Abstract
The relationship between oral health and atopic dermatitis (AD) remains unclear. Here we investigated the association between oral health status and AD using data from 634,299 subjects in the Korean Youth Risk Behavior Survey (KYRBS). Participants with oral symptoms were defined as those with any of following: sensitive teeth, toothache, bleeding gums or gum pain, and dry mouth. Current AD was determined by the question if participant had been diagnosed with AD from doctor within the past 12 months. We estimated the odds ratio (OR) for AD diagnosis according to the presence of oral symptoms. The OR for current AD, which is a dependent variable, was significantly increased in participants with oral symptoms, which are independent variables, in an adjusted model (OR, 1.27; 95% confidence interval [CI], 1.26–1.29; P < 0.001). In detailed analyses, all four oral symptoms were significantly associated with AD diagnosis: sensitive teeth (OR, 1.21; CI, 1.19–1.23; P < 0.001), bad breath (OR, 1.18; CI, 1.17–1.20; P < 0.001), toothache (OR, 1.18; CI, 1.16–1.20; P < 0.001), and bleeding gums (OR, 1.14; CI, 1.12–1.16; P < 0.001). In the presence of oral symptoms, the ORs for having two or more allergic diseases (AD, allergic rhinitis, and/or asthma) were higher than that of AD alone. In this study, oral symptoms appeared to be associated with AD in Korean adolescences.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD), a common allergic disease during childhood, is a chronic and relapsing inflammatory skin disease, often preceded by allergic rhinitis (AR) and asthma1. The prevalence of AD has been reported as 10–30% in children and 2–17% in adults2,3,4, and it has been increasing over the last 30 years not only in western countries, such as Western Europe and the United States, but also in Africa, Northern Europe, the Middle East, and East Asia5,6. In Korea, 13.5% of children and adolescents have AD7.
AD, AR, and asthma are typical “atopic” diseases, and are commonly associated with T helper type 2 (Th2) inflammatory pathway, which characterized by production of allergen-specific immunoglobulin E and Th2-type cytokines such as interleukin (IL)-4, IL-5, and IL-13, and eosinophilic infiltration8,9. Although a progression of allergic conditions in childhood, so called the “atopic march”, is well established8, there are still children and adolescents with a single allergic disease only. The mechanism of this susceptibility difference is not known exactly, but may arise from the differences in structural, genetic, environmental, and immunological factors. In case of AD, skin barrier dysfunction is considered as an important pathogenesis.
The pathophysiology of AD is recognized as complex and multifactorial, and still under investigation10. Both skin barrier dysfunction and immune dysregulation have been implicated as major pathophysiological mechanisms underlying the development of AD, and current concepts suggest that defective skin barrier function is a primary process driving AD, not a consequence of the disease10,11. Although many genetic mutations regarding AD have been identified, which involve skin barrier function, environmental sensing, innate and adaptive immune regulation, and tissue responses, the strongest genetic risk factor is a loss-of-function mutation in filaggrin (FLG), which plays an important role in maintenance of the skin barrier1. FLG aggregates keratin filaments in epithelial cells, and at least 20 mutations in FLG have been found in AD patients1,10,12,13.
A recent pediatric study found higher serum levels of FLG in patients with AR and asthma as well as AD, suggesting its potential pathological role in other atopic diseases not directly involving the skin14. In addition, immunohistochemical analyses have shown that FLG is expressed not only in the epidermis but also in the human oral mucosa15. Since disruption of barrier function could lead to frequent microbial infections and increased transepidermal water loss11, AD patients with structural skin abnormalities and disrupted integrity of the oral mucosa may have altered oral conditions, resulting in AD-associated oral manifestations that have yet to be documented fully. Since there was a large population-based national survey of adolescents in Korea, which included the questions on oral symptoms, such as sensitive teeth, toothache, bleeding or painful gums, and bad breath, we decided to investigate the association between the presence of AD and available oral symptoms in Korean adolescents using this data. Our hypothesis is that adolescents with oral symptoms may have a higher prevalence of AD.
Results
General characteristics
A total of 634,299 participants were included in this study, and the general characteristics of the total study population are presented in Table 1. The median age was 15.06 years, and the prevalence rates of physician-diagnosed current AD, AR, and asthma were 23.3%, 32.9%, and 9.0%, respectively. Of all participants, 59.8% reported that they had experienced oral symptoms. The detailed general characteristics according to the presence of allergic disease such as current AD, AR, and asthma are also presented in Table 1.
