10% fetal bovine serum (FBS). The FACTORIAL NR assays were conducted in a low-serum media containing 1% charcoal-stripped FBS (Hyclone, UT, USA).
The principle of FACTORIAL NR assay
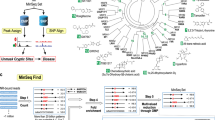
The FACTORIAL NR is a modular assay comprising one-hybrid reporter modules for each human NR. An individual module has a GAL4-NR expression vector paired with a GAL4 reporter transcription unit (RTU) (Fig. 1A). The GAL4-NR vector provides constitutive expression of a chimeric GAL4-NR protein (a fusion of the NR LBD with GAL4 DBD). The RTU has a reporter sequence under the control of GAL4 DBD binding promoter. The GAL4-NR/RTU pair acts as a classic one-hybrid GAL4-NR reporter15,16. The GAL4-NR proteins transactivate the RTU reporter proportionate to the NR LBD activity.
The FACTORIAL NR assay. (A) The GAL4-NR/RTU reporter module. The module comprises a GAL4-NR expression vector paired with a GAL4 reporter transcription unit (RTU). The module acts as a one-hybrid reporter construct producing RTU transcripts proportionate to NR LBD transcriptional activity. The RTU reporter sequence contains a restriction tag (the HpaI site). (B) The detection flowchart. The GAL4-NR/RTU modules are transiently transfected into separate pools of test cells. Transfected cells are mixed and plated into assay plate wells. After stimulation, total cellular RNA is amplified by RT-PCR, labeled by a fluorescent label, cut by HpaI enzyme and separated by capillary electrophoresis (CE). The CE profile mirrors the GAL4-NR activity.
To detect the FACTORIAL NR reporter modules, we profile the reporter RNA transcript using the homogeneous detection approach12. Under this approach, all RTUs have identical reporter sequences. To distinguish the RTU transcripts, the reporter sequences have the restriction tag (the HpaI site) placed at a different position (Fig. 1A).
The FACTORIAL NR detection entails RT-PCR amplification of reporter RNAs followed by HpaI digestion and DNA fragment separation by capillary electrophoresis (Fig. 1B). The homogeneous design ensures an equal detection efficacy across the reporters, thereby reducing the influence of experimental variables on the transcript profiles12.
The GAL4-NR/RTU modules
The assay comprises 50 reporter modules. There are forty-eight reporter GAL4-NR/RTU modules, one module for each human NR. Their NR LBDs have the entire coding LBD sequences with the hinge domains. The GAL4-NR protein expression is under the control of a constitutive SV40 promoter.
To control for ligand effects on the GAL4 promoter, we used the GAL4 reporter module with the expression vector lacking an NR LBD. For internal normalization, we used the TATA module without an expression vector. Its reporter sequence was under the control of a minimal TATA box promoter.
Validating the reporter modules
Prior to assembling the multiplex assay, we tested the functionality of individual reporter modules. All GAL4-NR expression vectors were sequence-verified. To assess the basal activity and NR ligand responsiveness, we used a traditional reporter gene assay with the secreted alkaline phosphatase (SEAP). The GAL4-NR vectors were transiently transfected along with the GAL4-SEAP reporter plasmid containing the SEAP cDNA. The SEAP activity was detected in cell growth medium following vendor’s protocol (Thermo Fisher Scientific, USA).
To test the functionality of GAL4-NR proteins with unknown NR ligands, we used two-hybrid cofactor interaction assays17 to determine whether the GAL4-NR proteins interact with their coactivators and corepressors to modulate reporter transcription. In these experiments, the expression plasmids contained the VP16 activation domain fused with NR coactivators (RIP140-VP16) and corepressors (NCOR-VP16 and SMRT-VP16). Note that adding the potent activating VP16 domain turns co-repressors into activators. In the case of GAL4- SHP (which has no known NR cofactors), we verified its expression by RT-PCR. The Table S1 and supplementary Fig. S1 summarize the test results.
The assay workflow
The assay was done following the previously described protocols 12 (Fig. 1B). The reporter modules were transiently transfected into separate pools of HepG2 cells using the TransIT-LT1 reagent (Mirus). Twenty-four hours later, cells were trypsinized and mixed in an equal proportion. The cell mix was plated into 12-well plates at 3 × 105 cells/well (each NR module being represented by approximately 6000 cells) in a 10% FBS growth medium. Each well represented an individual FACTORIAL NR assay. After the plating, cells were incubated overnight in a fresh low-serum (1% FBS) growth medium and treated with tested compounds for 24 h.
