Abstract
Early pubertal timing has been associated with adult diseases, and identifying preventable causes is of importance. In utero exposure to exogenous glucocorticoids, has been associated with changes in the reproductive hormonal axes in the children, which may influence pubertal timing. Exogenous glucocorticoids can be indicated for diseases such as asthma, allergy, skin diseases, as well as muscle and joint diseases. The aim was to explore the association between in utero exposure to glucocorticoids and pubertal timing in the children. This population-based study was conducted in the Puberty Cohort including 15,819 children, which is a sub-cohort of the Danish National Birth Cohort. Information on maternal glucocorticoid treatment was collected through interviews during pregnancy. Information on pubertal timing was obtained by questionnaires every 6 months throughout puberty, including Tanner Stages, axillary hair, acne, voice break, first ejaculation and menarche. The potential impact of confounding by indication was explored by stratifying on indication and treatment status. Overall, 6.8% of the children were exposed to glucocorticoids in utero. Exposure to glucocorticoids in utero was not associated with earlier puberty for neither boys nor girls with combined estimates of 0.4 months (95% CI: –1.5; 2.2) and –0.7 months (95% CI: –2.5; 1.2).
Similar content being viewed by others
Introduction
A trend towards earlier pubertal timing has been observed in the Western world since the 19th century. The trend is most prominent for girls, while it is more uncertain for boys due to the lack of data and the fact that male pubertal markers are difficult to accurately assess1. Genetics have a great impact on determining pubertal timing2. However, it cannot solely explain the apparent decline in pubertal timing as the genetic pool is considered to remain relatively stable. Obesity during childhood seems to explain part of the decline3. Endocrine disrupters4 and nutrition5 may as well contribute to this potential trend. Early puberty has been associated with diseases later in life including obesity, diabetes mellitus, cardiovascular diseases, breast cancer and testicular cancer6. Additionally, it has been associated with behavioural and emotional problems7. It is, therefore, of public health interest to identify potential causes of the decline in pubertal timing.
In utero exposures, such as medication, can impact the child’s health throughout life8. Medications containing glucocorticoids are commonly used for diseases, which also affect women in the fertile age, e.g. asthma, allergy, skin diseases, as well as more severe autoimmune diseases9. Studies have found an association between in utero exposure to glucocorticoids and altered hypothalamic-pituitary-adrenal-axis (HPA-axis) activity in infants and children10. Most studies have found decreased HPA-axis activity, which would be expected to accelerate pubertal timing through disinhibition of the hypothalamic-pituitary-gonadal-axis (HPG-axis)11. The HPG-axis, and indirectly the HPA-axis, as the two axes interact, regulate the initiation of pubertal timing, which makes a potential association between in utero exposure to glucocorticoids and pubertal timing plausible12,13.
The fetus is normally protected from large amounts of glucocorticoids by the placental enzyme, 11β-hydroxysteroid dehydrogenase type 2, however, the barrier may not be completely resistant14. In utero exposure to glucocorticoids has also been associated with metabolic- and endocrine diseases in the child15, which may be an intermediate factor on the path towards altered pubertal timing.
The aim of this study was to investigate the association between in utero exposure to exogenous glucocorticoids and pubertal timing in boys and girls in a large longitudinal cohort with multiple measurements of various markers of pubertal timing, while considering the indication for use of glucocorticoids in pregnancy.
Materials and Methods
Study population
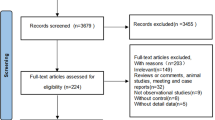
The study was conducted in the Puberty Cohort, which is a sub-cohort in the Danish National Birth Cohort (DNBC)16. The Puberty Cohort consists of live-born singleton children born between 2000 and 2003 of mothers recruited to the DNBC (Fig. 1). In the DNBC, interviews on maternal lifestyle, health and socioeconomic factors during pregnancy were carried out at approximately gestational weeks 12 and 30. Follow-up, including questions on health of the children, continued after birth at age 6 and 18 months, as well as 7 and 11 years. Of 56,641 eligible children, 22,439 children were invited to participate in the Puberty Cohort. The participants were sampled from 12 subgroups based on pre- and perinatal exposures, thought to be of relevance for pubertal development to ensure a broad exposure contrast in the Puberty Cohort. A randomly selected group of 8,000 of the 56,641 eligible children was added. Additionally, among the sampled children, we considered those with information on pubertal timing from the 11-year questionnaire in the DNBC as participants in this study. In total, 15,819 children (70%) returned at least one puberty questionnaire, and 15,769 (99.7%) of those had information on maternal glucocorticoid treatment during pregnancy.
