Abstract
Fear is an adaptive emotion that elicits defensive behavioural responses against aversive threats in animals. In mammals, serotonin receptors (5-HTRs) have been shown to modulate fear-related neural circuits in the basolateral amygdala complex (BLA). To understand the phylogenetic continuity of the neural basis for fear, it is important to identify the neural circuit that processes fear in other animals. In birds, fear-related behaviours were suggested to be processed in the arcopallium/amygdala complex and modulated by the serotonin (5-HT) system. However, details about the distribution of 5-HTRs in the avian brain are very sparsely reported, and the 5-HTR that is potentially involved in fear-related behaviour has not been elucidated. In this study, we showed that orthologs of mammalian 5-HTR genes that are expressed in the BLA, namely 5-HTR1A, 5-HTR1B, 5-HTR2A, 5-HTR2C, 5-HTR3A, and 5-HTR4, are expressed in a part of the chick arcopallium/amygdala complex called the dorsal arcopallium. This suggests that serotonergic regulation in the dorsal arcopallium may play an important role in regulating fear-related behaviour in birds. Our findings can be used as a basis for comparing the processing of fear and its serotonergic modulation in the mammalian amygdala complex and avian arcopallium/amygdala complex.
Similar content being viewed by others
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a conserved modulatory neurotransmitter across vertebrate and invertebrate species1. In mammals, 5-HT is involved in a variety of physiological and behavioural functions, including processing of emotions2. In particular, the 5-HT system has long been implicated in the regulation of fear-related behaviours in mammals by modulating the neural circuits of the amygdala3,4. To improve the understanding of the phylogenetic continuity of the neural basis for emotions, it is important to elucidate the neural circuit that processes fear-related behaviours in animals other than mammals. Birds are animals that can potentially serve as models to analyse the control of fear-related reactions5,6. Indeed, lesions of the arcopallium/amygdala complex—a term that has been introduced by Herold et al., to describe a region that combines arcopallium, the posterior pallial amygdala, the nucleus taeniae of the amygdala (TnA) and subpallial amygdaloid area7 —have been reported to reduce escape- and fear-related behaviour in birds8,9,10 and many reports have suggested that a large region of the arcopallium/amygdala complex could be partially homologous to the mammalian amygdala11,12. In addition, 5-HT/serotonin transporter systems have been suggested to modulate fear-related behaviours in chickens based on a combination of behavioural and functional polymorphisms13,14,15. However, because arcopallium/amygdala complex is a large and heterogenous brain region, the contribution of its individual subdivisions to the control of fear is unclear; furthermore, the neural circuits that are modulated by 5-HT for the fear processing in avian brains are largely unknown.
In mammals, the amygdala, which is comprised of a heterogeneous collection of nuclei derived from both the pallium and subpallium11,12, is a key brain region that is important for emotional processing, including the regulation of fear16. Under laboratory conditions, learned fear has been studied using Pavlovian fear conditioning16,17. In particular, the basolateral complex of the amygdala (BLA), which comprises the lateral (LA), basal (BA), and basomedial (BM) nuclei, has been well studied at the microcircuit level17,18,19. The LA receives sensory projections from outside the amygdala and projects to the BA, and the BA projects to the central nucleus of the amygdala (CeA) for output to the brainstem17,18,19. Every nucleus in the amygdala is densely innervated by fibres releasing the neurotransmitter 5-HT20. The released 5-HT acts on its downstream targets through multiple cell membrane receptors called 5-HT receptors (5-HTRs)1. To date, 14 distinct receptors have been classified into seven groups, namely 5-HTR1 to 5-HTR7, based on their structural, functional, and biochemical characteristics1,21. In the mammalian BLA, multiple 5-HTRs are expressed in various cell types, such as glutamatergic principal neurons and inhibitory interneurons22. Several 5-HTRs modulate the neurons that constitute the neural microcircuit that processes fear-related behaviours22,23,24,25,26,27,28.
On the contrary, to date, details about the distribution of 5-HTRs in avian brains are very sparsely reported, and the 5-HTR that is potentially involved in fear-related behaviour has not been elucidated. Therefore, the aim of the present study was to identify the potential 5-HTRs that are involved in the processing of fear-related behaviours in birds. In this study, we selected chick orthologs of six mammalian 5-HTR genes and performed in situ hybridisation in the chick telencephalon. We found that all the used orthologs of 5-HTRs were expressed in the dorsal arcopallium of chicks, which was proposed to be homologous to a part of mammalian BLA based on a detailed analysis of the embryonic origin and molecular profile (pattern of expression of morphogenetic genes during development)11,12,29. This result may support the proposed homology between dorsal arcopallium in birds and BLA in mammals. In addition, we found that 5-HTR2C and 5-HTR4 were preferentially expressed in a part of the TnA, suggesting the serotonergic modulation of the TnA. Our findings can be used as a basis for understanding the serotonergic modulation in the mammalian amygdala complex and avian arcopallium/amygdala complex.
Results
Selection of the chick orthologs of mammalian 5-HTR genes
We selected chick orthologs of six mammalian 5-HTR genes. Five of these mammalian orthologs, that is, 5-HTR1A, 5-HTR2A, 5-HTR2C, 5-HTR3A, and 5-HTR4, have been shown to be involved in the mammalian BLA microcircuit, which processes learned fear-related behaviour22,23,24,25,26,27,28. The mammalian ortholog of 5-HTR1B is certainly expressed in the BLA, but it has not yet been shown to be associated with fear-related behaviour30,31. The orthologs exhibited the following sequence similarities (protein and DNA) between chicks and mice: 5-HTR1A: 80.3% and 76.3%, respectively; 5-HTR1B: 86.7% and 79.4%, respectively; 5-HTR2A: 74.8% and 73.9%, respectively; 5-HTR3A: 70.9% and 71.7%, respectively; 5-HTR4: 84.3% and 80.5%, respectively. The following were the sequence similarities (protein and DNA) between chicks and tropical clawed frogs: 5-HTR2C: 81.4% and 80.5%, respectively. These 5-HTR characteristics are summarised in Table 1. When multiple transcript variants of the 5-HTRs were registered in the database, probes were designed to detect all of them in this study. We performed in situ hybridisation and analysed the expression patterns in the orthologs in the whole telencephalon of the chick.
