Abstract
The indiscriminate use of sedative drugs during endoscopy can pose multiple risks including cognitive impairment in advanced liver cirrhosis. However, the data are scarce regarding which sedative drugs are safest in these populations. The aim of this study was to evaluate the safety profiles including cognitive performance among midazolam, propofol, and combination therapy in advanced cirrhotic patients. This double-blind randomized controlled study included 60 consecutive advanced cirrhotic patients who underwent upper gastrointestinal endoscopy. The Stroop application was used to screen for cognitive impairment. Patients were randomly assigned to one of 3 groups, midazolam, propofol, or the combination group, and underwent Stroop test before and two hours after the completion of endoscopy. Hemodynamic safety and the subjective satisfaction score were also evaluated. Patients did not show significant changes in on-time or off-time on the Stroop test before and two hours after sedatives, and there was no significant difference among the 3 treatment groups. Also, there were no significant vital sign changes after sedatives. Time-to-recovery was longest in midazolam group, and patient awakening and patient memory were highest in propofol group. However, all 3 groups showed no difference in patient satisfaction, but the combination group was more preferred in terms of subjective satisfaction by physicians. Factors affecting worsened Stroop speed after sedatives were older age, low education level and high MELD score. All sedative methods using midazolam, propofol, or combination therapy showed similar safety profile in advanced cirrhosis, and were not associated with increased risk of cognitive impairment.
Similar content being viewed by others
Introduction
In patients with cirrhosis, the use of sedative drugs results in an increased risks of adverse events due to delayed hepatic clearance1. Besides the cardiopulmonary adverse events, acute deterioration of hepatic encephalopathy might occur2,3. The spectrum of neurocognitive impairment in liver cirrhosis ranges from unimpaired, minimal hepatic encephalopathy (MHE), to overt hepatic encephalopathy (OHE). Although MHE is a subclinical neurocognitive disorder without clinical symptoms, it is associated with impaired quality of life, employment, driving ability, and progression to OHE4,5,6.
Currently, the most frequently used methods to diagnose cognitive impairment include electroencephalography, critical flicker frequency, Continuous Reaction Time Test, Inhibitory Control Test, and computerized test batteries7. However, existing tests for detecting cognitive impairment are time consuming and costly, so they have not been widely used in clinical practice8. The Stroop test is one method of detecting hepatic encephalopathy, and has been shown to be an efficacious way to screen for cognitive impairment9. A previous study showed that the Stroop smartphone app was a short, valid, and reliable tool for use by cirrhotic patients10.
The use of midazolam and propofol in patients with liver cirrhosis has been investigated in a few studies. Although previous reports have suggested the use of propofol rather than midazolam in cirrhotic patients, propofol has no reversal agents and lacks analgesic effects. Recently, a balanced propofol sedation (BPS) method combining the advantages of both drugs has been recommended11. However, the safety and efficacy of the combination regimen compared to a propofol alone regimen remains controversial in cirrhotic patients12,13. Thus, we investigated the influence of midazolam, propofol, and midazolam plus propofol (BPS) on safety and efficacy during diagnostic upper GI endoscopy, to determine which method can best avoid cognitive impairment, using the Stroop test.
Results
Baseline characteristics
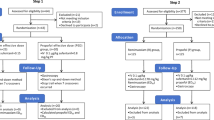
Between February 2018 and October 2018, 118 patients were screened and a total of 60 patients were randomized into the study. Flow chart is shown in Supplementary Fig. 114. The baseline characteristics of the patients enrolled in the study are reported in Table 1. The mean age of the patients was 52.63 ± 10.07 years, and 48 patients (80%) were male. When categorized by etiology, 34 patients (56.7%) had alcoholic cirrhosis and 26 patients (43.4%) had non-alcoholic cirrhosis. The median Model for End-Stage Liver Disease (MELD) score was 9.49 points and 37 patients (61.6%) were child-pugh class B or C. Any degree of ascites was observed in 34 patients (56.6%). Esophageal varices were found in 50 patients (83.3%), whereas gastric varices were found in 13 patients (23.7%).
