Abstract
Intermittent stress disrupts the circadian rhythm in clock genes such as Per2 only in peripheral organs without any effect on the central circadian clock in the suprachiasmatic nucleus. Here, the effect of restraint stress (RS) on circadian bladder function was investigated based on urination behavior and gene expression rhythms. Furthermore, PF670462 (PF), a Per2 phosphorylation enzyme inhibitor, was administered to investigate the effects on circadian bladder re-alignment after RS. Two-hour RS during the light (sleep) phase was applied to mice (RS mice) for 5 days. The following parameters were then examined: urination behaviors; clock gene expression rhythms and urinary sensory-related molecules such as piezo type mechanosensitive ion channel component 1 (Piezo1), transient receptor potential cation channel subfamily V member 4 (TRPV4), and Connexin26 (Cx26) in the bladder mucosa; Per2 expression in the excised bladder of Per2luciferase knock-in mice (Per2::luc); in vivo Per2 expression rhythms in the bladder of Per2::luc mice. Control mice did not show altered urination behavior in the light phase, whereas RS mice exhibited a higher voiding frequency and lower bladder capacity. In the bladder mucosa, RS mice also showed abrogated or misaligned Piezo1, TRPV4, Connexin26, and clock gene expression. The rhythmic expression of Per2 was also altered in RS mice both in excised- and in vivo bladder, compared with control mice. After PF administration, voiding frequency was reduced and bladder capacity was increased during the light phase in RS mice; the in vivo Per2 expression rhythm was also fully restored. Therefore, RS can alter circadian gene expression in the bladder during the light phase and might cause nocturia via changes in circadian bladder function due the dysregulation of clock genes. Amending the circadian rhythm therapeutically could be applied for nocturia.
Similar content being viewed by others
Introduction
Clock genes such as Per2, Bmal1, and Rev-erbα as representatives regulate transcriptional and translational mechanisms in organisms with circadian rhythms. Circadian gene expression, generated by clock genes, regulates many aspects of behavior and physiological processes involving various metabolic enzymes, channels, and receptors, and abnormalities in clock genes have been reported to be associated with various diseases1. Lower urinary tract functions are also associated with the circadian rhythm and clock gene regulation. The sensation of bladder fullness has a circadian rhythm correlated with the gene expression of urinary sensory-related molecules such as piezo type mechanosensitive ion channel component 1 (Piezo1), transient receptor potential cation channel subfamily V member 4 (TRPV4), and Connexin26 (Cx26) under the regulation of clock genes in the bladder mucosa, and lower urinary tract symptoms including nocturia are exacerbated with the dysregulation of urinary sensory-related molecules and clock gene abnormalities2,3,4,5,6,7.
People often encounter circadian misalignment associated with clock genes disorder (CMACD). These include not only living in modern society, which involves various stressors and inappropriate evening light-exposure, but also aging8,9,10,11,12. Further, shift workers are exposed to irregular sleep/wake cycles, which disturbs not only circadian rhythms involved in organ function but also clock genes at the epigenetic and transcriptional levels13. These CMACDs are linked to increased risks for physical and psychiatric disorders including cardiovascular disease, diabetes, obesity, cancer, and depression, among others14,15,16,17,18. Moreover, various factors have been reported to induce CMACD. For example, the administration of a high fat diet induces alterations to metabolic enzyme expression in the liver, which is accompanied by disruptions in the circadian expression of clock genes, resulting in disturbances in rhythmic liver metabolite concentrations19. The intestinal flora has been reported to show a circadian rhythm20. Accordingly, interruption of the circadian rhythm with respect to the bacterial flora can cause CMACD in the mouse liver21.
Furthermore, some types of intermittent stress such as restraint stress (RS) can disrupt the circadian rhythm of clock genes only in peripheral organs, without any effect on the central clock in the suprachiasmatic nucleus (SCN) in mice22. We hypothesized that RS could cause CMACD in the mouse bladder resulting in nocturia. In the present study, to reveal the effect of RS on alterations to circadian bladder function, we investigated its influence on urination behavior and gene expression rhythms in the mouse bladder.
Results
Restraint stress induces nocturia in mice
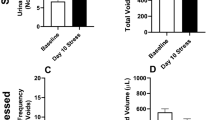
Body weights were not different between control and mice subjected to restraint stress (RS mice) (25.57 ± 0.54 vs. 24.44 ± 0.36 g, respectively, P = 0.10, by student’s t-test, N = 10 and 27 for control and RS mice, respectively). Actogram showed that the locomotor activity pattern that mouse was active in the dark and rest/sleep in the light period was the same between control and RS mouse (Supplementary Fig. 1). Total water intake volume (WIV) was also not different between control and RS mice (Fig. 1A); however, when WIV in the dark (active) and light (sleep) phases were individually compared, control mice did not show a difference, whereas in RS mice, WIV in the dark phase for RS3, 3 days after RS loading (Supplementary Fig. 2), was significantly lower than baseline values (Fig. 1B). Total urine volume (Uvol) and Uvol in the dark and light phases were not different in control and RS mice (Fig. 1C and 1D).