5-HTR1A expression in the chick pallium
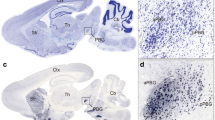
We performed in situ hybridisation analysis to reveal the 5-HTR1A expression pattern in the naïve chick brains on post-hatched day 1 (P1). When we observed the entire sections of the brain hemisphere, we did not detect a significant signal from A 12.8 to A 5.8 (defined by Kuenzel and Masson atlas32) (Sup Fig. S1). However, when we observed each section of the brain in detail, we detected cells showing strong signals that were sparsely distributed throughout the pallium (Fig. 1). On coronal section A 8.6, signals were detected in the hyperpallium (Fig. 1c,c’), mesopallium (Fig. 1d,d’), nidopallium (Fig. 1e,e’), and arcopallium (Fig. 1f,f’). On coronal section A 6.4, signals were detected in the hippocampus (Hp) (Fig. 1i,i’), area parahippocampalis (APH) (Fig. 1j,j’), area corticoidea dorsolateralis (CDL) (Fig. 1k,k’), mesopallium (Fig. 1l,l’), nidopallium (Fig. 1m,m’), and arcopallium (Fig. 1n,n’).
In situ hybridisation of 5-HTR1A in the P1 chick brains. DIG-labelled RNA antisense (a,c–f,g,i–n) and sense (a’,c’–f’,g’,i’–n’) 5-HTR1A probe was used for in situ hybridisation in P1 chick brain coronal sections. To evaluate the expression patterns of 5-HTR1A, sections of five chicks were analysed, and the representative levels of sections (A 8.6 and A 6.4) are shown. The levels of the sections are in accordance with the chick atlas by Kuenzel and Masson32. (b,h) Diagrams of coronal sections shown on panels (a) and (g), respectively. (c–n,c’–n’) Magnified views of brain areas are shown in the boxes in (b) and (h), respectively. A arcopallium; Aph area parahippocampalis; Cdl area corticoidea dorsolateralis; H hyperpallium; Hp hippocampus; LSt lateral striatum; M mesopallium; N nidopallium. Scale bars = 2.5 mm (a,a’,g,g’) and 100 µm (c–f), (c’–f’), (i–n), and (i’–n’).
5-HTR1B expression in the chick pallium
We examined the expression level of 5-HTR1B in sections A 13.6 to A 6.6 in the P1 chick brains (Fig. 2). Strong signals were detected in the whole mesopallium (Fig. 2a–f’, A 13.6 to A 6.6), the medial nidopallium (Fig. 2b–d,b’–d’, A 11.0 to A 8.4), lateral nidopallium (Fig. 2e–f,e’–f’, A 7.4 to A 6.6), and the whole arcopallium (Fig. 2e–f,e’–f’, A 7.4 to A 6.6). In addition, sparse signals were detected in the hyperpallium (Fig. 2a–d,a’–d’, A 13.6 to A 8.4), hippocampus (Fig. 2d–f,d’–f’, A 8.4 to A 6.6), APH (Fig. 2e–f,e’–f’, A 7.4 to A 6.6), CDL (Fig. 2e–f,e’–f’, A 7.4 to A 6.6). Signals were detected in the striatum and subpallial amygdaloid area (Fig. 2c–f, c’–f’, A 9.8 to A 6.6).
In situ hybridisation of 5-HTR1B in the P1 chick brains. DIG-labelled RNA antisense (a–f) and sense (a’–f’) 5-HTR1B probes were used for in situ hybridisation in coronal sections of P1 chick brains. To evaluate the expression patterns of 5-HTR1B, sections of eight chicks were analysed and representative images of four chick brain sections are shown. (a’’–f’’) Diagrams of coronal sections are shown on the rightmost panels. The levels of the sections (A 13.6 to A 6.6) are in accordance with the chick atlas by Kuenzel and Masson32. A arcopallium; Aph area parahippocampalis; Cdl area corticoidea dorsolateralis; E entopallium; H hyperpallium; Hp hippocampus; LSt lateral striatum; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala. Scale bar = 2.5 mm.
5-HTR2A expression in the chick pallium
We examined the expression level of 5-HTR2A in sections A 13.6 to A 5.8 and did not detect clear expression patterns when we observed the entire brain hemisphere sections (Sup Fig. S2). However, when we observed each brain region of the sections in detail (Fig. 3), we detected cells showing signals in the hippocampus (Fig. 3i,i’), lateral nidopallium (Fig. 3j,j’), dorsal arcopallium (Fig. 3k,k’), and intermediate arcopallium (Fig. 3l,l’) in section A 6.4 alone. We did not detect any signals in the remaining sections.
In situ hybridization of 5-HTR2A in the P1 chick brains. DIG-labelled RNA antisense (a,c–f,g,i–l) and sense (a’,c’–f’,g’,i’–l’) 5-HTR2A probes were used for in situ hybridisation in coronal sections of P1 chick brains. To evaluate the expression patterns of 5-HTR2A, sections of five chicks were analysed, and the representative levels of sections (A 8.6 and A 6.4) are shown. (b) and (h) Diagrams of coronal sections shown in panels (a) and (g), respectively. The levels of the sections (A 8.6 and A 6.4) are in accordance with the chick atlas by Kuenzel and Masson32. (c–l,c’–l’) Magnified views of brain areas shown in the boxes in (b) and (h), respectively. Arrowheads indicate signals. A arcopallium; Aph area parahippocampalis; DA dorsal arcopallium; H hyperpallium; Hp hippocampus; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala. Scale bars = 2.5 mm (a,a’,g,g’) and 100 µm (c–f), (c’–f’), (i–l), (i’–l’).