Hemodynamic changes and safety
Changes in vital signs before, during, and two hours after endoscopy are recorded in Supplementary Table 1 and Fig. 1. Two hours after endoscopy, systolic blood pressure (SBP) tended to be slightly lower in the M group (114 mmHg) and MP group (119 mmHg), compared to the P group (126 mmHg). However, the differences were not statistically significant between groups (P = 0.144; Fig. 1A). When SBP was compared before and after treatment for each drug, all groups showed no significant changes in SBP before or after the procedure. Heart rate (HR), tended to rise during the endoscopy (85 beat/min), but returned to baseline (78 beat/min) after the endoscopy. However, the differences among the groups were not clear (Fig. 1B). Diastolic blood pressure (DBP) and oxygen saturation did not show any significant change before, during, or after the procedure. In addition, there was no significant difference according to groups (Fig. 1C,D). None of the patients underwent major serious adverse events during the endoscopy, and no patients showed paradoxical response after sedative drug injection (Supplementary Table 2).
Stroop test results before and after sedation
The total Off-time and On-time from the Stroop test before and two hours after the endoscopy are described in Table 2 and Fig. 2. Median trials for five correct runs on the Stroop application are described in Supplementary Table 3. In all patients, pre-endoscopic Stroop On-time was significantly higher than Off-time (93.5 vs 76.9 seconds; P < 0.001), whereas the number of runs needed to complete five correct runs were similar between Off and On states. Two hours after the endoscopy, Off-time was changed from 76.9 to 74.7 seconds (P = 0.848) and On-time changed from 93.5 to 92.5 seconds (P = 0.457). There were no significant differences between pre-endoscopic and post-endoscopic examinations in the results of Off-time, On-time and Off-time plus On-time (Table 2). Next, we compared the changes in Stroop test results before and after endoscopy for each drug. In the M group, changes of Off-time (Fig. 2A, P = 0.681), On-time (Fig. 2B, P = 0.332), and Off-time plus On-time (Fig. 2C, P = 0.455) were not significantly different after sedation, and these results were similar in the P group (Off-time, P = 0.737; On-time, P = 0.204; Off-time plus On-time, P = 0.079) and the MP group (Off-time, P = 0.575; On-time, P = 0.108; Off-time plus On-time, P = 0.911).
Subjective satisfaction measurement
The time-to-recovery after endoscopy was analyzed. The M group took the longest time (27 minutes) to recover, followed by the MP group (15.0 minutes) and the P group (8.0 minutes). The difference between groups was statistically significant (P = 0.006). Next, we evaluated the procedure satisfaction of doctors, nurses, and patients after the endoscopic procedures (Table 3). In the physician group, the MP group showed the highest satisfaction (9.0 points), followed by the M group (8.0 points) and the P group (7.5 points) (P = 0.024). Nurses also showed a similar pattern, with higher satisfaction in the order of the MP group (8.5 points), the M group (8.0 points), and the P group (7.0 points), but the results were not statistically significant (P = 0.053). Patients were surveyed on four aspects. In patients, overall satisfaction did not show any difference among the groups (P = 0.365). Also, the recall of pain or discomfort did not show any significant difference (P = 0.127). However, patients who experienced temporary awakening or memory during endoscopy were significantly higher in the P group (both P < 0.001).
Factors affecting cognitive performance
Finally, we analyzed the factors affecting the cognitive performance using the results of Off-time plus On-time tests after sedation (Table 4). In multivariate analysis, older age [beta coefficients (B) 4.20, standard error (SE) 0.91; P < 0.001], low education level (B 85.17, SE 20.99; P < 0.001) and high MELD score (B 7.59, SE 1.84; P < 0.001) were associated with cognitive impairment showing an increased Off-time plus On-time after sedation. However, sedative drug and groups were not significant factors in univariate and multivariate analyses. Similar results were obtained when the outcome was changed from Off-time plus On-time to On-time (Supplementary Table 4).
Discussion
In terms of the safety of patients with liver cirrhosis, in addition to hemodynamic stability, cognitive impairment must be considered. Although most physicians are aware of the clinical significance of cognitive performance, the tests used to diagnose cognitive impairment, e.g. MHE, so far are expensive, time-consuming, require an expert to conduct, and have not been widely used in real clinical practice8. Recently, the Stroop application, a method that can be implemented very simply through a smartphone application, has been developed and compared with other tools (NCT, digit symbol test, block-design test), and verification has been reported. It is easier and simpler than other tests to diagnose cognitive impairment and has proven clinical utility in cirrhotic patients. In our study, even older patients performed a Stroop test with relative ease according to the examiner’s explanation.