Differences in voiding behavior after restraint stress (RS). (A) Water intake volume (WIV) over 24 h in control and mice subjected to restraint stress (RS mice). (B) WIV in the dark (active) and light (sleep) phase in control and RS mice. (C) Total urine volume (Uvol) in control and RS mice. (D) Uvol in the dark and light phase in control and RS mice. (E) Total voiding frequency (VF) in control and RS mice. (F) VF in the dark phase in control and RS mice. (G) VF in the light phase in control and RS mice. (H) Urine volume/voiding (Uvol/v) in control and RS mice. (I) Uvol/v in the dark phase in control and RS mice. (J) Uvol/v in the light phase in control and RS mice. The horizontal axis represents the day of measurement (Supplementary Fig. 2). Data are presented as means ± standard error (SE). D; The dark phase. L; the light phase. Numbers of mice are 10 for control mice and 27 for RS mice. Statistical analyses were performed by a one-way ANOVA with Bonferroni’s test. The Mann-Whitney U-test was used to compare differences in Uvol/v between active and sleep phases. A P value less than 0.05 was considered significant. *P < 0.05, **P < 0.01, n.s., not significant.
Total voiding frequency (VF) was unchanged in control mice; however, in RS mice this was significantly higher on RS5 compared to baseline levels (Fig. 1E). VF in the dark phase gradually increased and that on Day 5 was significantly higher than baseline levels of control mice. However, RS mice did not show any differences in VF during the dark phase (Fig. 1F). In contrast, VF in the light phase was not different in control mice. However, that in the light phase increased significantly after RS, compared to baseline values (Fig. 1G).
Urine volume/voiding (Uvol/v) in the dark phase was significantly smaller than that in the light phase in control mice. In contrast, differences in Uvol/v between the dark and light phase that were observed at baseline disappeared in RS mice (Fig. 1H). In control mice, a tendency of decreased Uvol/v in the dark phase was also observed and values at Day 5 were significantly smaller than baseline levels. However, in RS mice, no differences were observed (Fig. 1I). In contrast, Uvol/v in the light phase was not different in control mice. However, that in the light phase was significantly lower on Day 3 in RS mice (Fig. 1J). The representative traces were shown in Supplementary Fig. 3.
RS disrupts the gene expression rhythm in the mouse bladder mucosa
In control mice, the expression in Per2, Bmal1, and Rev-erbα showed a typical circadian rhythm. In contrast, the circadian rhythms of Per2 and Bmal1 were disrupted in RS mice, Rev-erbα expression rhythm maintained time-dependent variation in RS mice. However, the peak expression time was shifted forward and circadian Rev-erbα expression was also disrupted in RS mice compared to that in control mice (Fig. 2A).
Gene expression rhythms in the mouse bladder mucosa. (A) Clock gene mRNA expression rhythms in the mouse bladder mucosa in control and mice subjected to restraint stress (RS mice). (B) Piezo type mechanosensitive ion channel component 1 (Piezo1), transient receptor potential cation channel subfamily V member 4 (TRPV4), and Connexin26 (Cx26) mRNA expression rhythm in the mouse bladder mucosa in control and RS mice. The number of mice was 4 for both groups at each point. ZT; zeitgeber time. Statistical analyses were performed using a one-way ANOVA to compare differences among time points in each group. *P < 0.05, **P < 0.01, n.s., not significant. A two-way ANOVA with Bonferroni’s test was used to compare differences between control and RS mice at each time point. #P < 0.05, ##P < 0.01. Data are presented as means ± SE.
Urinary sensory-related molecules such as Piezo1, TRPV4, and Cx26 also showed circadian gene expression in control mice. However, the expression rhythm of Piezo1 and TRPV4 showed a time-dependent change in RS mice and the expression pattern was different from that in control mice. Regarding Cx26 expression, RS mice showed disrupted circadian rhythm (Fig. 2B).
RS induces the circadian misalignment of Per2 expression in the mouse bladder
Before comparing the Per2 expression rhythm in the ex vivo bladder between control and RS mice, we investigated how excision of the bladder could affect the gene expression rhythm. When the bladder was excised at both zeitgeber time (ZT) 8 and ZT20, which are the peak and nadir time of Per2 expression3, the expression rhythm was reset and a new circadian rhythm was established immediately after excision. The peak expression time in the bladders that were excised 12 h apart were similarly shifted by 12 h (Supplementary Fig. 4). Based on these findings, the Per2 expression rhythm was compared in the ex vivo bladder excised at ZT8 between control and RS mice. The circadian period was approximately 24 h in both groups. However, the amplitude of the circadian rhythm was significantly lower for RS mice (Fig. 3).