5-HTR2C expression in the chick pallium
We examined the expression level of 5-HTR2C in sections A 13.6 to A 6.6 in the P1 chick brains (Fig. 4). Strong signals were detected in the ventral and dorsal mesopallium (Fig. 4a–c,a’–c’, A 13.6 to A 10.6), entopallium (Fig. 4b–c,b’–c’, A 11.6 to A 10.6), basorostralis (Fig. 4b,b’, A 11.6), field L (Fig. 4e–f,e’–f’, A 7.4 to A 6.6), and TnA (Fig. 4e–f,e’–f’, A 7.4 to A 6.6). In addition, relatively weak signals were detected in almost the entire nidopallium (Fig. 4a–f,a’–f’, A 13.6 to A 6.6), the dorsal and intermediate arcopallium (Fig. 4e–f,e’–f’, A 7.4 to A 6.6), and the striatum and subpallial amygdaloid area (Fig. 2d–e,d’–e’, A 8.4 to A 7.4).
In situ hybridisation of 5-HTR2C in the P1 chick brains. DIG-labelled RNA antisense (a–f) and sense (a’–f’) 5-HTR2C probes were used for in situ hybridisation in coronal sections of P1 chick brains. To evaluate the expression patterns of 5-HTR2C, sections of seven chicks were analysed and representative images of three chick brain sections are shown. (a’’–f’’) Diagrams of coronal sections are shown on the rightmost panels. The levels of the sections (A 13.6 to A 6.6) are in accordance with the chick atlas by Kuenzel and Masson32. A arcopallium; Aph area parahippocampalis; B basorostralis; Cdl area corticoidea dorsolateralis; DA dorsal arcopallium; E entopallium; H hyperpallium; Hp Hippocampus; IA intermediate arcopallium; LSt lateral striatum; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala. Scale bar = 2.5 mm.
5-HTR3A expression in the chick pallium
A clear expression pattern was not detected when we observed the entire sections of brain hemisphere (Sup Fig. S3a–f’). When we observed each brain region of the sections in detail, cells showing strong signals were detected in the whole pallium (Fig. 5). In section A 8.4, cells showing strong signals were detected in the hyperpallium (Fig. 5c,c’), mesopallium (Fig. 5d,d’,l,l’), nidopallium (Fig. 5e,e’,m,m’), and arcopallium (Fig. 5f,f’,n,n’). In section A 6.8, cells showing strong signals were detected in the hippocampus (Fig. 5i,i’), APH (Fig. 5j,j’), and CDL (Fig. 5k,k’).
In situ hybridisation of 5-HTR3A in the P1 chick brains. DIG-labelled RNA antisense (a,c–f,g,i–n) and sense (a’,c’–f’,g’,i’–n’) 5-HTR3A probe was used for in situ hybridisation in P1 chick brain coronal sections. To evaluate the expression patterns of 5-HTR3A, sections of eight chicks were analysed, and representative images of chick brain sections are shown. (b) and (h) Diagrams of coronal sections shown on panels (a) and (g), respectively. Representative levels of sections (A 8.6 and A 6.4) are shown. The levels of the sections are in accordance with the chick atlas by Kuenzel and Masson32. (c–n,c’–n’) Magnified views of brain areas shown in the boxes in (b) and (h), respectively. A arcopallium; Aph area parahippocampalis; Cdl area corticoidea dorsolateralis; H hyperpallium; Hp hippocampus; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala. Scale bars = 2.5 mm (a,a’,g,g’) and 100 µm (c–f), (c’–f’), (i–n), (i’–n’).
5-HTR4 expression in the chick pallium
We examined the expression of 5-HTR4 in sections A 13.6 to A 5.6 (Sup Fig. S4) and detected signals in sections A 8.8 and A 6.6 (Fig. 6). We detected signals in the lateral striatum (LSt) (Fig. 6a,a’) in section A 8.8 and in the dorsal arcopallium (Fig. 6b,c,e,b’,c’,e’) and TnA (Fig. 6b,d,f,b’,d’,f’) in section A 6.6. We could not detect significant signals in the remaining sections.
In situ hybridization of 5-HTR4 in the P1 chick brains. DIG-labelled RNA antisense (a–f) and sense (a’–f’) 5-HTR4 probes were used for in situ hybridisation in P1 chick brain coronal sections. To evaluate the expression patterns of 5-HTR4, sections of six chicks were analysed, and a representative image is shown. (a’’–d’’) Diagrams of coronal sections are shown on the rightmost panels. The level of the sections (A 8.8 and A 6.6) is in accordance with the chick atlas by Kuenzel and Masson32. (c,d,c’,d’) Magnified views of brain areas are shown in the boxes in (b’’). (e,f,e’,f’) Magnified views of brain areas shown in the boxes in (c’’) and (d’’). A arcopallium; Aph area parahippocampalis; DA dorsal arcopallium; Hp hippocampus; LSt lateral striatum; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala. Scale bars = 2.5 mm (a,b,a’,b’), 500 µm (c,d,c’,d’), and 100 µm (e,f,e’,f’).
Comparison of the 5-HTRs-expression patterns in the arcopallium/amygdala complex region
All the examined 5-HTRs were expressed in the arcopallium/amygdala complex region as mentioned above. Subsequently, we compared the expression patterns of these HTRs in the arcopallium/amygdala complex region by using neighboring sections around A 6.6 to A 6.4 (Figs. 7 and 8). Cells showing 5-HTR1A signals were detected sparsely in both the arcopallium and TnA (Fig. 7a,a’, Fig. 8a,a’). 5-HTR1B signals were detected in the whole arcopallium, but no signal was detected in the TnA (Fig. 7b,b’, 8b,b’). 5-HTR2A signals were detected weakly in a part of the arcopallium but not in the TnA (Figs. 7c,c’, 8c,c’). 5-HTR2C signals were detected in the intermediate arcopallium and whole TnA (Figs. 7d,d’, 8d,d’). Cells with 5-HTR3A signals were detected sparsely and thoroughly in both the arcopallium and TnA (Figs. 7e,e’, 8e,e’). 5-HTR4 signals were detected in the whole TnA and not detected the TnA adjacent area of the arcopallium (Figs. 7f, f’, 8f,f’).
