Abstract
The development of insects is critically affected by temperature, which therefore plays an important role in the control of stored grain pests. Extreme temperature stress conditions lead to biological responses in mites, such as the synthesis of heat shock proteins. Tyrophagus putrescentiae (Tp) is a pest mite in stored grain that has negative effects on both economy and health. Since T. putrescentiae population dynamics are strongly influenced by temperature, in the present study we have cloned the cDNA of HSP70 and HSP90 (referred to as TpHSP70-1, TpHSP70-2 and TpHSP90) and determined their expression by fluorescence real time quantitative PCR. TpHSP70 and TpHSP90 showed high homology with similar genes in other species and the open reading frames of TpHSP70-1, TpHSP70-2 and TpHSP90 encoded proteins of 665, 661 and 718 amino acid residues, respectively. Under thermal stress, expression of TpHsp70-1 and TpHsp90 was up-regulated at higher temperatures, suggesting their role in the defense against thermal stress.
Similar content being viewed by others
Introduction
Storage mites are aeroallergens that can cause asthma and rhinitis in sensitised individuals1,2. In some cases, they may lead to anaphylaxis caused by the ingestion of contaminated food3,4. Tyrophagus putrescentiae is a mite that can be found worldwide in farms, laboratories, urban environments and food industries5,6 from where they have been successfully isolated7,8. T. putrescentiae is also associated to host bacterial communities or symbionts in the gut, fat body or other tissues9,10,11.
Normal growth and development in insects requires appropriate temperature, otherwise they stagnate and even die12. Their body temperature is close to that of their habitat, making them vulnerable to extreme temperatures13. High temperatures can cause developmental abnormalities or defects12,14. Since immature mites and other poikilotherms cannot efficiently regulate body temperature, the latter is usually the most important environmental factor that impacts in their development rate12,15.
In T. putrescentiae, the full normal pattern of development of early immature stages, egg, larva and protonymph, has been suggested to be seriously impaired under temperature stress and reproductive parameters and temperature are closely related16,17.
Heat shock proteins (Hsps) are molecular chaperones with crucial roles in protein folding and unfolding, aggregation, degradation and transport18. They are also important for the insect survival under thermal stress17,19. Indeed, when Drosophila was treated at 30–39 ℃, heat resistance correlated with Hsp70 gene expression at different stages20. When Hsp70 gene expression was inhibited by RNAi in Bemisia tabaci female, survival was lower than in the control group21. The literature reports the study of Hsp90 and Hsp70 genes of a few mites in the Tetranychidae. In Panonychus citri, although the three Hsp70 proteins were expressed under cold shock, only Hsp70-2 was up-regulated under heat shock, whereas Hsp90 gene was expressed under high temperature stress, suggesting that both Hsp90 and Hsp70-2 proteins play an important role in the adaptation to high temperatures22,23. Also, in Tetranychus cinnabarinus, Hsp70-1 and Hsp70-3 play a vital role in cold and heat stress24. Overall, these results suggest that Hsps play an important role in improving heat resistance of mites.
Herein, to understand the adaptation of T. putrescentiae to temperature stress we have cloned its full-length Hsp70 and Hsp90 cDNAs and measured their mRNA expression at different temperatures using real-time quantitative polymerase chain reaction (RT-qPCR).
Materials and methods
Mites
T. putrescentiae adults were collected from the storages at Nanchang suburb, Jiangxi Province, China. The mites were fed with wheat bran (Shangdong, China) in specially made plastic containers (18 × 11 × 8 cm) covered with a lid to prevent escape. A 3 cm diameter hole was shorn on the lid for ventilation25 T. putrescentiae of different ages were identified by our laboratory teachers using the Manual of Acarology 3rd Edition as a reference25. Climate-controlled incubators (RXZ-260B) were used to keep the rearing units for several generations at 25 ± 0.5 ℃ and 75 ± 5% relative humidity (controlled by a YADU ultrasonic humidifier) in the dark.