To date, there have been three randomized control trials for deterioration of cognitive performance after sedation. One study concluded that there was no difference in the incidence of MHE between midazolam and propofol15, and two studies concluded that midazolam increased MHE significantly compared to propofol16,17. In our study, deterioration was not observed before and after two hours of sedation in all three groups: midazolam, propofol, and the combination group. We suggest that the difference of the results for each study were because of the following reasons. First, the tests performed to detect cognitive impairment were different in all studies, so it is impossible to directly compare them. Second, the time of follow-up test after sedation was different in each study. We performed the follow-up Stroop test after two hours, in consideration of the half-life of midazolam and discharge time from the recovery room. However, other studies have conducted this test at 30 and 60 minutes, which is likely to result in worse effects for the midazolam group15,17. Third, drug doses and protocols tended to differ slightly from study to study. In fact, three studies conducted in Germany, India and Israel used a dose of propofol higher than our study (more than 150 mg vs. 50 mg). Asians seemed to reach the sedation level with relatively small doses compared to ethnicities represented in the three studies cited above. Because the body mass index and drug metabolism are different for different races, the protocol dose seems to be different for each race. Therefore, if race is different, direct comparison between studies may be difficult.
In our study, advanced age and deteriorated liver function were associated with worsened cognitive performance after sedatives. Factors reported in other studies were advanced cirrhosis, previous history of OHE10,18, and advanced ages19, similar to our study. Lower educated patients were also shown at increased risk factor for cognitive impairment in our study, and this is a new discovery not shown by other studies. In other studies, most of the enrolled patients were highly educated persons (median 14 years), and few studies have been conducted on patients with low education levels10. Taken together, age and education level should be considered in interpreting the results of the Stroop test.
Our study, unlike other studies, examined the satisfaction scores of medical staff and patients. Despite the fact that subjective satisfaction is a clinically important index, is has not been performed in many studies related to endoscopy in patients with liver cirrhosis. One study reported that patients prefer propofol rather than midazolam, in terms of subjective satisfaction20. However, in our study, all patients were satisfied with midazolam, propofol, and the drug combination, and there was no difference among the three groups. Meanwhile, in the medical staff, especially the physician group, the satisfaction of the drug combination was the highest. Patients who experienced temporary awakening were higher in the propofol group, and these items might also affect the satisfaction of the medical staff.
However, our study has the following limitations. First, only a single Stroop test was used as a diagnostic tool of cognitive impairment. Furthermore, exact cut-off for cognitive impairment was not presented. Second, no long-term consequences were evaluated in our patients. Third, this study included only upper GI endoscopy for screening varices, so the result might be different in colonoscopy or other type of endoscopic procedure. Forth, our study only recruited Asian patients and these findings may not be generalized for all different races.
In conclusion, neither midazolam only, propofol only, nor the combination protocol induced significant cognitive function changes in the Stroop test and showed similar safety profile in advanced cirrhosis. All three methods are hemodynamically safe and showed high patient satisfaction, and can be used clinically in patients with advanced cirrhosis.
Materials and Methods
Study design and patients
This was a single-center, prospective, double-blind randomized controlled trial at a tertiary referral hospital, performed from February 2018 to October 2018. This trial was registered in Clinical Research information Service (CRIS), which is a member of WHO International Clinical Trials Registry Platform (registration number KCT0002964, date of registration 31/01/2018). Reporting of the study conforms to Consolidated Standards of Reporting Trials (CONSORT) 2010 statement14. The inclusion criteria were admitted patients diagnosed with advanced liver cirrhosis between ages 19 and 75, who were to undergo diagnostic upper GI endoscopy to screen for varices. Exclusion criteria were (1) prior history of OHE; (2) evidence of current gastrointestinal bleeding; (3) American Society of Anesthesiologists (ASA) physical classification class IV or higher; (4) use of anti-convulsant drugs within four weeks before endoscopy that could be anticipated to have a reduced sedative effect; (5) patients who were allergic to study drugs, egg, or soybean; (6) patients who were illiterate or color-blind; and (7) patients who refused to participate in the study. The patients were randomly assigned in a 1:1:1 ratio to the midazolam alone group (M group), propofol alone group (P group), or midazolam plus propofol group (MP group) using computer-generated allocation sequences. The randomization of group was managed by a statistician (MJE) not participating in the endoscopic evaluation of the patients and blinded to the investigators and patients.
Sedation protocol
In the M group, 0.03 mg/kg or 2 mg of midazolam was given to patients aged <65 years or with a body weight >55 kg. In patients with >65 years old or with a body weight <55 kg, the initial dose was reduced by 20 percent. In the P group, 0.5 mg/kg of propofol was given intravenously initially. In patients >65 years old or with a body weight <55 kg, the initial dose was reduced by 50 percent. In the MP group, 0.03 mg/kg or 2 mg of midazolam and 20 mg of propofol were given initially. In patients >65 years old or with a body weight <55 kg, the dose of midazolam was reduced by 20%, and propofol was reduced by 50 percent. After the endoscopy, blood pressure, pulse oximetry, and heart rate were closely observed for all patients in the recovery room. The patients were discharged from the recovery room when the modified Aldrete score was scored at ≤921.