Comparison of the 5-HTRs-expression patterns in the arcopallium/amygdala complex in the P1 chick brains using neighboring sections. In situ hybridisation using DIG-labelled RNA antisense and sense 5-HTR1A (a) and (a’), 5-HTR1B (b) and (b’), 5-HTR2A (c) and (c’), 5-HTR2C (d) and (d’), 5-HTR3A (e) and (e’), and 5-HTR4 (f) and (f’) probes in coronal sections of P1 chick brains. (a’’–f’’) Diagrams of coronal sections are shown in the rightmost panels, respectively. A, arcopallium; TnA, nucleus taeniae of the amygdala. Scale bars = 500 µm.
Comparison of the 5-HTRs-expression patterns in the arcopallium/amygdala complex in the P1 chick brains using neighboring sections. Magnified views of the regions around the arcopallium and TnA boundaries are shown in Fig. 7a–f’. In situ hybridisation using DIG-labelled RNA antisense and sense 5-HTR1A (a) and (a’), magnified view of Fig. 7a,a’, 5-HTR1B (b) and (b’), magnified view of Fig. 7b,b’, 5-HTR2A (c) and (c’), magnified view of Fig. 7c,c’, and 5-HTR2C (d) and (d’), magnified view of Fig. 7d,d’, 5-HTR3A (e) and (e’), magnified view of Fig. 7e,e’, and 5-HTR4 (f) and (f’), magnified view of Fig. 7f,f’ probes in coronal sections of P1 chick brains. (a’’–f’’) Diagrams of coronal section are shown in the rightmost panels. A, arcopallium; TnA, nucleus taeniae of the amygdala. Scale bars = 100 µm.
Discussion
In the present study, we revealed the expression patterns of six orthologs of mammalian 5-HTRs, that is, 5-HTR1A, 5-HTR1B, 5-HTR2A, 5-HTR2C, 5-HTR3A, and 5-HTR4, in the telencephalon of chicks (Fig. 9). Previous studies have shown that all mammalian 5-HTRs orthologs are expressed in the mammalian BLA and are associated with fear processing in the rodent BLA, except for 5-HTR1B22,23,24,25,26,27,28,30,31. In this study, we showed that all the used orthologs of 5-HTRs were expressed in the dorsal arcopallium of chicks, which was proposed to be homologous to a part of mammalian BLA based on a detailed analysis of the embryonic origin and molecular profile (pattern of expression of morphogenetic genes during development) (Fig. 9)11,12,29; this suggests that the role of the dorsal arcopallium in fear-related behaviour is similar to that of the mammalian BLA. The arcopallium is a large, heterogenous brain region, but we are beginning to understand the contribution of its individual subdivisions to the regulation of fear-related behaviours10. Our findings support the proposal that the dorsal arcopallium is homologous to a part of the BLA based on developmental studies11,12,29.
Schematic summary of the expression patterns of the six 5-HTRs (5-HTR1A, 5-HTR1B, 5-HTR2A, 5-HTR2C, 5-HTR3A, 5-HTR4) in P1 chicks. Representative expression patterns in sections around A 6.6 to A 6.4 are represented by coloured areas (blue, 5-HTR1A; orange, 5-HTR1B; purple, 5-HTR2A; green, 5-HTR2C; yellow, 5-HTR3A; magenta, 5-HTR4). The darker the colour, the higher the level of gene expression. The dot pattern indicates that the expressed cells are distributed sparsely. The levels of the sections are in accordance with the chick atlas by Kuenzel and Masson32. A arcopallium; Aph area parahippocampalis; Cdl area corticoidea dorsolateralis; DA dorsal arcopallium; FL field L; Hp hippocampus; M mesopallium; N nidopallium; TnA nucleus taeniae of the amygdala.
The lateral nidopallium is also proposed to be partially homologous to the BLA based on a detailed analysis of the embryonic origin and gene expression profile11,12,29. Our data showed that 5-HTR4 was preferentially expressed in the dorsal arcopallium (Figs. 6b,b’ and Fig. 9) and that 5-HTR1B was expressed more abundantly in the dorsal arcopallium than in the lateral nidopallium (Figs. 2f,f’ and Fig. 9). This evidence suggests that the avian dorsal arcopallium and lateral nidopallium have an innervation by serotonergic fibres in common with the mammalian BLA, but the avian dorsal arcopallium resembles the mammalian BLA more closely than does the lateral nidopallium in terms of serotonergic modulation. In the absence of a neocortex in birds, the lateral nidopallium is often considered functionally analogous (but not homologous) to the prefrontal cortex of mammals because of its extensive reciprocal connections with many pallial areas; this implies an important role of the lateral nidopallium as a highly associative centre related to cognition33,34. The lateral arcopallium is another region that is suggested to be functionally and anatomically analogous to the mammalian BLA35. Similarly, 5-HTR1B was expressed less abundantly in the lateral arcopallium, and other 5-HTRs were expressed sparsely in the lateral arcopallium (Figs. 2f,f’ and Fig. 9), suggesting that the lateral arcopallium was modulated by 5-HT and that the avian dorsal arcopallium resembles the mammalian BLA more closely than does the lateral arcopallium in terms of serotonergic modulation. Collectively, our findings imply that the avian dorsal arcopallium is not only homologous but also functionally analogous to the mammalian BLA. Indeed, this notion is consistent with recent findings that the mammalian BLA correlates with the reptilian anterior dorsal ventricular ridge, a part of which is the counterpart of avian arcopallium, using single-cell transcriptome36.
We also found that 5-HTR1A, 5-HTR2C, 5-HTR3A, and 5-HTR4 were expressed in the TnA, whereas 5-HTR1B and 5-HTR2A were not expressed in the TnA but in the region of the arcopallium just adjacent to the TnA. The TnA mostly corresponds to the subpallial medial amygdala of mammals37,38,39,40. Similar to its mammalian counterpart, this region is functionally associated with a variety of social behaviours such as sexual behaviours41 and social interactions at large42,43. Our findings regarding the expression patterns of 5-HTRs in the TnA indicated the possibility that 5-HT plays a key role in shaping social responses. Because 5-HTR2C and 5-HTR4 were preferentially expressed in the TnA, analysing the roles of 5-HTR2C and 5-HTR4 in the TnA may lead to an understanding of the importance of 5-HT in social responses in birds. In this study, we used the Kuenzel and Masson atlas32,37 to determine the location of TnA although the precise location of the TnA in the avian brains is not consistent38,40,43. The location of the TnA was defined differently in the chicken atlases by Puelles et al.44 and the Kuenzel and Masson atlas32.