Total RNA was isolated using the PureLink RNA isolation kit (Invitrogen) and processed as described12. Briefly, RNA was reversely transcribed and amplified in a single PCR reaction tube using one pair of RTU primers, producing 702 bp PCR products 12. The products were labeled in an extension reaction with a 6-Fam-labeled primer and cut by HpaI. The minimal spacing of HpaI sites among the reporter sequences was of 5 bp to allow reliable separation of the DNA fragments by capillary electrophoresis (DNA Genetic Analyzer 3130xl (ABI)). As we showed previously, the HpaI-tagged reporters had essentially identical detection efficacy, ensuring the reproducibility of reporter transcript profiles regardless of broad variations in transfection efficacy, RNA degradation and PCR amplification12.
Data analysis
The output of the FACTORIAL NR assay is the CE electrophoregram that mirrors the GAL4-NR activity. To normalize CE profiles by different assays, we used the TATA module signals. Each tested NR ligand was assessed by three independent FACTORIAL NR assays, and the NR activity profiles were calculated as an average of the three replicates. To characterize NR ligand activity, we used differential NR activity profiles calculated by dividing the NR activity values for ligand-treated cells by those in vehicle-treated cells.
Statistical analysis
To compare NR activity profiles, we used the Pearson correlation coefficient (r) 12. The significance of individual NR responses was evaluated by Student’s t-test for the average values of three replicate assays. To assess the intraassay variability of individual NR endpoints, we used the coefficient of variation (CV) for multiple replicate assays within a single experiment using the formula below.
As the aggregate intraassay variability <CV> , we used average CV values across all significant NR responses. To assess assay quality, we used Z'-factor values for individual NR endpoints18. The baseline activity in vehicle-treated cells and the activity in stimulated cells provided the negative and positive control values, respectably. As the aggregate assay quality <Z'> , we used the average of Z'-factor values across all significant NR endpoints. To calculate EC50 values, we interpolated the concentration–response curves by the curve-fitting algorithms of the 4-Parameter Logistic (4PL) model, using the DRC package (v.2.5–12)19 of R software (v. 3.2.5)20, and the SciPy package of Python (v3.62.). The NR activity profile heatmaps were generated using the Matplotlib and Seaborn modules of Python.
Results
The FACTORIAL NR detection parameters
The baseline NR activity profile
In the absence of stimulation, the activity of most GAL4-NRs was within the tenfold range of the GAL4 reporter. The RORα,β,γ, RARα,β, ERRα,γ, CAR, and SF-1 reporters had a higher activity (suppl. Fig. S2) This high constitutive activity may stem from the presence of endogenous NR ligands or from activation of signaling pathways potentiating NR activity21.
The specificity
The lack of cross-reactivity between GAL4-NR/RTU modules is a built-in feature of the assay. The reporter modules are individually transfected prior to plating the pool of transfected cells into assay wells. To further test for the specificity, we used a series of specific ligands. The panel comprised physiological NR ligands for the vitamin D (VDR), progesterone (PR), estrogen (ER), farnesoid X (FXR), and thyroid hormone (THR) receptors (Fig. 2A). The statistical significance of individual NR responses was assessed by t-test. We have found that nonspecific responses (noise) were within 3 SD from the baseline, whereas statistically significant off-target responses (e.g., PXR activation by progesterone and chenodeoxycholic acid) were in agreement with literature data22,23.
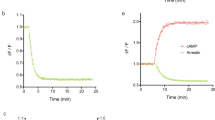
Profiling NR ligand activity by the FACTORIAL NR. The NR activity profiles for physiological (A) and synthetic (B) ligands. The NR activity profiles show the GAL4-NR activity in stimulated cells normalized by that in vehicle-treated cells. Bar graphs show NR activity fold-changes on a linear scale and radial graphs show log-transformed values. Each profile is an average of three independent FACTORIAL NR assays. Significant NR responses are marked (**P < 0.01; *P < 0.05). The Z’- factor and CV values for individual responses are averages of three independent assays. (C) The reproducibility of NR activity profiles for RXR agonist bexarotene in two independent experiments. Each profile is an average of three independent FACTORIAL NR assays. The profile similarity calculated as Pearson correlation coefficient r. The Z’-factor and CV values for individual responses are averages of three independent assays in one experiment. The aggregate < Z’ > and < CV > values are average across all significant responses.