Glucocorticoids
Information on glucocorticoid treatment was obtained through the two telephone interviews conducted during pregnancy. The women were asked about medication for specific diseases, including asthma, allergy, skin diseases and muscle or joint diseases (yes/no). If they replied yes, they were asked to report the pharmaceutical formulation and the specific drug name (either a list of often used medication was provided or the option to provide the name as free text). Further, they were asked about timing of their medication use in gestational weeks. Finally, the women were asked if they had taken any medication not yet mentioned. The questionnaires are available on DNBC’s website17.
The mothers were divided into three groups according to the reported formulation of glucocorticoids in order to make the most homogenous treatment groups: (1) Inhaled corticosteroids (ICS) and first-generation intranasal corticosteroids (INCS). (2) Second-generation INCS, cream, tablets, injections, suppositories, eye- and eardrops. (3) Non-treated (reference group). First generation INCS have a systemic absorption similar to ICS18,19 and were therefore grouped together, whereas second-generation INCS have a lower systemic absorption, and were, therefore, in a separate group20. A priori, it was expected that ICS and INCS were used every day as treatment guidelines recommend this21, which further makes the first treatment group more homogenous. The women were registered as treated independently of the duration of the treatment period, and more than one type of glucocorticoid used in the same week, did not double the exposure in the analyses.
To explore the risk of confounding by indication, information on diseases, for which glucocorticoids may be indicated, was obtained, including asthma, allergy, skin diseases and muscle or joint diseases. The women were divided into four groups based on their disease- and medication status: (1) Women with no disease and no treatment with glucocorticoids were included as the reference group. (2) Women with a disease who received no treatment. (3) Women with a disease who received treatment that did not contain glucocorticoids. (4) Women with a disease who received treatment containing glucocorticoids.
Puberty
Information on pubertal timing was obtained through half-yearly online questionnaires from 11.5 years of age until the child reached full sexual maturity or 18 years of age, whichever came first. The questions were based on a questionnaire used in the Avon Longitudinal Study of Parents and Children22. Additionally, information on pubertal timing was obtained from the 11-year questionnaire in the DNBC. The children were asked to evaluate their current stage of puberty on several pubertal markers, including genital hair and genital- and breast development according to Tanner Stages using short text descriptions and illustrations23,24, axillary hair (yes/no), acne (yes/no), voice break (yes – definitive (adult voice), yes – sometimes (voice break), no), first ejaculation (yes/no, age), and menarche (yes/no, age). When the child reached one pubertal marker, this specific question was not included in the next questionnaire. The questionnaire is available on DNBC’s website17.
Covariates
Directed acyclic graphs (DAGs)25 (Supplementary Fig. 1) were used to identify potential confounders in the study, which included maternal age, maternal body mass index (BMI), maternal age at menarche, smoking during first trimester and highest social class of the parents. Information on maternal age was obtained from the Danish Medical Birth Register, information on highest social class of the parents was obtained from Statistics Denmark and the remaining covariates were available from the DNBC questionnaires. For sub-analyses, length of gestation was obtained from the Danish Medical Birth Register, and childhood asthma was obtained from the 7-year questionnaire within the DNBC.
Statistical analyses
Data on pubertal markers were left, right or interval censored as information on current pubertal stage was reported half-yearly. Some had already reached different pubertal markers before returning the first questionnaire, which make these data left censored. Data on pubertal markers reached between two questionnaires were interval censored, and data on pubertal markers that were not obtained at the last questionnaire were right censored. Therefore, the data was analysed using a censored regression model from which the mean age difference (in months) at attaining the different pubertal markers was estimated for each exposure group with the unexposed as the reference group. The regression model assumed a normal distribution of age at attaining the given pubertal markers. This statistical strategy is recommended in studies when analysing pubertal development with multiple longitudinally collected measurements26. Hence, residuals were checked for normality in R (x64 3.3.1) by comparing the non-parametric cumulative incidence function based on the Turnbull Estimator, against the normal distribution. Moreover, a combined estimate for pubertal timing was estimated using a model for the combined association with all pubertal markers for each sex by using Huber-White robust variance estimation to handle multiple testing of correlated outcomes27,28. All analyses were stratified by sex. The statistical package, -intreg- in Stata/MP 15.1 (StataCorp LLC, College Station, Texas), was used to analyse data.