As for the expression pattern of 5-HTR1A, cells expressing it were sparsely distributed throughout the chick pallium, including the hyperpallium, mesopallium, nidopalliuim, arcopallium, Hp, APH, CDL. There was no difference in the density of 5-HTR1A-expressing neurones between the intra-pallial regions. However, previous studies using radioligand autoradiography revealed that the 5-HTR1A densities in the nidopallium and hyperpallium were higher than those in the Hp and arcopallium/amygdala complex in the pigeon pallium7,45. The difference in the 5-HTR1A signal density between the intra-pallial regions may reflect the density of the axonal terminals of 5-HT neurones projected from the midbrain. Normally, mRNA in the axonal terminals cannot be detected by in situ hybridisation, but receptor binding sites were detected both in the pre- and post-synaptic neurones using radioligand autoradiography30.
As for the expression pattern of 5-HTR1B, it was expressed in the whole mesopallium, arcopallium, striatum, and subpallial amygdaloid area and a part of hyperpallium, nidopallium, Hp, APH, CDL. In mammals, 5-HTR1B is distributed mainly in the basal ganglia regions and several cortical areas; it is also distributed in the amygdala30. Consistent with the mammalian 5-HTR1B distribution, the 5-HTR1B expression levels in the subpallial region seemed to be most densely distributed in the chick brain, suggesting the conserved modulatory function of 5-HTR1B in the striatum and subpallial amygdaloid area.
As for the expression pattern of HTR2A in rats, it was abundantly expressed in the prefrontal cortex, all other cortical areas, and other areas including the amygdala30,46,47. We could not detect the 5-HTR2A signals in the chicken hyperpallium, which is considered to be homologous to the mammalian neocortex48,49. This could have occurred because of the diversity of architecture between mammals and birds.
As for the expression pattern of 5-HTR2C, it is majorly expressed in the intercalated nidopallium (consisting of the basorostralis, entopallium, and field L50) and TnA in chickens. The intercalated nidopallium receives sensory projections from the thalamus, which suggests that serotonergic modulation through 5-HTR2C plays an important role in sensory input processing in the intercalated nidopallium in birds. In terms of TnA expression, high expression levels of 5-HTR2C have been observed in the rodent medial amygdala, which is considered to be the counterpart of the avian TnA51,52, suggesting the conserved roles of 5-HTR2C in the mammalian medial amygdala and avian TnA. For example, exposure to novel objects or novel environments is associated with the activation of TnA in avian species53,54. Avian species exhibit a reluctance to approach novel objects or novel environments (neophobia). Analysing serotonergic modulation via 5-HTR2C in TnA may lead to an understanding of the responsive neural circuits for the processing of neophobia, the fear-related responses to novelty in birds.
As for the expression pattern of 5-HTR3A, cells expressing it were distributed throughout the chick pallium, including the hyperpallium, mesopallium, nidopallium, arcopallium, Hp, APH, and CDL. Similarly, in mammals, 5-HTR3A expressing cells were distributed sparsely throughout the brain including the cortical areas30,55. In addition, many of the 5-HTR3A-expressing cells were shown as GABAergic interneurons. Especially in the amygdala, all 5-HTR3A-expressing cells were GABAergic30,56. Considering the similarity in the distribution patterns of mammalian and avian 5-HTR3A-expressing cells, we assumed that 5-HTR3A-expressing cells in avian arcopallium/amygdala complex are most likely to be GABAergic interneurons.
As for the expression pattern of 5-HTR4, it was expressed in the LSt, dorsal arcopallium, and TnA. In several mammalian species, it was revealed that the 5-HTR4-enriched cells are mainly distributed in the basal ganglia, except the globus pallidus and substantia nigra, and distributed in many pallial areas, including the amygdala30,57. Regarding the basal ganglia, similarities in the expression patterns of 5-HTR4 between mammals and birds suggest that the basal ganglia are subject to conserved serotonergic modulation via 5-HTR4.
In this study, we mainly focused on the control of fear-related behaviour as a physiological function in which the 5-HT system is potentially involved with in birds. Besides the regulation of fear-related behaviours by modulating the neural circuits of the amygdala, the 5-HT system is involved in a variety of physiological functions such as sleep, appetite, sensory processing, locomotor activity, cognition, and emotion in mammals2. We have shown that the 5-HTRs are found in a variety of brain regions other than the avian arcopallium/amygdala complex (Fig. 9). These other brain regions may be involved in the regulation of a variety of physiological and behavioural functions by modulating the neural circuits in birds.
In summary, we found that all the 5-HTRs used in this study were expressed in the dorsal arcopallium, which was proposed to be homologous to a part of the mammalian BLA11,12,29. This result may support the proposed homology between the dorsal arcopallium in birds and the BLA in mammals. In addition, we found that 5-HTR2C and 5-HTR4 were preferentially expressed in a part of the TnA, suggesting the serotonergic modulation of the TnA. Our findings can be used as a basis for understanding the serotonergic modulation in the mammalian amygdala complex and avian arcopallium/amygdala complex.
Methods
Animals
Fertilised eggs of domestic chicks (Gallus gallus domesticus, the Cobb strain) were purchased from a local dealer (3-M, Aichi, Japan). Eggs were incubated at Teikyo University (Kaga, Itabashi-ku, Tokyo). Animal experiments were carried out as described previously58,59. Newly hatched chicks (P0) were transferred to dark plastic enclosures in a dark warm cage at 30 °C for one day (P1). In this study, we used 5 chicks for the HTR1A condition, 8 for the HTR1B condition, 5 for the HTR2A condition, 7 for the HTR2C condition, 8 for the HTR3A condition, and 6 for the HTR4 condition (a total of 11 chicks). We summarized the information about the number of animals we used in this study in Supplemental Table S1. All procedures were reviewed and approved by the Committee on Animal Experiments of Teikyo University and conducted according to the guidelines of the national regulations for animal welfare in Japan.