Total RNA extraction and cDNA synthesis
300 female adults of T. putrescentiae were taken from the experimental population. Total RNA was extracted using the TRIzol method (Invitrogen, San Diego, CA, USA) and then treated with DNase I (Tiangen, Beijing, China)23,26. Concentration and purity of RNA was assessed with a NanoDrop2000 spectrometer (Thermo, USA) at 260 nm and 280 nm. Finally, the integrity of total RNA was tested using 1% agarose gel electrophoresis. The first strand cDNAs were obtained using the Reverse Transcription M-MLV Kit (TaKaRa, Tokyo, Japan): 1 µL of random 6-mers, 1 µL of dNTP mixture and 8 µL of total RNA were mixed and incubated at 65 ℃ for 5 min to improve reverse transcription efficiency. Then, 4 µL of 5 × PrimeScript II Buffer, 0.5 µL of RNase Inhibitor and 1 µL Primer Script II RTase were mixed with RNase-free water up to a final volume of 20 µL. Finally, the mixture was incubated at 45 ℃ for 50 min and at 70 ℃ for 15 min. The cDNA was stored at − 20 ℃ for subsequent experiments. Each sample was processed with three biological replicates.
Degenerate primers and amplification of cDNA
To amplify partial cDNA fragments of Hsp70 and Hsp90, degenerate primers were designed (Table 1) and used in PCR as described previously23,27. PCR reactions used 0.1 μg cDNA as template, 0.3 μM of each primer, 12.5 μL 2 × Taq polymerase Mix (Tiangen, Beijing, China) and ddH2O was added to a total volume of 25 μL.
The PCR programs were operated with the following cycling conditions: initial denaturation step of 3 min at 94 ℃ followed by 35 cycles of 94 ℃ (30 s), 49 ℃ (30 s), 72 ℃ (60 s) and 72 ℃ (10 min). PCR products were detected with 1% agarose gel. Bands with expected size were purified with a universal DNA purification kit. Purified DNA fragments were cloned into a pGEM-T Easy vector and transfected into Escherichia coli DH5α (Promega, Madison, WI, USA). DNA inserts of the recombinant clones were confirmed by PCR with the same degenerate primers used previously and by sequencing in both directions.
Rapid amplification of cDNA ends
The rapid amplification of cDNA ends (RACE) method was applied to obtain full-length cDNAs. Gene specific primers were designed (Table 1) using the identified Hsp70 and Hsp90 cDNA fragments, and 5′ and 3′-Full RACE Kits (TaKaRa, Tokyo, Japan) were used to amplify the 5′ and 3′-ends of the two genes. The first-round PCR program was pre-denaturation at 94 ℃(180 s) followed by 35 cycles of 94 ℃ (30 s), 60 ℃(30 s) and 72 ℃ (90 s) with a final extension at 72 ℃ (6 min). The second-round PCR program was the same as the first-round.
The PCR products from the 5′- and 3′-RACE reactions were cloned into the pGEM-T Easy vector and transfected into Escherichia coli DH5α cells (Promega, Madison, WI, USA). Six recombinant clones were identified by PCR amplification and sequenced (Sangon, Shanghai, China).
Confirmation of full-length cDNA sequences
After the 5′- and 3′-ends sequences were obtained, contigs were assembled with the Seqman software28 to produce the putative full length sequences of HSP70 and HSP90. Full length cDNAs were verified by amplification of the ORFs using the primers listed in Table 1. PCR products were cloned into a pGEM-T Easy vector and sequenced. PCR conditions and cloning methods were as described above.
Bioinformatics analysis
The sequences of TpHSP70 and TpHSP90 were blasted at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/) at both nucleotide and amino acid levels. Amino acid sequences were analyzed with the Expert Protein Analysis System (http://www.expasy.org/). Multiple alignments of TpHSP70 and TpHSP90 were analyzed in the DNAstar software (7.1 version). Neighbor-joining phylogenic trees were constructed with ClustalX 2.0 and MEGA 5.0 using the gene sequences of HSP70 and HSP90 (sequences shown in Table 2). The confidence of the branches was obtained using 1000 replicates’ Bootstrap analysis.
mRNA expression of TpHSP70 and TpHSP90 under thermal stress
Sample collection at different stages
The eggs of T. putrescentiae were separated with a 140 mesh sieve. These eggs continued to grow and develop and were observed every 12 h. After many generations, about 400 eggs, 400 larvae, 200 nymphs and 200 female adults were taken for total RNA extraction. Each experiment was repeated three times.