The assessment of cognitive performance
In this study, all patients underwent the Stroop test to evaluate the change of psychomotor function, which is one of the methods to screen for hepatic encephalopathy9,10. We used the Korean Color Word Stroop test (K-CWST, website, http://175.126.38.165), which is a modified version of the Encephalapp Stroop test translated into Korean. A trained nurse performed all tests using an iPAD. All patients underwent the K-CWST before endoscopy and 2 hours after endoscopy.
Outcome measurements
The primary outcome of this study was the change Stroop test results before and after endoscopy among three sedative protocols. As secondary outcomes, the time to recovery, hemodynamic profiles, and satisfaction scores of patients, nurses, and doctors were analyzed. After two hours, all patients were asked to complete questionnaires to assess overall satisfaction (0, no satisfaction; 10, full satisfaction), recall of pain or discomfort (0, none; 10, extreme), awakening (0, not awakened; 10, fully awakened), and memory (0, no memory; 10 full memory) using visual analogue scale. Also, the endoscopists and the assistant nurses, who were blinded to the chosen protocol, answered the questionnaires about overall satisfaction of the procedures (0, no satisfaction; 10, full satisfaction).
Sample size calculation
No studies using the Stroop task for evaluation of cognitive performance before and after sedative endoscopy have been reported. Therefore, we referred to a previous study using the number connection test (NCT) for evaluation of MHE before and after sedation with propofol and midazolam16. According to their data, the increase in NCT time was −9.5 seconds [95% confidence interval (CI), −15.7 to −4.6] for propofol and 11 seconds (95% CI, −1.2 to 16.1) for midazolam. We estimated an increase in Stroop test time of −9.5 ± 35.8 seconds for propofol and 11.0 ± 19.7 seconds for midazolam after endoscopy. For the combination of propofol and midazolam, we assumed that the increase in Stroop test time would be −2.67 ± 27.8 seconds, using weighted mean of propofol and midazolam. The resulting estimated sample size was 17 patients per group, or a total subject of 51 patients, with an alpha value of 0.05 and power of 90 percent. Considering a 15% drop out rate, 20 patients per group, or a total of 60 patients were required.
Statistical analysis
To compare the variables between independent groups, a Kruskal-Wallis test was conducted as appropriate. Statistical differences between the groups were investigated using the χ2 test or Fisher’s exact test for categorical variables and the Mann-Whitney U test, or Kruskal-Wallis test for continuous variables. The univariate and multivariate linear regression analysis was also used to find the factors affecting the results of the Stroop test after sedation. A two-tailed p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R (version 3.3.3, The R Foundation for Statistical Computing, Vienna, Austria), or SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Ethical approval and informed consent
Written informed consents for sedation and endoscopic procedures, and study protocol were obtained from all patients. This study was approved by the institutional review board of Soonchunhyang University Hospital, Bucheon, Korea (SCHBC-2017-12-017-001) and was registered in the Clinical Research Information Service by Korea Centers for Diseases Control and Prevention, Republic of Korea (KCT0002964). Also, all experiments were performed in accordance with relevant guidelines and regulations.
Data availability
All authors agreed to make materials, data and associated protocols promptly available to readers without undue qualifications in material transfer agreements.
References
McGuire, B. M. Safety of endoscopy in patients with end-stage liver disease. Gastrointestinal endoscopy clinics of North America 11, 111–130 (2001).
Vasudevan, A. E., Goh, K. L. & Bulgiba, A. M. Impairment of psychomotor responses after conscious sedation in cirrhotic patients undergoing therapeutic upper GI endoscopy. The American journal of gastroenterology 97, 1717–1721, https://doi.org/10.1111/j.1572-0241.2002.05831.x (2002).
Haq, M. M. et al. Midazolam for sedation during diagnostic or therapeutic upper gastrointestinal endoscopy in cirrhotic patients. European journal of gastroenterology & hepatology 24, 1214–1218, https://doi.org/10.1097/MEG.0b013e328356ae49 (2012).
Riggio, O. et al. A Model for Predicting Development of Overt Hepatic Encephalopathy in Patients With Cirrhosis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 13, 1346–1352, https://doi.org/10.1016/j.cgh.2014.12.025 (2015).
Ampuero, J. et al. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology 149, 1483–1489, https://doi.org/10.1053/j.gastro.2015.07.067 (2015).