Histological procedures
First, P1 chicks were deeply anaesthetised by intraperitoneal injection (0.40 mL/individual) of 1:1 solution of ketamine (10 mg/mL, ketalar-10, Sankyo Co., Tokyo, Japan) and xylazine (2 mg/mL, Sigma, St. Louis, Missouri, USA). Then, the chicks were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffered saline (pH 7.5) (PFA-PBS). Dissected brains were immersed in PFA-PBS overnight at 4 °C and placed in an 18% sucrose/PFA-PBS solution for cryoprotection for two days at 4 °C. Subsequently, brains with sucrose substitution were embedded in Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan), frozen immediately on dry ice, and stored at − 80 °C until use.
cDNA cloning and RNA probe preparation
Total RNA was extracted from the chick brain using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed with SuperScript III kit (Invitrogen, Carlsbad, CA, USA) using an oligo (dT) primer, according to the manufacturer’s protocol. RT-PCR was performed using the following gene specific primer (forward and reverse) pairs: HTR1A: 5′-AACACTACCTCCCCAGAACG-3′ and 5′-TCCCCGTTGCTTTTCTTCTG-3′, respectively; HTR1B: 5′-TCACGTGGCTGGGATATCTC-3′ and 5′-CACTGCCACTTCTCACACAC-3′, respectively; HTR2A: 5′-GTGTTTAAGAAAGGCCACTGC-3′ and 5′-TTCCCTGGACTGATGCTTCC-3′, respectively; HTR2C: 5′-CTGACTGTCCAGGTGCTACA-3′ and 5′-TCCATTGACCAACGCTTACA-3′, respectively; HTR3A: 5′-TCCAGAACCTCAAGCCCATC-3′ and 5′-CCGTAGTGGTTCAGTTTGGC-3′, respectively; HTR4: 5′-ATGTGAGTTCGAGTGAGGGC-3′ and 5′-GTCTTGGCAGCTTTGGTCTC-3′, respectively. PCR products were subcloned into the pGEM-T easy vector (Promega, Madison, WI, USA). The sequences that were the target products were confirmed by the Sanger method. Plasmids containing the cDNA fragment for HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, and HTR4 were amplified by PCR with an M13 primer pair. The amplicons containing the T7 and SP6 promoter sites were purified using a PCR purification kit (Qiagen, Valencia, CA, USA). The digoxigenin (DIG)-labelled sense and antisense RNA probes were prepared by in vitro transcription using a DIG RNA labelling kit (Roche, NJ).
Sectioning and in situ hybridisation
The frozen brain blocks were cut into 18 µm-thick sections using a cryostat (Leica CM3050S or Leica CM1850, Leica Biosystems, Nußloch, Germany). Serial coronal sections were prepared from level A 14.4 to A 5.6 of the Kuenzel and Masson’s atlas32. In situ hybridisation was performed as described previously with some modifications60,61. Briefly, brain sections were re-fixed in 4% PFA-PBS and pre-treated and hybridised with DIG-labelled riboprobes at 70 °C. After stringent washes, hybridised probes were detected immunohistochemically with alkaline phosphatase-conjugated anti-DIG antibody (1:1,000; Roche, NJ). To visualise the signals, a chromogenic reaction with a nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate was performed at room temperature for the following duration: HTR1A, 15.5–38 h; HTR1B, 14.5–17 h; HTR2A, 15.5–39 h; HTR2C, 15.5–39 h; HTR3A, 13–37.5 h; and HTR4, 15.5–37.5 h. In every experiment, sense probes were used as negative controls.
Imaging and data processing
Digital photographs of all brain sections on each slide glass were obtained semi-automatically with NanoZoomer 2.0HT or NanoZoomer XR systems (Hamamatsu Photonics, Shizuoka, Japan). The microscopic fields of interest were cropped using NDP.view2 software (ver. 2.7.25, https://www.hamamatsu.com/, Hamamatsu Photonics, Shizuoka, Japan). The entire cropped images were converted to 8-bit, and the brightness and contrast of the entire cropped images were adjusted using ImageJ (https://imagej.nih.gov/ij/).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Marin, P. et al. Classification and signaling characteristics of 5-HT receptors: toward the concept of 5-HT receptosomes. Handb. Behav. Neurosci. 31, 91–120 (2020).
Kandel, E., Schwartz, J. & Jessell, T. Principles of neural science (McGraw-hill, New York, 2000).
Deakin, J. F. W. & Graeff, F. G. 5-HT and mechanisms of defence. J. Psychopharmacol. 5, 305–315 (1991).
Lawther, A. J., Hale, M. W. & Lowry, C. A. Serotonin and the neurobiology of anxious states. Handb. Behav. Neurosci. 31, 505–520 (2020).
Papini, M. R., Penagos-Corzo, J. C. & Perez-Acosta, A. M. Avian emotions: comparative perspectives on fear and frustration. Front. Psychol. https://doi.org/10.3389/Fpsyg.2018.02707 (2019).
Rosa Salva, O., Mayer, U. & Vallortigara, G. Roots of a social brain: developmental models of emerging animacy-detection mechanisms. Neurosci. Biobehav. Rev. 50, 150–168. https://doi.org/10.1016/j.neubiorev.2014.12.015 (2015).
Herold, C., Paulitschek, C., Palomero-Gallagher, N., Güntürkün, O. & Zilles, K. Transmitter receptors reveal segregation of the arcopallium/amygdala complex in pigeons (Columba livia). J. Comp. Neurol. 526, 439–466 (2018).
Phillips, R. E. & Youngren, O. M. Unilateral kainic acid lesions reveal dominance of right archistriatum in avian fear behavior. Brain Res. 377, 216–220. https://doi.org/10.1016/0006-8993(86)90861-9 (1986).
Lowndes, M. & Davies, D. C. The effect of archistriatal lesions on “open field” and fear/avoidance behaviour in the domestic chick. Behav. Brain Res. 72, 25–32. https://doi.org/10.1016/0166-4328(95)00026-7 (1995).
Saint-Dizier, H. et al. Subdivisions of the arcopallium/posterior pallial amygdala complex are differentially involved in the control of fear behaviour in the Japanese quail. Brain Res. Bull. 79, 288–295. https://doi.org/10.1016/j.brainresbull.2009.03.004 (2009).