Thermal stress treatment
Two hundred female adult mites were transferred to 1.5 mL centrifuge tubes sealed with a 0.45 µm filter membrane (BBI, China) for ventilation. In total, ten tubes with 200 mites each were exposed to 0, 5, 10, 15, 20, 30, 33, 36, 39, and 42 ℃, respectively for 1 h, where mites kept at 25 ℃ were used as control group. Three replicates were used for each group.
qPCR of TpHSPs
qPCR assays were performed on Fx960 Real-time Quantitative PCR (BIO-RAD, USA) with alpha-tubulin gene from T. putrescentiae (GenBank accession number: AY986760) as endogenous reference. Each PCR reaction was mixed with 10 µL TB green, 7.8 µL ddH2O, 1.0 µL cDNA, 0.4 µL Rox dye and 0.4 µL of each primer. The thermal cycling profile consisted of an initial denaturation at 95 ℃ (5 min) and 40 cycles at 95 ℃ (10 s) and 60 ℃ (20 s). For each gene specific primer, three independent replicates were performed, each repeated three times. The expression levels of TpHSP70 and TpHSP90 were calculated with the 2−ΔΔCt method29.
Data analysis
Data was represented as the mean ± SE (standard error) for all data sets. The data were then subjected to a one-way analysis of variance (ANOVA) using SPSS 26.0 (Chicago, IL, USA). Differences between means were tested using the Duncan’s test for multiple comparisons. Differences were considered statistically significant at the 5% level (p < 0.05).
Results
Sequence analysis of TpHSP70 and TpHSP90 genes
The complete cDNA sequences of TpHSP70-1 (GenBank accession number: KR479867) and TpHSP70-2 (GenBank accession number: KR479868) were 2494 (ORF of 1,998) and 2354 bp (ORF 1984 bp) long, respectively (Fig. 1A, B). The TpHSP70-1 cDNA included a 186 bp 5′ untranslated region (UTR) and a 310 bp 3′ UTR. TpHSP70-1 encodes a 665 amino acid protein with a calculated molecular weight of 72.72 kDa and an isoelectric point of 5.21. The TpHSP70-2 cDNA included a 168 bp 5′ untranslated region and a 173 bp 3′ UTR. TpHSP70-2 encodes a 661 amino acid protein with a calculated molecular weight of 72.75 kDa and an isoelectric point of 5.29. For both proteins, a possible consensus signal sequence for polyadenylation (AATAAA) was located 45 bp upstream of the poly (A) tail and both have three motifs typical of the Hsp70 proteins family.
The complete cDNA of the TpHSP90 gene was deposited in GenBank with accession number KJ820823 and consisted of 2538 bp with an ORF of 2157 bp, which encoded a 718 amino acid protein (Fig. 1C). The TpHSP90 cDNA included a 165 bp 5′ untranslated region located upstream of the putative start codon (ATG) and a 216 bp 3′ UTR located downstream of the stop codon. The mature protein had a calculated molecular weight of 82.79 kDa with an isoelectric point of 4.92. A possible consensus signal sequence for polyadenylation (AATTAAA) was located 15 bp upstream of the poly (A) tail. The typical histidine kinase-like ATPase domain, ubiquitous in all Hsp90 family members, was located at the position of 37–181. TpHSP90 contained the five typical motifs observed in Hsp90 proteins: NKEIFLRELISNASDALDKIR, LGTIAKSGT, IGVFGVGFYSAYLIAD, IKLYVRRVFI and GVVDSEDLPLNISRE. The C-terminal "MEEVD" motif, which is specific to the of Hsp90 family (cytoplasmic type). Comparative analysis showed that the amino acids of HSP90 in T. putrescentiae presented high similarity of 79–81% with Hsp90 in other species.