Risk of having current AD according to the presence of oral symptoms
We examined the association between the presence of oral symptoms and the risk for current AD in Korean adolescents. Without adjusting for potential confounders, the ORs for current AD were significantly higher in those with oral symptoms (OR, 1.33; 95% confidence interval [CI], 1.32–1.35; P < 0.001) than in those without such symptoms. The ORs for current AD + AR, AD + AR + asthma, AR alone, and asthma alone were also significantly increased in participants with oral symptoms than in those without them (Supplementary Table 1). When adjusted for confounders using model 1 (age and sex) and model 2 (age, sex, region of residence, family income, and smoking, stress, daily tooth brushing frequency, teeth scaling experience, soft drinks/soda consumption, and snack foods consumption), the ORs remained significantly higher in participants with oral symptoms (Fig. 1). In model 2, the OR for current AD, AR, and asthma simultaneously (OR, 1.43; 95% CI, 1.36–1.50; P < 0.001) was higher than the ORs of current AD alone (OR, 1.27; 95% CI, 1.25–1.29; P < 0.001), AR alone (OR, 1.28; 95% CI, 1.26–1.30; P < 0.001), and asthma alone (OR, 1.22; 95% CI, 1.19–1.25; P < 0.001), respectively. Thus, the association between oral symptoms and current AD was significant using both adjustment models, and this relationship was also observed in AR, asthma, and their combination with AD.
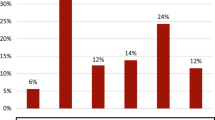
Risk for allergic diseases according to the presence of oral symptoms. When adjusted for confounders using model 2 (age, sex, region of residence, family income, smoking, stress, daily tooth brushing frequency, teeth scaling experience, soda/soft drink consumption, and snack foods consumption), the odds ratio (ORs) of current atopic dermatitis (AD) were significantly higher in those with oral symptoms than in those without such symptoms. The ORs increased with the combination of other allergic diseases, such as allergic rhinitis (AR) and/or asthma, compared to current AD alone. For all OR values, P < 0.001. A P-value less than 0.008 is significant after Bonferroni correction for multiple comparison. Abbreviations: AD, atopic dermatitis; AR, allergic rhinitis.
In addition, we found that the prevalence of oral symptoms were higher in the 15–17 age group than in the 12–14 age group (62.5 ± 0.1% vs. 55.5 ± 0.1%, P < 0.001), and conversely, the OR for AD was higher in the 12–14 age group (OR, 1.30; 95% CI, 1.27–1.33, P < 0.001) than in the 15–17 age group (OR, 1.25; 95% CI, 1.22–1.28; P < 0.001). More detailed analysis is described in Supplementary Table 2.
Differences in risk of having current AD, AR, and asthma according to specific oral symptoms
We also evaluated whether there were differences in the risk of having current AD, AR, and asthma according to each oral symptom (sensitive teeth, toothache, bleeding gums, and bad breath). All oral symptoms were significantly related with each of the allergic diseases (i.e., current AD, AR, and asthma) and their combination (Table 2). In model 2, the ORs for current AD with each oral symptom were higher in the following order: sensitive teeth (OR, 1.21; 95% CI, 1.19–1.22; P < 0.001), bad breath (OR, 1.18; 95% CI, 1.16–1.20; P < 0.001), toothache (OR, 1.16; 95% CI, 1.14–1.18; P < 0.001), and bleeding gums (OR, 1.14; 95% CI, 1.12–1.16; P < 0.001). In addition, the ORs for the combination of current AD, AR, and asthma in all oral symptoms were higher than those for current AD alone. Each oral symptom was significantly associated with the risk for current AD alone, AR alone, and asthma alone, and the ORs for the combination of these allergic diseases were higher than for any single allergic disease.
In age-subgroup analysis, all four oral symptoms were also associated with the presence of allergic diseases in both age group, and the ORs were also high in the order of sensitive teeth, bad breath, toothache, and bleeding gums in both age group. The ORs for allergic diseases were higher in the 12–14 age group than that in 15–17 age group (Supplementary Table 2).