Figure 2B shows NR activity profiles for synthetic NR agonists of androgen (AR), pregnane X (PXR), peroxisome proliferator-activated receptor gamma (PPARγ), glucocorticoid (GR), and liver X (LXR) receptors. The profiles were entirely consistent with the reported data. For example, in addition to the primary activity at the LXRα and LXRβ, LXR agonist T0901317 had significantly activated the PXR24 while not affecting other reporters. Dexamethasone had significantly activated its primary target GR and the mineralocorticoid (MR) receptor25,26. More profiles for specific NR ligands are shown by supplementary Fig. S3. Therefore, the FACTORIAL NR assay specifically responded to the selective ligands.
The assay variability and quality
To assess the intraassay variability, we used the CV coefficient for multiple replicate assays within a single experiment. The CV values for the prototypical ligands (as shown by Figs. 2A,B) varied from 5.04 to 10.9%, with an average value of 7.23%.
The assay quality was assessed by the Z’-factor that characterizes the separation of the induced and baseline reporter activity and the likelihood of false positives/negatives18. To calculate Z' values for individual endpoints, we used data of three independent replicate assays of the same experiment. The Z' factor values for Fig. 2A,B data were in the range from 0.52 to 0.82 with an average value of 0.73. That exceeds the excellent quality criterion (Z' > 0.5) 18.
The reproducibility
For the reproducibility assessment, we compared the NR activity profile for polyvalent NR ligands from different experiments. As a quantitative measure, we used the Pearson correlation coefficient (r). As an example, Fig. 2C shows the signatures for a synthetic RXR agonist bexarotene27. The NR activity profiles were faithfully reproduced (r = 0.991) in experiments performed over several months. The signature endpoints (RXRs, Nur77, and NURR1) were in agreement with others' data28,29.
Assessing NR ligand-receptor interactions
Using the FACTORIAL NR in a concentration–response format, we assessed the EC50 values for ligand-receptor interactions. The concentration–response data for selective NR ligands fitted the classic Hill equation sigmoid curves (Fig. 3). More concentration–response data are shown by supplementary Fig. S4. The inferred EC50 values agreed with the published data by others (Table 1). Therefore, the FACTORIAL NR permitted accurate quantitative evaluation of ligand-receptor interactions.
Examining NR antagonists
The rapid turnover of reporter RNA makes the FACTORIAL NR assay particularly well-suited for detecting inhibited NR responses to NR antagonists. Figure 4A shows the NR activity profiles for ERRα, RORγ, and ER antagonists. The high basal activity of ERRα and RORγ reporters (suppl. Fig. S2) allowed assessing their antagonists without additional stimulation. The NR activity profiles agreed with the literature data on these antagonists. The single NR response to RORγ antagonist GSK805 reflected its primary activity30 (Fig. 4Aa). The ERRα antagonist XCT79027 had inhibited the primary target (ERRα) and activated the PPARγ (Fig. 4Ab). The PPARγ response may be explained by XCT790 effects on PPARγ coactivator 1-alpha (PGC-1α)31.
Assessing NR antagonists by the FACTORIAL NR assay. (A) The NR activity profiles for RORγ (a), ERRα (b), and ER (c) antagonists after a 24-h incubation. The log-transformed fold-changes of NR activity in antagonist- vs. vehicle-treated cells are shown. (c) To assess ER antagonist 4-HT, cells were stimulated with ER agonist 17β-estradiol (E2). The differential NR activity profile (c) shows NR activity changes in cells treated with the combination of E2/4-HT vs. that in E2-treated cells. The (a–c) profiles are average of three independent replicate FACTORIAL NR assays. (B) The concentration-responses of the primary NR targets of RORγ (a), ERRα (b), and ER (c) antagonists in FACTORIAL NR assay. The responses show the percentage of the baseline activity in vehicle-treated (a,b). or (c) E2-stimulated cells. The inferred EC50 values are average data of three independent FACTORIAL NR assays.