Homology analysis of TpHSP70 and TpHSP90
A BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search of GenBank revealed that TpHSP70-1 and TpHSP70-2 belong to the HSP70 family and that TpHSP90 belongs to the Hsp90 family. Multiple sequence alignments showed that the deduced amino acid sequences of TpHSP70-1 and TpHSP70-2 share high similarity with three HSP70s from Tetranychus cinnabarinus, Ixodes scapularis, Bombyx mori, Drosophila melanogaster and Homo sapiens (Fig. 2A). Phylogenetic analyses showed that TpHSP70-1 and TpHSP70-2 belong to the cytoplasmic and the endoplasmic reticulum types of the phylogenetic tree, respectively (Fig. 3A).
Phylogenetic tree of HSP70 (A) and HSP90 (B) from T. putrescentiae and other species. Constructed by the neighbor joining method based on amino acid sequences. Numbers at each branch indicate the percentage of times, and a node is supported in 1,000 bootstraps pseudo-replication by neighbor joining.
The deduced amino acid sequence of TpHSP90 shared high similarity with five HSP90s from T. cinnabarinus, I. scapularis, B. mori, D. melanogaster and H. sapiens (Fig. 2B). Among these, TpHSP90 showed the highest similarity to Hsp90 from I. scapularis (81% identity), and lowest similarity with HSP90 from D.melanogaster (77% identity). HSP90 homology was high within the arthropods, especially in the signature regions of the Hsp90 family. The relationships between HSP90s displayed in the phylogenic tree are consistent with the traditional taxonomy of these species (Fig. 3B). HSP90 from T. putrescentiae and T. cinnabarinus cluster together earlier than I. scapularis, M. occidentalis and N. cucumeris (Fig. 3B).
Expression of TpHSP70 and TpHSP90 in different stages of development
Tubulin was used as a reference gene to measure the mRNA expression level of TpHSP90, TpHSP70-1 and TpHSP70-2 in different developmental stages of T. putrescentiae: egg, larva, protonymph, tritonymph and adult. The mRNA expression levels of all three genes increased with the development of the mites, but their genes’ expression levels varied greatly (Fig. 4). Although the expression levels of the TpHSP70-2 gene changed with developmental stage, no significant differences were observed in the expression levels of TpHSP70-1 gene and TpHSP90 genes across developmental stages. The expression level of TpHSP70-1, TpHSP70-2 and TpHSP90 genes was highest in the protonymph, tritonymph and in the larva, respectively. Thus overall expression of three TpHSPs was highest in the immature stages, and lower in the egg and adult stage. Interestingly, expression of heat shock protein genes was higher in female mites than in male mites.
Stage-specific TpHSP70-1, TpHSP70-2 and TpHSP90 expression in T.putrescentiae. The mRNA expression level of TpHSP70-1, TpHSP70-2 and TpHSP90 genes in different developmental stages including egg, protonymph, deutonymph, tritonymph and adult stages of T.putrescentiae was measured by fluorescent real-time quantitative PCR. Values are the mean ± SD (n = 200). The different letters above the bars (a–f) indicate a significant difference in the means as assessed using Duncan’s multiple comparison tests (P < 0.05).
Dependency of TpHSP90 and TpHSP70 expression on temperature
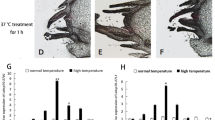
The expression patterns of TpHSP90 and TpHSP70 mRNA were examined at low temperatures. Expression of TpHSP70-1 gene peaked at 10℃, where it was 6.05 times higher than in the control group. The TpHSP70-2 gene peaked at 20℃. Finally, TpHSP90 gene peaked at 0 ℃, but no differences were observed between 5, 10, 15 and 25 ℃ (Fig. 5A). At high temperatures, expression of TpHSP70-1 gene peaked at 39 ℃ (Fig. 5B) where expression was 19.73 times higher than in the control. The expression of TpHSP70-2 gene peaked at 30 ℃, whereas at other temperatures expression was the same as in the control. Finally, TpHSP90 gene expression peaked at 42 ℃ (4.17 times higher than in the control group).