Discussion
In this study, we found that the presence of oral symptoms was associated with increased risk for physician-diagnosed current AD in Korean adolescents aged 12–18 years. The oral symptoms in the questionnaire included sensitive teeth, toothache, bleeding gums, and bad breath, which may indicate broken teeth or worn tooth enamel, dental caries, gingivitis or periodontitis, and dry mouth or gum disease, respectively. When analyzed for each oral symptom, all four oral symptoms were also significantly associated with current AD (Fig. 2). A further analysis revealed the ORs for having two or more allergic diseases, such as AD + AR, AD + asthma, and AD + AR + asthma, were higher than that of AD alone in participants with oral symptoms. When compared 12–14 age group and 15–17 age group, the ORs for AD and/or other allergic diseases were higher in the 12–14 age group, which showed a lower prevalence of oral symptoms than 15–17 age group. There was no significant difference in the prevalence of AD between the age subgroup, so the cause of this age-related difference is uncertain.
Schematic diagram of self-reported oral symptoms and each corresponding oral condition. We inferred the corresponding oral conditions from each survey question, and each OR value of current AD diagnosis was significantly higher for all four oral symptoms. *Adjusted for age, sex, region of residence, family income, smoking, stress, daily tooth brushing frequency, teeth scaling experience, soda/soft drink consumption, and snack foods consumption. †P < 0.001. Abbreviations: AD, atopic dermatitis; OR, odds ratio.
Previous studies regarding oral manifestations in AD, most of which have been cross-sectional studies, have suggested that the following oral manifestations may be associated with AD: increased susceptibility to cariogenic activity, odontogenic focal infection, and reactivation of herpes simplex virus (HSV) type 1 followed by release into the oral cavity16,17,18,19. According to a study based on a national survey that enrolled 91,642 children aged 0–17 years in the United States, eczema was associated with toothache (P < 0.0001), broken teeth (P = 0.01) and bleeding gums (P < 0.0001) but not with dental caries20. This study differed from our study in the questionnaire items, directly including the presence of dental caries and broken teeth.
A study of 21,792 Japanese children aged 6–15 years indicated no significant associations between dental caries and allergic diseases, such as AD, AR, and asthma21. In this study, dental caries were defined as having one or more decayed or filled teeth on oral examination21. The different results from our study may arise from objective oral findings which is more strict outcomes than subjective oral symptoms. However, the GUSTO birth cohort study, which also included oral examination, found that a risk of early childhood caries (ECC), a chronic diet-mediated infectious oral disease at 2 or 3 years of age, was associated with the development of AD within the first year of birth22. This birth cohort study suggests the presence of a common pathologic mechanisms between AD and ECC, which is ectodermal defects during tissue development22. AD with positive skin prick test (SPT) results showed higher OR value than total reported-AD or AD with negative SPT results, which may exclude simple rashes or other skin condition other than true AD by combining SPT results22. Since our study investigated self-reported diseases only, there is a possibility that skin conditions other than AD may be included, resulting lower OR values of AD than GUSTO study which present OR value up to 3 in AD with positive SPT.
In addition, according to a study using National Health Insurance Database of Taiwan, the prevalence of AR, but not asthma, was associated with oral diseases such as dental caries, periodontitis, and gingivitis in the early 20s23. Similarly, Taiwanese children aged 1–9 years showed positive association between the frequency of clinical visit for dental caries and AR, but not asthma24. In Taiwanese studies, AR and asthma were analyzed by including each other in the adjustment model, while our study performed analysis by categorizing as AR, AR + asthma, etc. Although previous studies and our study have showed some inconsistent results, oral symptoms and AD seem to be associated in children and adolescents. The inconsistencies may be due to difference in study design, enrolled population (age and race), and definition and determination of each dental condition (different questionnaire or the presence of the oral examination).