Sampling weights were applied to account for the non-random sampling of participants. Selection weights were used to overcome the risk of selection bias due to non-participation, where the prior mentioned covariates were included, as well as the exposure variable. Sampling and selection weights were inverse probability weights that were multiplied and used to reweight the analyses. Robust standard errors were included due to the use of sampling and selection weights, as well as clustering of siblings29,30.
Different sub-analyses were performed. First, to examine possible dose-response effects, a sub-analysis was performed, where the number of gestational weeks treated with ICS or first-generation INCS was included as a continuous variable in units of five weeks up to gestational week 28. In total, 15,184 participants were included in this analysis. Second, to explore the risk of confounding by indication, women who were treated with glucocorticoids for a disease not of interest (n = 6), women who were treated with other formulations than ICS or first-generation INCS (n = 585), and women with missing data in either disease status or medication use (n = 188) were not included in the analysis. In total, 14,990 participants were included in this analysis. Third, to consider the risk that glucocorticoids given to women at risk of preterm birth, children born before week 34 + 0 (n = 395) were excluded from the main analysis. At last, additionally adjustments were performed to explore childhood asthma by adjusting for a dichotomous childhood asthma variable (yes/no) and also to explore childhood BMI, the children’s BMI obtained from the 7-year questionnaire were included.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Committee for Biomedical Research Ethics in Denmark has approved data collection in the DNBC ((KF) 01-471/94). A written informed consent was obtained from mothers at recruitment including both mother’s and child’s participation until the children turned 18 years of age. This study was approved by the steering committee of the DNBC (2012-04, 2015-47 and 2017-06) and the Danish Data Protection Agency (2012-41-0379 and 2015-57-0002). As data to the study was gathered and approved prior to this study, further approval was not needed.
Results
In total, 15,769 (70%) mother-child pairs were included in the main analysis. Of these, 6.8% were exposed to glucocorticoids in utero, with the most common being ICS (27.8%), first-generation INCS (19.7%) and cream (43.6%). The reported frequencies of different diseases of interest among mothers were asthma 6.4%, allergy 14.6%, skin diseases 6.8% and muscle or joint diseases 11.5%. As expected, mothers reporting use of glucocorticoids were more likely to have a disease of interest compared to non-treated (Table 1). A large number of women reported one of the diseases, but did not report any treatment for it (17.3%).
We found no difference in age at attaining the different pubertal markers in the crude or the adjusted main analysis for neither boys nor girls (Figs. 2 and 3, Supplementary Table 1), and the estimates for the combined association between all pubertal markers did not show any difference in pubertal timing for boys (0.4 (95% confidence interval (CI): −1.5; 2.2) months) or girls (−0.7 (95% CI: −2.5; 1.2) months). No association between the duration of exposure and timing of puberty for neither boys nor girls was found (Supplementary Table 2). Excluding children born before gestational week 34 + 0 (Supplementary Table 3. Combined estimate boys: 0.4 (95% CI: −1.5; 2.2), combined estimate girls: −0.7 (95% CI: −2.6; 1.1)), adjusting for childhood asthma (Supplementary Table 4. Combined estimate boys: 0.5 (95% CI: −1.6; 2.6), combined estimate girls: −1.7 (95% CI: −3.8; 0.4)) or adjusting for childhood BMI (Supplementary Table 5. Combined estimate boys: 0.6 (95% CI: −1.5; 2.7), combined estimate girls: −1.7 (95% CI: −3.8; 0.4)) did not essentially change the results obtained in the main analysis.
Adjusted mean monthly age differences in attaining different pubertal markers in boys across exposure groups according to pharmaceutical formulation with 95% CI. ICS: inhaled corticosteroids. INCS1: first-generation intranasal corticosteroids. Others include second-generation intranasal corticosteroids, cream, tablets, injections, suppositories, eye- and eardrops. N = 7,676.