(A) Comparative quantitative RT-PCR analyzed of the relative expression of TpHSP70-1, TpHSP70-2 and TpHSP90 at low temperatures. Control: 25 ℃; Low heat shock temperature: 0, 5, 10, 15, 20 ℃. (B) Comparative quantitative RT-PCR analyzed of the relative expression of TpHSP70-1, TpHSP70-2 and TpHSP90 at high temperatures. Control: 25 ℃; High heat shock temperature: 30, 33, 36, 39, 42 ℃. Each temperature treatment was three replicates. Data were represented as the mean ± SD (n = 200). Letters above columns indicate levels of difference significance at P < 0.05. The same letters are not significantly different, P > 0.05.
Discussion
In the present study, we cloned the three full-length cDNAs of HSP70-1, HSP70-2 and HSP90 genes and evaluated their expression in response to thermal stress, in the hope to understand how they help withstand extreme temperatures. Our results show that TpHsp70-1 plays the most important role at low temperatures, whereas at high temperatures (heat stress), both TpHsp70-1 and TpHsp90 are important. Expression of HSPs helps organisms to adapt to high temperatures as shown by many studies, e.g., in D. melanogaster19, S. exigua30, T. cinnabarinus24,28,31 and P. citri23,32.
Under cold stress conditions, we found that TpHSP70-1 and TpHSP70-2 failed to express, consistent with Hsp70 expression results in T. cinnabarinus24,28, P. citri24 and D. melanogaster19. This suggests other mechanisms in T. putrescentiae to help resist low temperatures, such as the synthesis of trehalose, polyols and other small molecules, antioxidant reactions and production of other heat shock proteins24.
The expression of T. putrescentiae TpHSP70-1 and TpHSP90 genes was up-regulated at higher temperatures and improved heat resistance. In particular, the relative mRNA expression level of TpHSP70-1 at 39 ℃ was 19.03 times higher than the control group. The survival of Bactrocera tryoni’s eggs and larvae at 46 ℃ is significantly lower than the control33 and it has been suggested that exposure of Ceratitis capitata to sublethal temperatures of 42 ℃ for 1 h enhanced heat resistance34. The heat resistance of Anastrepha suspensa raised at 30 ℃ was higher than in those raised at 20 ℃35. Lastly, after a short period of high temperature stress, survival time was prolonged in Cydia pomonella exposed at lethal temperatures36. The synthesis of heat shock protein begins to decline after a certain threshold32. We found that TpHSP70-1 expression was highest at 39 ℃ and decreased at 42 ℃, which may reflect proximity to that threshold23,37. Therefore, heat shock proteins can protect biological cells only to a certain extent37: induced high expression of heat shock proteins can improve insect heat resistance, but this expression affects the synthesis of other proteins in the insect body, which results in shortened life span and reduced fertility27,38. Indeed, our results show that the number of eggs laid by T. putrescentiae decreased significantly after exposure to temperatures above 39 ℃.
Heat shock protein genes are also involved in normal physiological activities and in fertility. These include the folding of new peptide chains to form mature proteins, the formation of gametes and cell differentiation39. We show that the transcription and expression level of the HSP90 gene of T. putrescentiae depends on the developmental stage, being higher in the larval stage. This indicates that this gene is also involved in the regulation of growth and development. Expression of Hsp70 fluctuated with the development period, suggesting its involvement in normal physiological activities and reproductive development.
Finally, the mRNA expression level of heat shock protein genes in the female adult was higher than in the male, consistent with Grapholita molesta results27, and hinting that the ability to cope with environmental temperature stress is higher in females.
In conclusion, new TpHSP90, TpHSP70-1and TpHSP70-2 genes sequences were isolated from T. putrescentiae, and their phylogeny with other mites was inferred. Their expression levels varied with the developmental stages and the highest expression observed was in the immature mite, suggesting that TpHSPs genes are involved in the regulation of growth and development. These three TpHSPs genes are important for T. putrescentiae to defend against temperature and are closely related to mortality. Our study helps to understand the resistance of mites and other insects to environmental stress, and guides T. putrescentiae management by using different temperatures in crops.
References
Liao, E. C., Ho, C. M., Yin, S. C. & Tsai, J. J. Immune responses to Tyrophagus putrescentiae induced airway inflammation in mice. J. Investig. Allergol. Clin. Immunol. 23, 20–29 (2013).