Barrier dysfunction may contribute to increased susceptibility to microbial infection of both skin and the oral cavity. In this regard, there are several possible explanations for the relationship between AD and oral symptoms. AD patients are susceptible to cutaneous infection and colonization, particularly by Staphylococcus aureus and HSV25. Such vulnerability of AD patients to infection is based on skin barrier dysfunction caused by defects in structural proteins such as FLG, and immune dysregulation, including decreased levels of antimicrobial peptides, which may also result in susceptibility to oral cavity infection1,25. Similarly, the pathomechanism of ECC also includes some structural defects in tooth formative stages caused by genetic mutation, leading to increased susceptibility to dental caries26. For example, the distal-less homeobox (Dlx-3) gene is important for both enamel formation and epidermal differentiation, and some polymorphisms of genes, such as mannose-binding lectin 2 (MBL2) and toll-like receptor 2 (TLR2), are associated with both ECC and AD22,27. In addition, FLG, a key structural protein involved in barrier function, is expressed not only in the epidermis but also in the human oral mucosa15, which may cause more infections in the oral cavity. Therefore, increased susceptibility to infections caused by structural gene defects may be one of the mechanisms underlying the association between oral symptoms such as dental caries and AD.
Periodontitis is also an infectious disease, mainly caused by Porphyromonas gingivalis, Treponema denticola, and Actinobacillus actinomycetemcomitans28,29. In the human body, the second largest number of microbiota lives in the oral cavity, and when oral microbiota dysbiosis occurs, proliferation of the above-described anaerobic gram-negative bacteria trigger the immune response of the host, causing chronic inflammation and periodontal tissue destruction30,31. Periodontal diseases include gingivitis, which is more common in children, and periodontitis, which is more prevalent in adults28. Bleeding gums or gum pain may indicate periodontal disease, and are main signs of gingivitis28. Our findings suggest that gingivitis may be associated with increased risk for current AD, and this may also be explained by susceptibility to infection due to structural defects. When pathogens are propagated in the gingival crevice, proinflammatory cytokines and matrix metalloproteinases are released, and then local inflammation and periodontal destruction occur28. Microbial antigens and proinflammatory cytokines could enter the systemic circulation and cause systemic inflammation as well as local inflammation32. This suggests the possibility of oral lesions as an aggravating factor for AD. Unfortunately, we could not determine the association between oral symptoms and the severity of AD due to the limitations of the questionnaire. However, a previous study indicated the improvement of symptoms in AD patients treated for odontogenic focal infections for 3 months, even when there were no complaints regarding oral symptoms18. Further studies are required to clarify the association between oral manifestations and AD severity, and the disease course of AD after treatment of oral lesions.
Our study also showed that self-reported bad breath was associated with current AD. Bad breath may be a sign of not only gum disease but also dry mouth, which is due to mouth breathing or xerostomia29. Although, a decreased salivary flow was observed in asthma and AR patients, it is unclear whether this finding is related to the pathological mechanism of the disease itself, or a result of the treatment of the disease, because some medications, such as antihistamines and inhalers, can cause xerostomia33,34. There are no studies that have directly investigated salivary flow in AD patients, but there has been a study that demonstrated significantly increased OR for mouth breathing in AD patients aged 2–6 years even after adjusting for the coexistence of AR or asthma35. Decreased saliva resulting from mouth breathing or antihistamines could increase susceptibility to oral infection because saliva plays an important role in host defense against oral pathogens36. In addition, Candida albicans, which is a commensal yeast normally present in the oral cavity, may be transformed to a pathogenic form, resulting in the developing of oral lesions, because AD patients are often treated with immunosuppressants or antibiotics and may also have xerostomia19,37. A decrease in IgA levels has been also found in gingival tissue of asthma patients, which plays an important role in immune defense at mucosal levels, thus this may be involved with susceptibility to oral infection in allergic patients38. Furthermore, as observed in the FLG-defective skin, oral mucosa with defective FLG may also be susceptible to dryness and infection leading to dental caries19,39.
This study had some limitations. First, it was based on the results of a self-reported survey and no physical examinations or objective tests were performed. Second, although we found significant associations between patient-reported oral symptoms and current AD, our investigation could not reveal causal links due to the limitations of the study design. Since nebulizers, inhalers and oral medications such as antihistamines may affect xerostomia or other oral conditions, it is also a limitation that the analysis conducted without medication history. Additionally, the relationships between oral symptoms and severity of AD or aggravation of AD could not be established. Finally, because of its large samples size and small effect size, there is also a risk of overpowered study which may be vulnerable to an inflated false positive rate.