The ER antagonist 4-hydroxytamoxifen (4-HT) is the major active metabolite of tamoxifen32. Since ER reporters had low basal activity (suppl. Fig. S3), we stimulated cells with ER agonist 17β estradiol (E2). The NR activity profile for 4-HT showed an inhibition of ERα and ERβ and ERRγ (Fig. 4Ac). The ERRγ response to 4-HT was consistent with the reported data33.
Using the assay in a concentration–response format, we determine EC50 values for the NR antagonists (Fig. 4Ba–c). The inferred values were in agreement with the literature data (Table 1).
These results demonstrate excellent quality (Z’ factor), low intraassay variability (CV), high reproducibility (r), and the robustness of the FACTORIAL NR assay. Furthermore, these data show the assay’s capability for evaluating both NR agonists and antagonists.
Examining polypharmacological NR ligands
We used a diverse panel of polypharmacological PR and PPAR ligands, including drugs, nutritionals, and environmental chemicals.
PR agonists
Progestins are synthetic analogs of the hormone progesterone, widely used in contraception pills and hormone replacement therapy34. The primary progestin target is the PR receptor, but they have activities at other NRs.
Here, we evaluated NR activity profiles for a progestin panel that included etonogestrel (ETG), gestodene (GST), medroxyprogesterone acetate (MEDA), norgestimate (NGS), levonorgestrel (LVG), ethynodiol diacetate (ETD). The Fig. 5 heatmap exemplifies progestins’ NR activity profiles (for more data see suppl. Fig. S5A–C). These profiles were faithfully reproduced (r > 0.96) in experiments conducted over the period of several months (Fig. 6).
Assessing the polypharmacology of progestins. The heatmap shows NR activity profiles for progestins after a 24 h incubation with indicated concentrations. The fold-induction NR activity values in progestins vs. vehicle-treated cells are shown. Progest. progesterone; ETG etonogestrel, GST gestodene; MEDA medroxyprogesterone acetate; NGS norgestimate; LVG levonorgestrel; ETD ethynodiol diacetate; PRG progesterone.
The reproducibility of progestins’ NR activity profiles. The NR data of two independent experiments are shown. Each profile is an average of three independent replicate FACTORIAL NR assays. All significant individual NR responses are marked by asteriscs (**P < 0.01; *P < 0.05). The similarity of NR activity profiles is calculated as the Pearson correlation coefficient r.
The most common off-target responses involved the AR, ER, GR, and PXR receptors, which agreed with the literature data35,36,37,38 (Fig. 5). These effects varied among progestins, e.g., NGS had a weak activity at the AR and GR; MEDA showed no estrogenic activity; ETD activated the FXR receptor (Fig. 5 and suppl. Fig. S5A–C).
The inferred on-target activity EC50 values at the PR varied among progestins from 0.048 to 0.95 nM (Fig. 7A,B). The potency of synthetic progestins was similar or higher as compared to that of progesterone (Fig. 7B, S5B).
Evaluating the NR activity of progestins. (A) The concentration–response of progestins’ primary target (the PR). The data shown as the percentage of the maximal PR activation by the progestins. Average data of three independent replicate FACTORIAL NR assays are shown. (B) The inferred EC50 values for the primary progestin activity. (C) A competitive mode assay to assess off-target activity mechanisms. The graphs show log-transformed NR activity fold-changes (in progestin- vs. vehicle-treated cells) after a 24-h treatment with LVG. Blue line shows the NR activity profile for LVG. Red line: the NR activity profile for LVG in the presence of ER inhibitor 4-HT (a) or AR inhibitor FT (b). Each is the average profile of three independent replicate FACTORIAL NR assays.
Our data illustrate the diversity of endocrine disrupting properties of synthetic progestins. The polypharmacology may account for the differential clinical side effects35,36. These data also demonstrate the utility of the FACTORIAL NR for selecting optimal drug candidates with appropriate activity profiles.
To examine the underlying mechanisms of the off-target effects, we used the assay in a competitive mode. An example of this approach is the study of levonorgestrel (LVG). The off-target LVG activity was at the PXR, AR, ERα, and ERβ receptors (Fig. 5). These responses can stem from direct effects of ligand binding or from activation of cellular pathways that potentiate the NR activity. To distinguish between these possibilities, we assessed these responses in the presence of NR antagonists. The ER antagonist 4-HT had inhibited ERα and ERβ activation, but not other NR responses (Fig. 7Ca). (For other progestins’ data see suppl. Fig. S7). Akin to that, AR antagonist flutamide selectively inhibited LVG effects at the AR (Fig. 7Cb). Therefore, ER and AR activation were direct effects of LVG receptor binding.