Fernandez-Caldas, E., Puerta, L. & Caraballo, L. Mites and allergy . Chem. Immunol. Allergy 100, 234–242 (2014).
Liao, E. C., Lin, Y. H., Chiu, C. L., Lin, T. C. & Tsai, J. J. Identification of allergenic component Tyr p 8 from Tyrophagus putrescentiae and cross-reactivity with Der p 8. Clin. Vaccine. Immunol. 20, 506–512 (2013).
Yu, S. J., Liao, E. C. & Tsai, J. J. House dust mite allergy: Environment evaluation and disease prevention. Asia. Pac. Allergy. 4, 241–252 (2014).
Duek, L., Kaufman, G., Palevsky, E. & Berdicevsky, I. Mites in fungal cultures. Mycoses 44, 390–394 (2000).
Solarz, K., Senczuk, L., Maniurka, H., Cichecka, E. & Peszke, M. Comparisons of the allergenic mite prevalence in dwellings and certain outdoor environments of the Upper Silesia (southwest Poland). Int. J. Hyg. Environ. Health. 210, 715–724 (2007).
Smrz, J. & Jungova, E. The ecology of a field population of Tyrophagus putrescentiae (Acari: Acaridida). Pedobiologia 33, 183–192 (1989).
Rozej, E. et al. Mite species inhabiting commercial bumblebee (Bombus terrestris) nests in Polish greenhouses. Exp. Appl. Acarol. 56, 271–282 (2012).
Hubert, J. et al. Detection and identification of species-specific bacteria associateed with synanthropic mites. Microb. Ecol. 63, 919–928 (2012).
Kopecky, J., Nesvorna, M., Mareckova-Sagova, M. & Hubert, J. The effect of antibiotics on associated bacterial community of stored product mites. PLoS ONE 9, e112919 (2014).
Brown, A. N. & Lloyd, V. K. Evidence for horizontal transfer of Wolbachia by a Drosophila mite. Exp. Appl. Acarol. 66, 301–311 (2015).
Trullas, S. C., van Wyk, J. H. & Spotila, J. R. Thermal melanism in ectotherms. J. Therm. Biol. 32(5), 235–245 (2007).
Rinehart, J. P., Yocum, G. D. & Denlinger, D. L. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 25(4), 330–336 (2000).
Mironidis, G. K. & Savopoulou, S. M. Effects of heat shock on survival and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) adults. J. Therm. Biol. 35(2), 59–69 (2010).
Fields, P. G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. 28(2), 89–118 (1992).
Ismael, S. R. & Pedro, C. Development and survival of Tyrophagus putrescentiae (Acari: Acaridae) at constant temperatures. Environ. Entomol. 30(6), 1082–1089 (2001).
Tiwari, S., Thakur, R. & Shankar, J. Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol. Res. Int. 2015, 11 (2015).
Xu, Q. et al. Three heat shock proteins from Spodoptera exigua: gene cloning, characterization and comparative stress response during heat and cold shocks. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 159(2), 92–102 (2011).
Bettencourt, B. R., Hogan, C. C. & Nimali, M. Polyglutamine expansion in Drosophila: thermal stress and Hsp70 as selective agents. J. Biosci. 32(3), 537–547 (2007).
Lü, Z. C. & Wan, F. H. Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius). J. Exp. Biol. 214(5), 764–769 (2011).
Xin, T. et al. Gene cloning and expression of heat shock protein gene from Aleuroglyphus ovatus and its response to temperature stress. Int. J. Acarol. 44(7), 279–287 (2018).
Yang, L.-H., Jiang, H.-B., Liu, Y.-H., Dou, W. & Wang, J.-J. Molecular characterization of three heat shock protein 70 genes and their expression profiles under thermal stress in the citrus red mite. Mol. Biol. Rep. 39, 3585–3596 (2012).
Li, M., Lu, W. C., Feng, H. Z. & He, L. Molecular characterization and expression of three heat shock protein70 genes from the carmine spider mite, Tetranychus cinnabarinus (boisduval). Insect Mol. Biol. 18(2), 183–194 (2010).