Martinez-Garcia, F., Novejarque, A. & Lanuza, E. Evolution of the amygdala in vertebrates. In Evolution of Nervous Systems. A Comprehensive Reference (ed. Kaas, J. H.) 255–334 (Elsevier Academic Press, Amsterdam, 2007).
Medina, L., Abellan, A., Vicario, A., Castro-Robles, B. & Desfilis, E. The amygdala. In Evolution of Nervous Systems Vol. 1 (ed. Kaas, J.) (Elsevier Inc, Amsterdam, 2017).
Krause, E. T., Kjaer, J. B., Luders, C. & Van, L. P. A polymorphism in the 5’-flanking region of the serotonin transporter (5-HTT) gene affects fear-related behaviors of adult domestic chickens. Behav. Brain Res. 330, 92–96. https://doi.org/10.1016/j.bbr.2017.04.051 (2017).
Van Phi, V., Krause, E. T. & Phi-Van, L. Modulation of fear and arousal behavior by serotonin transporter (5-HTT) genotypes in newly hatched chickens. Front. Behav. Neurosci. 12, 284 (2018).
Krause, E. T., Kjaer, J. B., Dudde, A., Schrader, L. & Phi-Van, L. Fear but not social behaviour is affected by a polymorphism in the 5’-flanking region of the serotonin transporter (5-HTT) gene in adult hens. Behav. Brain Res. 361, 50–53 (2019).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184. https://doi.org/10.1146/annurev.neuro.23.1.155 (2000).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292. https://doi.org/10.1038/nature14188 (2015).
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned Fear. Neuron 82, 966–980. https://doi.org/10.1016/j.neuron.2014.04.042 (2014).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331. https://doi.org/10.1038/nrn3945 (2015).
Linley, S. B., Olucha-Bordonau, F. & Vertes, R. P. Pattern of distribution of serotonergic fibers to the amygdala and extended amygdala in the rat. J. Comp. Neurol. 525, 116–139. https://doi.org/10.1002/cne.24044 (2017).
Hannon, J. & Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213. https://doi.org/10.1016/j.bbr.2008.03.020 (2008).
Bocchio, M., McHugh, S. B., Bannerman, D. M., Sharp, T. & Capogna, M. Serotonin, amygdala and fear: assembling the puzzle. Front. Neural. Circuit https://doi.org/10.3389/Fncir.2016.00024 (2016).
Cheng, L. L., Wang, S. J. & Gean, P. W. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur. J. Neurosci. 10, 2163–2172. https://doi.org/10.1046/j.1460-9568.1998.00229.x (1998).
Chen, A., Hough, C. J. & Li, H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience 119, 53–63. https://doi.org/10.1016/S0306-4522(03)00076-9 (2003).
Jiang, X. L. et al. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacol 34, 410–423. https://doi.org/10.1038/npp.2008.71 (2009).
Yamamoto, R., Hatano, N., Sugai, T. & Kato, N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT2C receptor. Neuropharmacology 82, 49–58. https://doi.org/10.1016/j.neuropharm.2014.03.007 (2014).
Rainnie, D. G. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J. Neurophysiol. 82, 69–85 (1999).
Huang, Y. Y. & Kandel, E. R. 5-hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J. Neurosci. 27, 3111–3119. https://doi.org/10.1523/Jneurosci.3908-06.2007 (2007).
Martinez-Garcia, F. & Lanuza, E. Evolution of vertebrate survival circuits. Curr. Opin. Behav. Sci. 24, 113–123. https://doi.org/10.1016/j.cobeha.2018.06.012 (2018).
Vilaró, M. T., Cortés, R., Mengod, G. & Hoyer, D. Distribution of 5-HT receptors in the central nervous system: an update. Handb. Behav. Neurosci. 31, 121–146 (2020).
O’Leary, O. F., Codagnone, M. G. & Cryan, J. F. Revisiting the behavioral genetics of serotonin: relevance to anxiety and depression. Handb. Behav. Neurosci. 31, 665–709 (2020).
Kuenzel, W. J. & Masson, M. A Stereotaxic Atlas of the Brain of the Chick (Gallus Domesticus) (Johns Hopkins University Press, Baltimore, 1988).
Güntürkün, O. The avian “prefrontal cortex” and cognition. Curr. Opin. Neurobiol. 15, 686–693. https://doi.org/10.1016/j.conb.2005.10.003 (2005).
Güntürkün, O. & Bugnyar, T. Cognition without cortex. Trends Cogn. Sci. 20, 291–303. https://doi.org/10.1016/j.tics.2016.02.001 (2016).
Xin, Q., Ogura, Y., Uno, L. & Matsushima, T. Selective contribution of the telencephalic arcopallium to the social facilitation of foraging efforts in the domestic chick. Eur. J. Neurosci. 45, 365–380. https://doi.org/10.1111/ejn.13475 (2017).
Tosches, M. A. et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science https://doi.org/10.1126/science.aar4237 (2018).
Reiner, A. et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 473, 377–414 (2004).
Yamamoto, K., Sun, Z., Wang, H. B. & Reiner, A. Subpallial amygdala and nucleus taeniae in birds resemble extended amygdala and medial amygdala in mammals in their expression of markers of regional identity. Brain Res. Bull. 66, 341–347 (2005).
Yamamoto, K. & Reiner, A. Distribution of the limbic system-associated membrane protein (LAMP) in pigeon forebrain and midbrain. J. Comp. Neurol. 486, 221–242 (2005).
Hanics, J., Teleki, G., Alpar, A., Szekely, A. D. & Csillag, A. Multiple amygdaloid divisions of arcopallium send convergent projections to the nucleus accumbens and neighboring subpallial amygdala regions in the domestic chicken: a selective pathway tracing and reconstruction study. Brain Struct. Funct. 222, 301–315 (2017).
Ikebuchi, M., Hasegawa, T. & Bischof, H. J. Amygdala and socio-sexual behavior in male zebra finches. Brain Behav. Evol. 74, 250–257. https://doi.org/10.1159/000264660 (2009).