Adjusted mean monthly age differences in attaining different pubertal markers in girls across exposure groups according to pharmaceutical formulation with 95% CI. ICS: inhaled corticosteroids. INCS1: first-generation intranasal corticosteroids. Others include second-generation intranasal corticosteroids, cream, tablets, injections, suppositories, eye- and eardrops. N = 8,093.
In the sub-analysis exploring confounding by indication, a tendency towards earlier pubertal timing was observed for sons and daughters of mothers who had one of the diseases of interest, but who did not receive treatment (Figs. 4 and 5, Supplementary Table 6, Combined estimate boys: −0.9 (95% CI: −1.7; 0.0), combined estimate girls: −1.1 (95% CI: −1.9; −0.2)). The association was most pronounced for girls in Tanner Breast stage 5 and Tanner Pubic Hair stage 5, with estimates of mean monthly age differences at −2.0 (95% CI: −4.0; 0.1) and −2.5 (95% CI: −4.3; −0.7). These associations moved towards the null for women treated for their disease (Combined estimate boys: 0.4 (95% CI: −0.9; 1.7), combined estimate girls: −0.9 (95% CI: −2.2; 0.4)), including treatment with glucocorticoids (Combined estimate boys: 0.5 (95% CI: −1.4; 2.3), combined estimate girls: −0.9 (95% CI: −2.8, 1.0)).
Adjusted mean monthly age differences in attaining different pubertal markers in boys across exposure groups according to disease and treatment status with 95% CI. Diseased refers to asthma, allergy, skin diseases or muscle and joint diseases, treatment refers to treatment for the disease without glucocorticoids. N = 7,297.
Adjusted mean monthly age differences in attaining different pubertal markers in girls across exposure groups according to disease and treatment status with 95% CI. Diseased refers to asthma, allergy, skin diseases or muscle and joint diseases, treatment refers to treatment for the disease without glucocorticoids. N = 7,693.
Discussion
In this study, in utero exposure to glucocorticoids was not associated with pubertal timing across various pubertal markers for boys and girls, and no association between the duration of exposure and pubertal timing was observed. However, a tendency towards earlier pubertal timing was observed among sons and daughters of mothers reporting to have one of the diseases of interest (asthma, allergy, skin diseases and muscle or joint diseases) without being treated compared to children of mothers with a disease who received treatment. However, these differences were small and must be interpreted with caution.
No previous studies among humans have investigated the association between in utero exposure to glucocorticoids and pubertal timing in the children. However, one experimental rat study have suggested that exposure to glucocorticoids was associated with delayed puberty31. Animal studies may not be comparable with human studies due to differences in neurodevelopmental profiles across species32, no juvenile pause in rats and the study used a high dose of glucocorticoids which is different from our study. Previous human studies have examined the HPA-axis activity through basal cortisol levels and reactivity to stress after exposure to glucocorticoids in utero10,33. In general, these studies found decreased HPA-axis activity in infancy and focused on glucocorticoids given to pregnant women at risk of preterm birth, which are more potent than the glucocorticoids being evaluated in our study. Moreover, they are often given once or twice late in pregnancy rather than continuously throughout pregnancy, and, therefore, differences may be observed. However, few studies have also investigated the use of ICS15 and INCS34. A large study within the DNBC found an association between in utero exposure to ICS and metabolic and endocrine diseases in children around age 6 years15.
Strengths of the study include the large study population and information on various pubertal markers with continuous follow-up for boys and girls. Both information on treatment and puberty was self-reported, which introduces the risk of misclassification. However, interviews and questionnaires were conducted near the event, which reduces the risk of recall bias. A validation study within the DNBC conclude that women are more likely to report medicine for chronic diseases rather than medicine for local use or short-term treatment, which implies that women treated for e.g. asthma may be more likely to report use of medication compared to women treated with local corticosteroids for a skin disease35. Due to the risk of underreporting, it cannot be ruled out that women in the untreated group may be truly treated, and, thereby, the results would be biased towards the null. Some women may also have been treated with glucocorticoids due to risk of preterm birth, but since exclusion of children born before week 34 + 0 did not change the results, we consider this to be of minor importance in the study. However, we cannot exclude that there may be women who were treated with glucocorticoids and following giving birth to a full-term baby, but we consider this proportion to be limited.