Sánchez-Ramos, I. & Castaera, P. Effect of temperature on reproductive parameters and longevity of Tyrophagus putrescentiae (Acari: Acaridae). Exp. Appl. Acarol. 36, 93–105 (2005).
Krantz, G. W. & Walter, D. E. (Eds.) A manual of acarology 3rd edn 807. (Texas Tech University Press, 2009).
Chen, D. S., Jin, P. Y. & Hong, X. Y. The complete mitochondrial genome of Tetranychus truncatus Ehara (Acari: Tetranychidae). Mitochondrial DNA 27, 1482–1481 (2014).
Feng, Y., Dearen, T., Cama, V. & Xiao, L. 90-kilodalton heat shock protein, Hsp90, as a target for genotyping Cryptosporidium spp. known to infect humans. Eukaryot. Cell. 8(4), 478–482 (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 4, 402–408 (2001).
Feng, H. et al. Molecular charac-terisation and expression of a heat shock protein gene (HSP90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval). J. Insect Sci. 10, 1–14 (2010).
Colinet, H., Siaussat, D., Bozzolan, F. & Bowler, K. Rapid decline of cold tolerance at young age is associated with expression of stress genes in Drosophila melanogaster. J. Exp. Biol. 216(2), 253–259 (2013).
Tian, H. et al. Molecular cloning of heat shock protein gene Hsp90 and effects of abamectin and double-stranded RNA on its expression in Panonychus citri (Trombidiformes: Tetranychidae). Fla. Entomol. 98(1), 37–43 (2015).
Beckett, S. J. & Evans, D. E. The effects of thermal acclimation on immature mortality in the Queensland fruit fly Bactrocera tryoni and the light brown apple moth Epiphyas postvittana at a lethal temperature. Entomol. Exp. Appl. 82(1), 45–51 (1997).
Jang, E. B. Heat shock proteins and thermotolerance in a cultured cell line from the Mediterranean fruit fly, Ceratitis capitata. Arch. Insect Biochem. Physiol. 19(2), 93–103 (1992).
Hallman, G. J. Mortality of third-instar Caribbean fruit fly (Diptera: Tephritidae) reared at three temperatures and exposed to hot water immersion or cold storage. J. Econ. Entomol. 87(2), 405–408 (1994).
Wang, S. et al. Thermal death kinetics and heating rate effects for fifth-instar Cydia pomonella (Lepidoptera: Tortricidae). J. Stored Prod. Res. 38(5), 441–453 (2002).
W.-W. Liu, P. Yang, X.-M. Chen, D.-L. Xu, and Y.-H. Hu. Cloning and expression analysis of four heat shock protein genes in Ericerus pela (Homoptera: Coccidae). J. Insect Sci. 14(142), 1–9 (2014). https://doi.org/10.1093/jisesa/ieu032
Huang, L. H., Chen, B. & Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 53(12), 1199–1205 (2007).
Huot, J. et al. Hsp27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 56(2), 273–279 (1996).
Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3(5), 294–299 (1994).
Acknowledgements
This work was supported by National Natural Science Foundation of China (31760621, 31460553), Natural Science Foundation of Jiangxi Province, China (20161ACB20003, 20181BAB20405), Leadership in Major Subjects Project of Jiangxi Province (20172BCB22004), Jiangxi Key Research & Development Plan, China (20161BBF60117), Jiangxi Provincial Department of Education Technology plan (KJLD14014), Jiangxi Provincial Department of Education Science and Technology research project (GJJ14167). The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Contributions
J.W. and B.X. conceived the research and drafed the manuscript. S.Q.Q. performed experiment. M.R.J., X.Y.L. wrote the main manuscript text and T.R.X., Z.W.Z. prepared figures and Tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Que, Sq., Liu, X. et al. Characteristic and expression of Hsp70 and Hsp90 genes from Tyrophagus putrescentiae and their response to thermal stress. Sci Rep 11, 11672 (2021). https://doi.org/10.1038/s41598-021-91206-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91206-2
This article is cited by
-
Heat shock protein 70 reflected the state of inhabited fish response to water quality within lake ecosystem
International Journal of Environmental Science and Technology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.