Per2 bioluminescence over time in the ex vivo mouse bladder. (A) Per2 expression rhythms in individual ex vivo mouse bladders for control and mice subjected to restraint stress (RS mice). (B) Mean values. The number of mice was 6 for both groups. ZT; zeitgeber time. Black arrow indicates the time of 15 μM forskolin administration, to confirm the viability of the excised bladder. Circadian period, a vs a’: 25.38 ± 0.30 vs 26.12 ± 0.33 h; P = 0.34. b vs b’: 24.35 ± 0.21 vs 25.12 ± 0.31 h; P = 0.29 based on the Mann-Whitney U-test. Amplitude in control and RS mice between the first nadir and peak: 30300 ± 6494 vs 15310 ± 2465 (photons/min), P = 0.045; between the second nadir and peak: 15661 ± 2695 vs 4876 ± 2011 (photons/min); P = 0.023 based on the Mann-Whitney U-test.
In in vivo imaging of mouse bladder, Per2-bioluminescence showed a circadian rhythm in both control and RS mouse bladders. However, the peak in Per2 expression, which was observed at ZT18 in control mice, was changed to ZT0 in RS mice. The amplitude of circadian rhythm was also lower in RS mice than in control mice (Fig. 4).
Per2 bioluminescence in the in vivo mouse bladder. (A) Images of Per2 expression in control and mice subject to restraint stress (RS mice). (B) The total region of interest (ROI) quantification of Per2::Luc bioluminescence in the bladder of control and RS mice. The amplitude from nadir to peak was 1.349 ± 0.002 × 107 (photons/min) in control mice and 0.737 ± 0.219 × 107 (photons/min) in RS mice; P = 0.049 based on the Mann-Whitney U-test. Three mice were used for each time point. ZT; zeitgeber time. Statistical analyses were performed using a one-way ANOVA with a Bonferroni’s test to compare differences among the time points in each group. A P value less than 0.05 was considered significant. *P < 0.05, n.s., not significant.
PF670462 (PF) ameliorates circadian bladder dysfunction and nocturia induced by RS
Next, the voiding behavior was compared between RS mice and RS mice administered 10 mg/kg PF (RS + PF mice). In this study, 100 μL deionized water (DW) was administered to RS mice intraorally for 5 days as a control. The body weight was the same between the two groups. (24.53 ± 0.57 vs. 25.41 ± 0.52 g, P = 0.16 based on a Student’s t-test; N = 26 and 24 for RS and RS + PF mice, respectively). Total WIV decreased significantly in RS + PF mice (Fig. 5A). However, both groups exhibited decreasing WIV in the dark phase and there were no changes in the light phase (Fig. 5B).
Voiding behavior after PF670462 (PF) administration. (A) Water intake volume (WIV) for 24 h in mice subjected to restraint stress (RS mice) and RS loading + PF administered mice (RS + PF mice). (B) WIV in the dark (active) and light (sleep) phases in RS and RS + PF mice. (C) Total urine volume (Uvol) in RS and RS + PF mice. (D) Uvol in the dark and light phase in RS and RS + PF mice. (E) Total voiding frequency (VF) in RS and RS + PF mice. (F) VF in the dark phase in RS and RS + PF mice. (G) VF in the light phase in RS and RS + PF mice. (H) Urine volume/voiding (Uvol/v) in RS and RS + PF mice. (I) Uvol/v in the dark phase in RS and RS + PF mice. (J) Uvol/v in the light phase in RS and RS + PF mice. The horizontal axis represents the day of measurement (Supplementary Fig. 1). Data are presented as the means ± standard error (SE). D; the dark phase. L; the light phase. Numbers of mice are 26 in RS mice and 24 in RS + PF mice. Statistical analyses were performed using a one-way ANOVA with a Bonferroni’s test. The Mann-Whitney U-test was used to compare the differences between two groups. A P value less than 0.05 was considered significant. *P < 0.05, **P < 0.01, n.s., not significant.
Total Uvol gradually increased and was significantly higher on RS5, as compared to baseline levels, in RS mice. In contrast, the increase in total Uvol was observed only on RS3 in RS + PF mice (Fig. 5C). Uvol in the dark and light phase was also significantly higher than baseline levels in RS mice. However, in the dark phase, Uvol did not show differences in RS + PF mice. Moreover, Uvol in the light phase was higher on RS3 than baseline levels in RS + PF mice, but then decreased significantly on RS5 (Fig. 5D).