Another validation study in the Puberty Cohort36 showed some misclassification on the self-reported pubertal staging compared to clinical assessment, but the estimated agreements were reported as fair to moderate indicating that self-reported staging is still a useful tool. Self-reported puberty staging is time and cost saving with high participation rates, and gives the opportunity to analyse large cohorts which is needed especially for boys within research on pubertal timing. The potential misclassification in this study is expected to be non-differential given that information on exposure and outcome was obtained independently of each other, as pregnant women were not aware of their unborn child’s future pubertal timing, and the children would not be prone to answer questions on pubertal stages according to their mothers’ treatment with glucocorticoids.
Glucocorticoids of various formulations differ in systemic absorption, for example tablets37 versus cream38, and may therefore not have the same effect on pubertal timing. We primarily focused on the group exposed to ICS and first-generation INCS, and one should be careful when concluding upon the results for the other formulations. The association between duration of treatment and pubertal timing was explored by using information on which gestational weeks the women were treated. No associations were found in this analysis, which may be due to lack of exposure contrast, as a large proportion (49%) had been treated every gestational week until week 28. The number of treated weeks was used as a surrogate marker for the treatment dose. It may also be relevant to consider timing of treatment, as the fetus may be more vulnerable to exposures during the first trimester39. The data used in this study concerns the first two trimesters, and the majority have been exposed every week; therefore, a trimester specific analysis was not performed.
Although the study has a relatively high participation rate, about 70%, risk of selection bias must be considered. Glucocorticoid exposure was similar between participants (6.8%) and non-participants (5.5%). Therefore, we do not expect that selection bias has biased our findings. Still, selection weights were included in the analyses to reduce the risk of selection bias.
It is challenging to separate the effect of the medicine from the disease itself, but results from our sub-analysis showed that the disease itself seems to be associated with earlier puberty for both boys and girls, rather than the use of glucocorticoids. There might be different explanations for this. First, untreated disease may be associated with earlier puberty. Second, diseases treated with glucocorticoids showed no clear effect on pubertal timing, which may be due to glucocorticoids delaying pubertal timing and, thereby, cancel out the effect of the disease. This potential explanation is contradictory to the initial hypothesis, which suggests that glucocorticoids accelerate pubertal timing. However, from this study we are not able to rule out that other unknown biological mechanisms may be at play. Third, the results can be chance findings. Either way, the results must be interpreted with caution as the estimated differences were small. In general, studies find it safer to use ICS for asthma, rather than refusing treatment during pregnancy, when evaluating health implications for the child40. This recommendation was supported by the results for pubertal timing in the children obtained in this study.
In conclusion, in utero exposure to glucocorticoids was not associated with pubertal timing in the children. No association between duration of treatment with glucocorticoids and pubertal timing was observed. The results are reassuring for women who suffer from diseases where glucocorticoids are indicated.
Data availability
The dataset analysed in the study is not publicly available due to national data security legislation on sensitive personal data.
References
Ong, K. K., Ahmed, M. L. & Dunger, D. B. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 254-255, 8–12 (2006).
Wohlfahrt-Veje, C. et al. Pubertal Onset in Boys and Girls Is Influenced by Pubertal Timing of Both Parents. J Clin Endocrinol Metab. 101, 2667–74 (2016).
Reinehr, T. & Roth, C. L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 3, 44–54 (2019).
Greenspan, L. C. & Lee, M. M. Endocrine disrupters and pubertal timing. Curr Opin Endocrinol Diabetes Obes. 25, 49–54 (2018).
Villamor, E. & Jansen, E. C. Nutritional Determinants of the Timing of Puberty. Annu Rev Public Health. 37, 33–46 (2016).
Golub, M. S. et al. Public health implications of altered puberty timing. Pediatrics. 121(Suppl 3), S218–30 (2008).
Mendle, J., Turkheimer, E. & Emery, R. E. Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Developmental review: DR. 27, 151–171 (2007).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 359, 61–73 (2008).
Coutinho, A. E. & Chapman, K. E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 335, 2–13 (2011).
Tegethoff, M., Pryce, C. & Meinlschmidt, G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev. 30, 753–89 (2009).
Oyola, M. G. & Handa, R. J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 20, 476–494 (2017).