In summary, we found that oral symptoms, such as sensitive teeth, toothache, bleeding gums or gum pain, and bad breath, were associated with increased risk for current AD. Although the magnitudes of OR in our study seem to be not strong, it is still significant, and we believe it suggests substantial association between oral symptoms and AD. This study alone is insufficient to determine whether altered oral condition and AD are sharing a common pathogenic pathway, or whether these findings are more likely results or complications of AD, and other allergic diseases. We provide plausible explanations as follows: sensitive teeth, toothache, bleeding gums, and bad breath may be caused by structural defects of enamel and oral epidermis/mucosa resulting increased susceptibility to oral infections, and bad breath may be the results of decreased salivary flow due to mouth breathing or antihistamines, and toothache, bleeding gums, and bad breath may also be induced by change in normal microbiota by the use of immunosuppressants in AD patients. These oral symptoms seem to be interconnected. We therefore suggest that children and adolescents with AD should undergo regular dental examinations and receive appropriate treatment as necessary.
Methods
Study population
Since 2005, the Korea Centers for Disease Control and Prevention (CDC) has annually conducted a large-scale web-based survey to monitor health risk behaviors of Korean middle and high school students, known as the Korea Youth Risk Behavior Web-based Survey (KYRBS)40. KYRBS adopts a stratified, clustered, multi-stage (geographic area, school size, and grade) probability sampling design and the response rate has been high as over 95%40. This survey is therefore considered a nationally representative sample of general Korean adolescents aged 12–18 years40,41. The KYRBS was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (Statistics Korea, approval No.11758). All participants signed online informed consent forms40. All methods in the study were carried out with relevant guidelines and regulations.
We analyzed the data from nine survey years (KYRBS 2009–2017) including 638,475 individuals. Among them, we excluded the participants who did not answer any of the following: region of residence, family income, smoking, stress, school performance, oral symptoms, and the physician-diagnosed AD, AR, and asthma. Finally, a total of 634,299 participants was enrolled in this study.
Variable definitions
The following question was used for each participant to determine current AD, AR, and asthma: “Have you been diagnosed with AD (or AR or asthma) by a doctor within the last 12 months?” Oral symptoms were assessed using the question “Have you experienced any of the following symptoms during the last 12 months?”: aching teeth when you drink hot or cold beverages; throbbing and pulsating tooth pain; bleeding or painful gums; or bad breath. We considered participants with any of the above four symptoms as having an oral symptom. The region of residence was classified as large city, medium or small city, and rural area. For family income, level of stress, and school performance, each participant made a self-assessment by responding to questions “How would you describe your academic performance (or family income/level of stress)?” into one of the following five levels: highest, upper-middle, middle, lower-middle, and lowest. Smoking status was classified as current cigarette smoking or not. The number of tooth brushing per day and tooth scaling within the last year were also evaluated. According to the weekly frequency of consumption of soft drinks/soda or snack foods, the participants were classified as low (0–2 times/week), medium (3–4 times/week) or high (more than 5 times/week) soft drinks/soda or snack foods consumers, respectively.
Although validity tests have not been performed for all variables, Korea CDC conducted a validity test for the self-reported height, weight and smoking status, and the results showed a good validity.
Statistical analyses
Sampling weights, stratification, and clusters provided in the KYRBS data set were incorporated into the analysis, to account for the complex KYRBS survey design and obtain proper estimates and their standard errors. The differences in general characteristics of the participants according to the presence of each atopic disease were analyzed with a t-test using the complex samples general linear model for continuous variables and Pearson χ2 test with Rao-Scott adjustment for categorical variables. To estimate ORs for each of AD, AR, and asthma and their combinations (AD + AR, AD + asthma, and AD + AR + asthma) according to the presence of oral symptoms, we performed binary logistic regression (univariate and multivariate analyses) with a complex sampling design in the following ways: no adjustment, confounder adjustment for age and sex (model 1), and for age, sex, region of residence, family income, smoking, stress, daily tooth brushing frequency, teeth scaling experience, soda/soft drink consumption, and snack foods consumption (model 2). Potential co-variates for model 2 were adopted based on previous studies on the epidemiology and risk of allergic diseases using large-scale nationwide population-based data42,43,44, and then final co-variates were selected via univariate analyses which determined each variable’s significance. Multicollinearity among independent variables also checked by the calculation of the variance inflation factor (VIF)45.