PPAR agonists
We have evaluated four classes of PPAR agonists, including nutritional ligands (omega-3 fatty acids); pharmacological PPARγ agonists (glitazones); selective agonists of PPARα and PPARδ; and environmental PPAR ligands (organotins). To capture the maximal range of off-target activities, we used high concentrations of these ligands (Fig. 8A).
Assessing the polypharmacology of PPAR agonists. (A) NR activity profiles after a 24 h incubation with indicated concentrations of PPAR ligands. The heatmap shows fold-induction NR activity values in stimulated vs. vehicle-treated cells. Each profile is an average of three independent replicate FACTORIAL NR assays. EPA eicosapentaenoic acid; DHA docosahexaenoic acid; Cig ciglitazone; Pio pioglitazone; Trog troglitazone; Rosi rosiglitazone; TBT tributyltin; TPT triphenyltin; ACT azocyclotin; CYH cyhexatin. Organotins were used at 0.1 µM as they were cytotoxic at higher concentrations; all other inducers were at 5 µM. (B) Concentration–response of PPAR isoforms to PPARα agonist GW7647 and PPARδ agonist GW0742. Average data of three independent replicate FACTORIAL NR assays. (C) Examining off-target activity mechanisms for PPAR ligands. The blue line graphs show NR activity fold-changes in response to Pio (at 5 µM) (a) or TBT (0.1 µM) (b), (c) vs. vehicle-treated cells. The red line graphs show NR activity profiles for the PPAR ligands in the presence of PPAR inhibitor T0070907 (2 µM) (a), (b) or RXR inhibitor UVI3003 (2 µM) (c) vs. vehicle-treated cells. Average profiles of three independent replicate FACTORIAL NR assays are shown.
The PPAR ligands produced four distinct signature clusters. The first cluster comprised signatures of most common omega-3 acids, the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)39. Their NR activity profiles were characterized by PPARγ and PPARα responses, which was consistent with the literature data40.
The second cluster comprised antidiabetic drugs pioglitazone, ciglitazone, troglitazone, and rosiglitazone. All the glitazones had induced PPARγ activation, reflecting their primary activity41. Another common response to glitazones, the PXR activation, was also in agreement with the literature data42. Interestingly, one drug (pioglitazone) had activated the PPARα; this response was further confirmed by competitive experiments (see Fig. 8Ca).
The third cluster comprised selective agonists of PPARα (GW59073540 and GW764741) and PPARδ (GW074242 and GW50151643). At low concentrations, GW7647 and GW0742 selectively activated PPARα and PPARδ, respectively (Fig. 8B), which was consistent with their selectivity 43,44. At a high (5 µM) concentration, all these compounds activated PPARα, δ, and γ, and PXR. In addition, GW501516 showed a weak estrogenic activity. The concentration-responses curves fitted the classic Hill’s equation (Figs. 8B and S8A). The inferred EC50 values for these compounds agreed with the literature data (Table 1).
The fourth signature cluster comprised widely used industrial chemicals organotins45. The consensus signature comprised responses of multiple NRs, including PPARγ, NURR1, and RXRα, β, and γ (Figs. 8A and S6). These organotin-induced responses were in agreement with the literature data45,46. We have also detected some specific features, e.g., PPARδ and PXR activation by tributyltin chloride. Our data support the notion that the endocrine disrupting activity significantly contributes to organotins’ toxicity45,46. These data also illustrate the utility of FACTORIAL NR for assessing polypharmacological environmental contaminants.
To examine the underlying mechanisms for the NR responses to the PPAR ligands, we used the assay in a competitive mode (Figs. 8C and S8B). To this end, we used a selective PPARγ antagonist T007090747 that acts as a pan-PPAR inhibitor at higher concentrations. The addition of T0070907 had inhibited the PPARγ and PPARα responses to pioglitazone (Fig. 8Ca) and PPARδ activation by tributyltin (TBT) (Fig. 8Cb). Therefore, these responses stemmed from direct binding of these compounds to the PPAR LBDs.