Mayer, U., Rosa-Salva, O. & Vallortigara, G. First exposure to an alive conspecific activates septal and amygdaloid nuclei in visually-naive domestic chicks (Gallus gallus). Behav. Brain Res. 317, 71–81. https://doi.org/10.1016/j.bbr.2016.09.031 (2017).
Mayer, U., Rosa-Salva, O., Loveland, J. L. & Vallortigara, G. Selective response of the nucleus taeniae of the amygdala to a naturalistic social stimulus in visually naive domestic chicks. Sci. Rep. https://doi.org/10.1038/S41598-019-46322-5 (2019).
Puelles, L., Martinez-de-la-Torre, M., Watson, C., Martinez, S. & Paxinos, G. The Chick Brain in Stereotaxic Coordinates and Alternate Stains 1st edn. (Academic Press, Cambridge, 2007).
Herold, C., Palomero-Gallagher, N., Güntürkün, O. & Zilles, K. Serotonin 5-HT(1A) receptor binding sites in the brain of the pigeon (Columba livia). Neuroscience 200, 1–12. https://doi.org/10.1016/j.neuroscience.2011.10.050 (2012).
Bombardi, C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. Brain Res. 1370, 112–128. https://doi.org/10.1016/j.brainres.2010.11.055 (2011).
Bombardi, C. Neuronal localization of the 5-HT2 receptor family in the amygdaloid complex. Front. Pharmacol. https://doi.org/10.3389/Fphar.2014.00068 (2014).
Fernandez, A. S., Pieau, C., Reperant, J., Boncinelli, E. & Wassef, M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development 125, 2099–2111 (1998).
Puelles, L. et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 424, 409–438 (2000).
Jarvis, E. D. et al. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J. Comp. Neurol. 521, 3614–3665. https://doi.org/10.1002/cne.23404 (2013).
Li, Q. et al. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J. Neurochem. 84, 1256–1265. https://doi.org/10.1046/j.1471-4159.2003.01607.x (2003).
Bonn, M., Schmitt, A. & Asan, E. Double and triple in situ hybridization for coexpression studies: combined fluorescent and chromogenic detection of neuropeptide Y (NPY) and serotonin receptor subtype mRNAs expressed at different abundance levels. Histochem. Cell Biol. 137, 11–24. https://doi.org/10.1007/s00418-011-0882-3 (2012).
Perez, E. C. et al. Object and food novelty induce distinct patterns of c-fos immunoreactivity in amygdala and striatum in domestic male chicks (Gallus gallus domesticus). Behav. Brain Res. 381, 112453. https://doi.org/10.1016/j.bbr.2019.112453 (2020).
Morandi-Raikova, A. & Mayer, U. The effect of monocular occlusion on hippocampal c-Fos expression in domestic chicks (Gallus gallus). Sci. Rep. 10, 7205. https://doi.org/10.1038/s41598-020-64224-9 (2020).
Koyama, Y., Kondo, M. & Shimada, S. Building a 5-HT3A receptor expression map in the mouse brain. Sci. Rep. https://doi.org/10.1038/Srep42884 (2017).
Mascagni, F. & McDonald, A. J. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience 144, 1015–1024. https://doi.org/10.1016/j.neuroscience.2006.10.044 (2007).
Vilaro, M. T., Cortes, R. & Mengod, G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions. J. Comp. Neurol. 484, 418–439. https://doi.org/10.1002/cne.20447 (2005).
Yamaguchi, S. et al. Gene expression profile in cerebrum in the filial imprinting of domestic chicks (Gallus gallus domesticus). Brain Res. Bull. 76, 275–281. https://doi.org/10.1016/j.brainresbull.2008.02.002 (2008).
Yamaguchi, S. et al. Up-regulation of microtubule-associated protein 2 accompanying the filial imprinting of domestic chicks (Gallus gallus domesticus). Brain Res. Bull. 76, 282–288. https://doi.org/10.1016/j.brainresbull.2008.02.010 (2008).
Yamaguchi, S., Aoki, N., Matsushima, T. & Homma, K. J. Wnt-2b in the intermediate hyperpallium apicale of the telencephalon is critical for the thyroid hormone-mediated opening of the sensitive period for filial imprinting in domestic chicks (Gallus gallus domesticus). Horm. Behav. 102, 120–128. https://doi.org/10.1016/j.yhbeh.2018.05.011 (2018).
Fujita, T. et al. The chick pallium displays divergent expression patterns of chick orthologues of mammalian neocortical deep layer-specific genes. Sci. Rep. 9, 20400. https://doi.org/10.1038/S41598-019-56960-4 (2019).
Acknowledgements
This work was supported by the Teikyo University Research Encouragement Grant (T.F.), Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (S.Y., 24590096, 15K07945, 18K06667; N.A., 24790089, 20K06915; T.M., 25291071, 18K07351; C.M., 20K16472 and K.J.H., 26440182, 17K07492, 20K06747), Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)) (T.M., K.J.H, T.F, 19KK0211), the Uehara Memorial Foundation (S.Y.), the Sagawa Foundation for Promotion of Cancer Research (S.Y.), a Grant-in-Aid for Scientific Research on Innovative Areas “Memory dynamism” (26115522), “Adaptive circuit shift” (15H01449) and “Evolinguistics” (20H05012) from the Ministry of Education, Culture, Sports, Science and Technology (K.J.H.), the Naito Foundation (K.J.H.), and the Japan Foundation for Applied Enzymology (K.J.H.). We thank Mr. S. Ishii, Mr. T. Toyoda, Mr. R. Suzuki, and Mr. M. Yumoto (Teikyo University, Faculty of Pharmaceutical Sciences) for technical assistance. All the drawings for brain sections in figures were drawn by T.F. and S.Y.
Author information
Authors and Affiliations
Contributions
T.F. and S.Y. designed the study and performed the research; T.F., N.A., C.M., E.F., K.J.H., and S.Y. analysed the data; and T.F., N.A., C.M., T.M., K.J.H., and S.Y. wrote the paper. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, T., Aoki, N., Mori, C. et al. The dorsal arcopallium of chicks displays the expression of orthologs of mammalian fear related serotonin receptor subfamily genes. Sci Rep 10, 21183 (2020). https://doi.org/10.1038/s41598-020-78247-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78247-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.