For subgroup analyses according to the presence of each oral symptom, ORs for AD alone, AR alone, asthma alone, and their combinations were also obtained by similar logistic regression analyses with complex sampling. Values are expressed as means ± standard deviations (SD) or proportions ± standard errors (SE), and P value < 0.05 was taken to indicate significance. For multiple comparison, we determined proper P value via the Bonferroni correction. All statistical analyses were performed using SPSS version 22.0 (IBM, Armonk, NY).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Boguniewicz, M. & Leung, D. Y. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol. Rev. 242(1), 233–246 (2011).
Larsen, F. S. & Hanifin, J. M. Epidemiology of atopic dermatitis. Immunol. Allergy Clin. 22(1), 1–24 (2002).
Shaw, T. E., Currie, G. P., Koudelka, C. W. & Simpson, E. L. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J. Invest. Dermatol. 131(1), 67–73 (2011).
Sacotte, R. & Silverberg, J. I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 36(5), 595–605 (2018).
Deckers, I. A. et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE 7(7), e39803 (2012).
Al-Afif, K. A. M. et al. Understanding the burden of atopic dermatitis in Africa and the Middle East. Dermatol. Therapy. 9(2), 223–241 (2019).
Lee, J. H. et al. Prevalence of atopic dermatitis in Korean children based on data from the 2008–2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol. Res. 8(1), 79–83 (2016).
Hill, D. A. & Spergel, J. M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 120(2), 131–137 (2018).
Skevaki, C. & Renz, H. Advances in mechanisms of allergic disease in 2017. J. Allergy Clin. Immunol. 142(6), 1730–1739 (2018).
Weidinger, S. & Novak, N. Atopic dermatitis. Lancet (London, England). 387(10023), 1109–1122 (2016).
David Boothe, W., Tarbox, J. A. & Tarbox, M. B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 1027, 21–37 (2017).
Bieber, T. Atopic dermatitis. N. Engl. J. Med. 358(14), 1483–1494 (2008).
Oyoshi, M. K., He, R., Kumar, L., Yoon, J. & Geha, R. S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 102, 135–226 (2009).
Rasheed, Z. et al. Markers of atopic dermatitis, allergic rhinitis and bronchial asthma in pediatric patients: correlation with filaggrin, eosinophil major basic protein and immunoglobulin E. Clin. Mol. Allergy: CMA. 16, 23 (2018).
De Benedetto, A., Qualia, C. M., Baroody, F. M. & Beck, L. A. Filaggrin expression in oral, nasal, and esophageal mucosa. J. Invest. Dermatol. 128(6), 1594–1597 (2008).
Hannuksela, A. & Vaananen, A. Predisposing factors for malocclusion in 7-year-old children with special reference to atopic diseases. Am. J. Orthodontics Dentofacial Orthopedics 92(4), 299–303 (1987).
Yoshida, M. & Amatsu, A. Asymptomatic shedding of herpes simplex virus into the oral cavity of patients with atopic dermatitis. J. Clin. Virol. 16(1), 65–69 (2000).
Igawa, K., Nishioka, K. & Yokozeki, H. Odontogenic focal infection could be partly involved in the pathogenesis of atopic dermatitis as exacerbating factor. Int. J. Dermatol. 46(4), 376–379 (2007).
Oliveira, A. D. T. et al. Oral Aspects Identified in atopic dermatitis patients: a literature review. Open Dentistry J. 12, 424–434 (2018).
Silverberg, J. I. & Simpson, E. L. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatric Allergy Immunol. 24(5), 476–486 (2013).
Tanaka, K., Miyake, Y., Arakawa, M., Sasaki, S. & Ohya, Y. Dental caries and allergic disorders in Japanese children: the Ryukyus Child Health Study. J. Asthma 45(9), 795–799 (2008).
Kalhan, T. A. et al. Atopic dermatitis and early childhood caries: results of the GUSTO study. J. Allergy Clin. Immunol. 139(6), 2000–2003 (2017).
Ho, S. W., Lue, K. H. & Ku, M. S. Allergic rhinitis, rather than asthma, might be associated with dental caries, periodontitis, and other oral diseases in adults. PeerJ. 7, e7643 (2019).
Chuang, C. Y., Sun, H. L. & Ku, M. S. Allergic rhinitis, rather than asthma, is a risk factor for dental caries. Clin. Otolaryngol. 43(1), 131–136 (2018).