To assess TBT-induced responses, we used a selective RXR antagonist UVI300348. Its addition had inhibited the activation of both RXRs and NURR1 (Fig. 8Cc). Therefore, RXR activation by TBT was mediated by a direct LBD binding, whereas the NURR1 response had involved an RXR-dependent mechanism, presumably the NURR1-RXR dimerization49.
Summarily, these results demonstrate the utility of FACTORIAL NR assay for the evaluation of polypharmacological NR ligands. Specifically, we have shown that the assay afforded clear-cut identification of their multiple NR targets and permitted accurate quantitative assessments of the EC50 values. Moreover, the assay provided valuable mechanistic insights into ligands’ activity.
Discussion
Currently, reporter gene assays are the gold standard for NR ligand evaluation8. However, most of these assays evaluate only a single NR response. Because of that, screening NR ligands across multiple receptors necessitates large assay panels. That requires protracted assay development, time, and expense. Moreover, these screens may not always be appropriately controlled, complicating the analysis across experimental variables50,51.
The FACTORIAL NR obviates the main limitation of traditional low-content reporter gene assays. Using the transcription-based detection enabled parallel assessment of multiple reporters in a single-well format. In addition, the homogeneous design has equalized the detection efficacy across the reporters, thereby drastically diminishing the influence of variable experimental conditions12. Another essential feature is the use of transient transfection to eliminate the unpredictable effects of the host genome on the reporters.
The inherent robustness has translated into excellent assay quality (< Z’ > = 0.73 > and low intraassay variability (< CV > = 7.2%). Furthermore, the obtained NR activity profiles were faithfully reproduced (r > 0.96) in experiments conducted over a several-month period. Thus, these signatures afforded unequivocal identification of NR targets for polypharmacological ligands. Furthermore, the FACTORIAL NR enabled quantitative assessments of ligand-receptor interactions. What is important, the inferred EC50 values agreed with the literature data. However, it should be noted that the EC50 estimates by different groups often differed by orders of magnitude (Table 1). That may be explained by differences in the reporter assays and experimental conditions. In this regard, the advantage of the FACTORIAL NR assay is that it permits parallel evaluation of EC50 values for multiple ligand-receptor interactions, thereby minimizing the interassay variability.
Another advantage of the FACTORIAL NR over the traditional reporter gene assays is rapid turnover of reporter RNAs. That results in faster responsiveness, which is particularly useful for detecting NR inhibition and evaluating NR antagonists.
Our previous publications have shown the ramifications of a prototype multiplex NR assay (trans-FACTORIAL) for assessing environmental chemicals14, water pollution13, and NR drugs52. However, the prototype assay covered only a fraction of human NRs. In this regard, the FACTORIAL NR enables profiling NR ligands across the entire human NRome, thus permitting comprehensive environmental and pharmacological NR ligands evaluation. Notably, the FACTORIAL NR provides valuable mechanistic insights into NR ligand activity. As we showed, using the assay in a competitive mode permitted distinguishing direct and indirect effects of polypharmacological ligands on multiple NR targets (Figs. 7C, 8C, suppl. Figs. S7, S8B).
Like any assay, the FACTORIAL NR has its limitations. The advantage of using ectopically expressed GAL4-NR proteins is that they permit assessing NR ligands’ activity across the entire NRome. On the other hand, this approach does not capture the complete spectrum of compounds’ effects on the endogenous receptors, e.g., on NR transcription, post-transcriptional modifications, splice variants, receptor biosynthesis, and metabolism. Also, the GAL4-NR proteins may not detect the allosteric NR modulators binding the NR protein outside of the LBD region.
To address these deficiencies, we use a complementary multiplex reporter assay, the cis-FACTORIAL (a.k.a. FACTORIAL TF). Unlike the FACTORIAL NR, the cis-FACTORIAL assay comprises a set of reporters that enable profiling the activity of endogenous TF families12,14,53. However, the cis-FACTORIAL does not allow distinguishing the individual NR responses (i.e., of α, β, γ, δ receptors). Besides, the cis-assay’s content is limited by the NRs expressed by a particular cell type. Therefore, combining the FACTORIAL NR and cis-FACTORI AL assays provides the most comprehensive evaluation of compounds’ acti vity.
Data availability
The authors declare that the all data needed to support the conclusions of this study are available in the paper and the supplementary information. The raw data are available from the corresponding author upon reasonable request.