Seckl, J. R. & Holmes, M. C. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 3, 479–88 (2007).
Ebling, F. J. The neuroendocrine timing of puberty. Reproduction. 129, 675–83 (2005).
Braun, T., Challis, J. R., Newnham, J. P. & Sloboda, D. M. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr Rev. 34, 885–916 (2013).
Tegethoff, M. et al. Inhaled glucocorticoids during pregnancy and offspring pediatric diseases: a national cohort study. Am J Respir Crit Care Med. 185, 557–63 (2012).
Olsen, J. et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health. 29, 300–7 (2001).
DNBC. Danish National Birth Cohort https://www.dnbc.dk/data-available (2018).
Sastre, J. & Mosges, R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol. 22, 1–12 (2012).
Daley-Yates, P. T. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 80, 372–80 (2015).
Chong, L. Y. et al. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 4, Cd011993 (2016).
Chauhan, B. F., Chartrand, C., & Ducharme, F. M. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database Syst Rev. Cd009611 (2013).
Fraser, A. et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 42, 97–110 (2013).
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch Dis Child. 44, 291–303 (1969).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 45, 13–23 (1970).
Shrier, I. & Platt, R. W. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 8, 70 (2008).
Euling, S. Y. et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 121 Suppl 3, S172–91 (2008).
Huber, P. J. The behavior of maximum likelihood estimates under nonstandard conditions. in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics. Berkeley, Calif.: University of California Press. (1967)
White, H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 48, 817–838 (1980).
Hernan, M. A., Hernandez-Diaz, S. & Robins, J. M. A structural approach to selection bias. Epidemiology. 15, 615–25 (2004).
Brix, N., et al. Maternal Smoking During Pregnancy and Timing of Puberty in Sons and Daughters: A Population-Based Cohort Study. Am J Epidemiol. (2018).
Smith, J. T. & Waddell, B. J. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 141, 2422–8 (2000).
Moisiadis, V. G. & Matthews, S. G. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol. 10, 391–402 (2014).
McGowan, P. O. & Matthews, S. G. Prenatal Stress, Glucocorticoids, and Developmental Programming of the Stress Response. Endocrinology. 159, 69–82 (2018).
Alhussien, A. H., Alhedaithy, R. A. & Alsaleh, S. A. Safety of intranasal corticosteroid sprays during pregnancy: an updated review. Eur Arch Otorhinolaryngol. 275, 325–333 (2018).
Olesen, C. et al. Do pregnant women report use of dispensed medications? Epidemiology. 12, 497–501 (2001).
Ernst, A. et al. Self-assessment of pubertal development in a puberty cohort. J Pediatr Endocrinol Metab. 31, 763–772 (2018).
Pickup, M. E. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 4, 111–28 (1979).
Chi, C. C., Lee, C. W., Wojnarowska, F., & Kirtschig, G. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. Cd007346 (2009).
Sachdeva, P., Patel, B. G. & Patel, B. K. Drug use in pregnancy; a point to ponder! Indian journal of pharmaceutical sciences. 71, 1–7 (2009).
Labor, S. et al. What is safe enough - asthma in pregnancy - a review of current literature and recommendations. Asthma Res Pract. 4, 11 (2018).
Acknowledgements
The work was supported by the Independent Research Fund Denmark (2015-4183-00152). The funding source had no involvement in the study.
Author information
Authors and Affiliations
Contributions
S.A.S. planned the study in close collaboration with A.E., L.L.H.L., N.B. and C.H.R.-H. S.A.S. performed data management and statistical analyses with guidance from A.E. and N.B. Discussion and interpretation of results were discussed among S.A.S., A.E., L.L.H.L., N.B., A.G-S. and C.H.R.-H. S.A.S. wrote the first draft for the manuscript, and A.E., L.L.H.L., N.B., A.G.-S. and C.H.R.-H. provided constructive comments. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Author Cecilia Høst Ramlau-Hansen has received research grants from the Independent Research Fund Denmark. The other authors declare that they have no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sand, S.A., Ernst, A., Lunddorf, L.L.H. et al. In Utero Exposure to Glucocorticoids and Pubertal Timing in Sons and Daughters. Sci Rep 9, 20374 (2019). https://doi.org/10.1038/s41598-019-56917-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56917-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.