Boguniewicz, M. & Leung, D. Y. Recent insights into atopic dermatitis and implications for management of infectious complications. J. Allergy Clin. Immunol. 125(1), 4–13 (2010).
Vieira, A. R., Gibson, C. W., Deeley, K., Xue, H. & Li, Y. Weaker dental enamel explains dental decay. PLoS ONE 10(4), e0124236 (2015).
Duverger, O. & Morasso, M. I. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J. Cell. Physiol. 216(2), 337–346 (2008).
Arbes, S. J. Jr. & Matsui, E. C. Can oral pathogens influence allergic disease?. J. Allergy Clin. Immunol. 127(5), 1119–1127 (2011).
Perugia, C. et al. Atopic dermatitis and dental manifestations. Giornale italiano di dermatologia e venereologia 152(2), 122–125 (2017).
Kinane, D. F., Stathopoulou, P. G. & Papapanou, P. N. Periodontal diseases. Nat. Rev. Disease Primers 3, 17038 (2017).
Minty, M. et al. Oral microbiota-induced periodontitis: a new risk factor of metabolic diseases. Rev. Endoc. Metab. Disorders 20(4), 449–459 (2019).
Hirschfeld, J. & Kawai, T. Oral inflammation and bacteremia: implications for chronic and acute systemic diseases involving major organs. Cardiovasc. Hematol. Disord.: Drug Targets 15(1), 70–84 (2015).
Lenander-Lumikari, M., Laurikainen, K., Kuusisto, P. & Vilja, P. Stimulated salivary flow rate and composition in asthmatic and non-asthmatic adults. Arch. Oral Biol. 43(2), 151–156 (1998).
Elad, S., Heisler, S. & Shalit, M. Saliva secretion in patients with allergic rhinitis. Int. Arch. Allergy Immunol. 141(3), 276–280 (2006).
Yamaguchi, H. et al. Association between mouth breathing and atopic dermatitis in Japanese children 2–6 years old: a population-based cross-sectional study. PLoS ONE 10(4), e0125916 (2015).
Heo, S. M. et al. Host defense proteins derived from human saliva bind to Staphylococcus aureus. Infect. Immun. 81(4), 1364–1373 (2013).
Portela, M. B. et al. Differential recovery of Candida species from subgingival sites in human immunodeficiency virus-positive and healthy children from Rio de Janeiro, Brazil. J. Clin. Microbiol. 42(12), 5925–5927 (2004).
Ostergaard, P. A. IgA levels, bacterial carrier rate, and the development of bronchial asthma in children. Acta pathologica et microbiologica Scandinavica Sect. C Immunol. 85(3), 187–195 (1977).
Smith, S. A. & Dale, B. A. Immunologic localization of filaggrin in human oral epithelia and correlation with keratinization. J. Invest. Dermatol. 86(2), 168–172 (1986).
Kim, Y. et al. Data resource profile: the Korea Youth Risk Behavior Web-based Survey (KYRBS). Int. J. Epidemiol. 45(4), 1076–1076e (2016).
Bae, J. et al. Test-retest reliability of a questionnaire for the Korea Youth Risk Behavior Web-based Survey. J. Prevent. Med. Public Health 43(5), 403–410 (2010).
Cheng, H. M. et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J. Allergy Clin. Immunol. 133(4), 1048–1055 (2014).
Park H, Kim K. Association of perceived stress with atopic dermatitis in adults: a population-based study in Korea. Int. J. Environ. Res. Public Health. 2016;13(8).
Park, S., Choi, H. S. & Bae, J. H. Instant noodles, processed food intake, and dietary pattern are associated with atopic dermatitis in an adult population (KNHANES 2009–2011). Asia Pac. J. Clin. Nutr. 25(3), 602–613 (2016).
Kutner, M. H., Nachtsheim, C. & Neter, J. Applied Linear Regression Models 4th edn. (McGraw-Hill/Irwin, Boston, 2004).
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2019M3E5D3073365).
Author information
Authors and Affiliations
Contributions
J.S.S. contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.S.Y. contributed to conception, design, data analysis and interpretation, critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shim, JS., Yang, MS. Identification of oral symptoms associated with atopic dermatitis in adolescents: Results from the Korea national representative survey 2009–2017. Sci Rep 10, 19461 (2020). https://doi.org/10.1038/s41598-020-76532-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76532-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.