References
Francis, G. A., Fayard, E., Picard, F. & Auwerx, J. Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 65, 261–311 (2003).
Evans, R. M. & Mangelsdorf, D. J. Nuclear receptors, RXR, and the Big Bang. Cell 157, 255–266 (2014).
Alexander, S. P. H. et al. The concise guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br. J. Pharmacol. 170, 1652–1675 (2013).
Gronemeyer, H., Gustafsson, J.-A. & Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 3, 950–964 (2004).
Burris, T. P. et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 65, 710–778 (2013).
Hopkins, A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. https://doi.org/10.1038/nchembio.118 (2008).
Anighoro, A., Bajorath, J. & Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 57, 7874–7887 (2014).
Raucy, J. L. & Lasker, J. M. Current in vitro high throughput screening approaches to assess nuclear receptor activation. Curr. Drug Metab. 11, 806–814 (2010).
Willemsen, P. et al. Use of reporter cell lines for detection of endocrine-disrupter activity. Anal. Bioanal. Chem. 378, 655–663 (2004).
Grimaldi, M. et al. Reporter cell lines to evaluate the selectivity of chemicals for human and zebrafish estrogen and peroxysome proliferator activated Î3 receptors. Front. Neurosci. 9, 212 (2015).
Pikaart, M. J., Recillas-Targa, F. & Felsenfeld, G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 12, 2852–2862 (1998).
Romanov, S. et al. Homogeneous reporter system enables quantitative functional assessment of multiple transcription factors. Nat. Methods 5, 253–260 (2008).
Blackwell, B. R. et al. Potential toxicity of complex mixtures in surface waters from a nationwide survey of United States streams: Identifying in vitro bioactivities and causative chemicals. Environ. Sci. Technol. 53, 973–983 (2019).
Martin, M. T. et al. Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA’s ToxCast program. Chem. Res. Toxicol. 23, 578–590 (2010).
Hollenberg, S. M. & Evans, R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell 55, 899–906 (1988).
Webster, N. J., Green, S., Jin, J. R. & Chambon, P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell 54, 199–207 (1988).
Tyree, C. M. & Klausing, K. The mammalian two-hybrid assay for detection of coactivator-nuclear receptor interactions. in Novel Anticancer Drug Protocols. 175–184. https://doi.org/10.1385/1-59259-380-1:175. (Humana Press, 2003).
Zhang, J.-H., Chung & Oldenburg. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Strebig, J. C. Package ‘drc’ Title Analysis of Dose-Response Curves. (2016).
R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 14 Apr 2021.
Mukherjee, S. & Mani, S. Orphan nuclear receptors as targets for drug development. Pharm. Res. 27, 1439–1468 (2010).
Kliewer, S. A. et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92, 73–82 (1998).
Carazo, A. et al. Acetylated deoxycholic (DCA) and cholic (CA) acids are potent ligands of pregnane X (PXR) receptor. Toxicol. Lett. 265, 86–96 (2017).
Mitro, N., Vargas, L., Romeo, R., Koder, A. & Saez, E. T0901317 is a potent PXR ligand: Implications for the biology ascribed to LXR. FEBS Lett. 581, 1721–1726 (2007).
Grossmann, C. et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: A comparison of their glucocorticoid and mineralocorticoid properties. Eur. J. Endocrinol. 151, 397–406 (2004).
Sedlák, D., Paguio, A. & Bartůněk, P. Two panels of steroid receptor luciferase reporter cell lines for compound profiling. Comb. Chem. High Throughput Screen. 14, 248–266 (2011).
Lowe, M. N. & Plosker, G. L. Bexarotene. Am. J. Clin. Dermatol. 1, 245–250 (2000) ((discussion 251–2)).
McFarland, K. et al. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem. Neurosci. 4, 1430–1438 (2013).
Giner, X. C., Cotnoir-White, D., Mader, S. & Lévesque, D. Selective ligand activity at Nur/retinoid X receptor complexes revealed by dimer-specific bioluminescence resonance energy transfer-based sensors. FASEB J. 29, 4256–4267 (2015).
Xiao, S. et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Willy, P. J. et al. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc. Natl. Acad. Sci. U. S. A. 101, 8912–8917 (2004).
Murphy, C. S., Langan-Fahey, S. M., McCague, R. & Jordan, V. C. Structure-function relationships of hydroxylated metabolites of tamoxifen that control the proliferation of estrogen-responsive T47D breast cancer cells in vitro. Mol. Pharmacol. 38, 737 (1990).