Roman, E. et al. Minimal hepatic encephalopathy is associated with falls. The American journal of gastroenterology 106, 476–482, https://doi.org/10.1038/ajg.2010.413 (2011).
Weissenborn, K. Diagnosis of minimal hepatic encephalopathy. Journal of clinical and experimental hepatology 5, S54–59, https://doi.org/10.1016/j.jceh.2014.06.005 (2015).
Bajaj, J. S., Etemadian, A., Hafeezullah, M. & Saeian, K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology 45, 833–834, https://doi.org/10.1002/hep.21515 (2007).
Allampati, S. et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. The American journal of gastroenterology 111, 78–86, https://doi.org/10.1038/ajg.2015.377 (2016).
Bajaj, J. S. et al. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology 58, 1122–1132, https://doi.org/10.1002/hep.26309 (2013).
Committee, A. So. P. et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointestinal endoscopy 87, 327–337, https://doi.org/10.1016/j.gie.2017.07.018 (2018).
McQuaid, K. R. & Laine, L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointestinal endoscopy 67, 910–923, https://doi.org/10.1016/j.gie.2007.12.046 (2008).
Kim, E. H., Park, J. C., Shin, S. K., Lee, Y. C. & Lee, S. K. Effect of the midazolam added with propofol-based sedation in esophagogastroduodenoscopy: A randomized trial. Journal of gastroenterology and hepatology 33, 894–899, https://doi.org/10.1111/jgh.14026 (2018).
Schulz, K. F., Altman, D. G., Moher, D. & Group, C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Bmj 340, c332, https://doi.org/10.1136/bmj.c332 (2010).
Agrawal, A., Sharma, B. C., Sharma, P., Uppal, R. & Sarin, S. K. Randomized controlled trial for endoscopy with propofol versus midazolam on psychometric tests and critical flicker frequency in people with cirrhosis. Journal of gastroenterology and hepatology 27, 1726–1732, https://doi.org/10.1111/j.1440-1746.2012.07231.x (2012).
Riphaus, A., Lechowicz, I., Frenz, M. B. & Wehrmann, T. Propofol sedation for upper gastrointestinal endoscopy in patients with liver cirrhosis as an alternative to midazolam to avoid acute deterioration of minimal encephalopathy: a randomized, controlled study. Scandinavian journal of gastroenterology 44, 1244–1251, https://doi.org/10.1080/00365520903194591 (2009).
Khamaysi, I. et al. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. Journal of hepatology 54, 72–77, https://doi.org/10.1016/j.jhep.2010.06.023 (2011).
Dhiman, R. K. et al. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Digestive diseases and sciences 55, 2381–2390, https://doi.org/10.1007/s10620-010-1249-7 (2010).
Levy, L. J., Bolton, R. P. & Losowsky, M. S. The use of the visual evoked potential (VEP) in delineating a state of subclinical encephalopathy. A comparison with the number connection test (NCT). Journal of hepatology 5, 211–217 (1987).
Weston, B. R. et al. Nurse-administered propofol versus midazolam and meperidine for upper endoscopy in cirrhotic patients. The American journal of gastroenterology 98, 2440–2447, https://doi.org/10.1111/j.1572-0241.2003.08668.x (2003).
Aldrete, J. A. & Kroulik, D. A postanesthetic recovery score. Anesthesia and analgesia 49, 924–934 (1970).
Acknowledgements
We thank A. Ri Song, R.N., Song Ah Jeong, R.N., Sun A. Moon, R.N. and the rest of the nursing staff for their support and assistance with procedures. We thank Eun-Ae Jung (Librarian, Medical Library, Soonchunhyang University Bucheon Hospital) for carefully proofreading the manuscript. This work was supported by Soonchunhyang university research fund.
Author information
Authors and Affiliations
Contributions
Conceptualization, Writing- Original Draft: Yoo J.J. and Goong H.J. Methodology: Yoo J.J. and Kim Y.S. Formal analysis: Moon J.E. Investigation and Resources: Kim S.G. Supervision: Kim Y.S. Approval of final manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, JJ., Goong, H.J., Moon, J.E. et al. Safety profile of sedative endoscopy including cognitive performance in liver cirrhosis: A double-blind randomized controlled trial. Sci Rep 9, 16798 (2019). https://doi.org/10.1038/s41598-019-52897-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52897-w
This article is cited by
-
Pre-procedural Preparation and Sedation for Gastrointestinal Endoscopy in Patients with Advanced Liver Disease
Digestive Diseases and Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.