Coward, P., Lee, D., Hull, M. V. & Lehmann, J. M. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc. Natl. Acad. Sci. U. S. A. 98, 8880–8884 (2001).
Schindler, A. E. et al. Classification and pharmacology of progestins. Maturitas 46(Suppl 1), S7–S16 (2003).
Runnalls, T. J., Beresford, N., Losty, E., Scott, A. P. & Sumpter, J. P. Several synthetic progestins with different potencies adversely affect reproduction of fish. Environ. Sci. Technol. 47, 2077–2084 (2013).
Louw-du Toit, R., Perkins, M. S., Hapgood, J. P. & Africander, D. Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy. Biochem. Biophys. Res. Commun. 491, 140–146 (2017).
Jordan, V. C., Jeng, M. H., Catherino, W. H. & Parker, C. J. The estrogenic activity of synthetic progestins used in oral contraceptives. Cancer 71, 1501–1505 (1993).
Koubovec, D., Ronacher, K., Stubsrud, E., Louw, A. & Hapgood, J. P. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol. Cell. Endocrinol. 242, 23–32 (2005).
Davidson, M. H. Omega-3 fatty acids: New insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr. Opin. Lipidol. 24, 467–474 (2013).
Calder, P. C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 142, 592S-599S (2012).
Hauner, H. The mode of action of thiazolidinediones. Diabetes. Metab. Res. Rev. 18(2), S10–S15 (2021).
Singh, S. K., Yende, A. S., Ponnusamy, K. & Tyagi, R. K. A comprehensive evaluation of anti-diabetic drugs on nuclear receptor PXR platform. Toxicol. Vitr. 60, 347–358 (2019).
Brown, P. J. et al. Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg. Med. Chem. Lett. 11, 1225–1227 (2001).
Sznaidman, M. L. et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)–synthesis and biological activity. Bioorg. Med. Chem. Lett. 13, 1517–1521 (2003).
Grün, F. & Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147, s50–s55 (2006).
Grün, F. et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 20, 2141–2155 (2006).
Lee, G. et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J. Biol. Chem. 277, 19649–19657 (2002).
Zhu, J. et al. The unexpected teratogenicity of RXR antagonist UVI3003 via activation of PPARγ in Xenopus tropicalis. Toxicol. Appl. Pharmacol. 314, 91–97 (2017).
Wallén-Mackenzie, Å. et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes Dev. 17, 3036–3047 (2003).
Gray, L. E. et al. Endocrine screening methods workshop report: Detection of estrogenic and androgenic hormonal and antihormonal activity for chemicals that act via receptor or steroidogenic enzyme mechanisms. Reprod. Toxicol. 11, 719–750 (1997).
Rotroff, D. M. et al. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ. Health Perspect. 121, 7–14 (2013).
Whitehead, G. S. et al. Therapeutic suppression of pulmonary neutrophilia and allergic airway hyperresponsiveness by a RORγt inverse agonist. JCI Insight 5, 14 (2019).
Medvedev, A. et al. Evaluating biological activity of compounds by transcription factor activity profiling. Sci. Adv. 4, eaar4666 (2018).
Acknowledgements
This work was supported by the NIH/NIGMS grant 1R44GM125469 to Attagene. The views expressed in this paper are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of the U.S. Environmental Protection Agency.
Author information
Authors and Affiliations
Contributions
A.M. and S.S.M. are co-inventors of the Factorial technology. A.M. and S.S.M. designed and supervised experiments, analyzed and interpreted data. M.M. constructed the GAL4-NR reporter modules and performed experiments. A.M., L.M., E.M., A.G., L.R., M.Z., K.G., and B.L. performed experiments. E.M. performed quality control, S.S.M., A.M. and K.A.H. interpreted data; S.S.M. wrote the manuscript with contribution of A.M. and K.A.H.
Corresponding author
Ethics declarations
Competing interests
S.S.M., A.M., E.M., L.M., and M.M. are shareholders of Attagene. Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Medvedev, A., Moeser, M., Medvedeva, L. et al. Comprehensive assessment of NR ligand polypharmacology by a multiplex reporter NR assay. Sci Rep 12, 3115 (2022). https://doi.org/10.1038/s41598-022-07031-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07031-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.