Total VF increased significantly from baseline in both mice (Fig. 5E). VF in the dark phase was the same in RS mice. However, in the dark phase on RS5, VF was higher than baseline levels and that on RS3 in RS + PF mice (Fig. 5F). Further, VF in the light phase increased significantly from baseline in both groups. However, the increase in VF in the light phase from baseline was significantly lower in RS + PF mice than in RS mice on RS3 and RS5 (both P < 0.0001 between RS and RS + PF mice, based on Mann-Whitney U-test; Fig. 5G).
Uvol/v in the light phase was higher than that in the dark phase at baseline in both groups. Although this variation disappeared in RS mice on RS3 and RS5, RS + PF mice maintained higher Uvol/v in the light, compared to that in the dark phase at these time points (Fig. 5H). Uvol/v in the dark phase did not show any differences in both groups (Fig. 5I). However, Uvol/v in the light phase was lower on RS5 compared to baseline levels in RS mice and in contrast, RS + PF mice did not show differences in Uvol/v during the sleep phase (Fig. 5J). The representative traces were shown in Supplementary Fig. 3.
Circadian misalignment in the mouse bladder after RS is ameliorated by PF
Based on the in vivo imaging of mouse bladders in the RS + PF group, Per2-bioluminescence showed a circadian rhythm (Fig. 6). Peak Per2 expression, which was observed at ZT18 in control mice and shifted to ZT0 in RS mice (Fig. 4), was restored to the timing observed in control mice in Fig. 4. Moreover, the amplitude of Per2 oscillation also seemed to restore to the control levels depicted in Fig. 4B.
Effects of PF670462 (PF) on the circadian rhythm in the mouse bladder. The images and total region of interest (ROI) quantification of Per2::Luc bioluminescence based on the in vivo imaging of mouse bladders from the restraint stress loading + PF administered mice (RS + PF mice). Three mice were used for each time point. ZT; zeitgeber time. Statistical analyses were performed using a one-way ANOVA with a Bonferroni’s test to compare differences among the time points. A P value less than 0.05 was considered significant. n.s., not significant.
Discussion
The present study demonstrated that intermittent RS increases VF and reduces Uvol/v in the light (sleep) phase in mice. However, this treatment did not affect Uvol in the dark and light phase. Furthermore, RS mice exhibited alterations to the circadian rhythm of clock genes in the bladder, as well as Piezo1, TRPV4, and Cx26 expression in the bladder mucosa, which could induce abnormal circadian bladder function and cause nocturia. Interestingly, these circadian misalignments in the RS mouse bladder were amended to control rhythms and nocturia was ameliorated after PF administration.
In organisms, stress-related information is properly integrated through neural networks in the central nervous system (CNS). Then, minimized stress responses are necessary to maintain homeostasis via stress effectors such as the hypothalamic-pituitary-adrenal axis and the sympathetic and parasympathetic arms23. The secretion of vasopressin from the pituitary gland, one of the factors that regulates Uvol in the kidney, increases after chronic stress24. In the present study, locomotor activity pattern (Supplementary Fig. 1) and Uvol did not change in control and RS mice (Fig. 1C and 1D). Furthermore, if water intake behavior was considered a rhythmic daily activity, no differences were observed in total WIV, as well as WIV in the dark and light phases, between control and RS mice; however, the RS group showed lower WIV in the dark only on RS3 (Fig. 1A and 1B). This suggests that 5 days of RS does not affect the central clock in SCN, as reported previously22. Differences in WIV and Uvol were observed between RS and RS + PF mice (Fig. 5A–D), unlike the results shown in in Fig. 1A–D. Accordingly, the administration of 100 µL of DW, administered to RS mice and used as a solvent for PF, could represent too large of a volume for mice; however, the differences in Uvol were larger in the light phase than in the dark phase (Fig. 5D). This is thought to be indicative of the fact that drinking during the sleep phase affects nocturia and/or nocturnal polyuria25.
The differences in VF and Uvol/v in control mice were more prominent in the dark phase than in the light phase. In contrast, RS mice showed the opposite results (Fig. 1F, 1G, 1I, and 1J). These results suggest that RS affects voiding behavior only in the light phase. We measured voiding behavior for 6 days in metabolic cages. Such a long period of housing could have been a stressor for mice and induces the differences in voiding behavior in the dark phase26. However, 12 h dark/light cycles, the pivotal circadian entrainment regulators, would create robust circadian rhythms in mice, which was represented by consecutive circadian locomotor activity (Supplementary Fig. 1) and WIV even in the RS2,8. Probably, the 12 h dark/light cycle would limit the effect of the stress to the light phase. Therefore, RS mice showed nocturia pattern and did not show the voiding differences in the dark phase. RS might be more stimulating than housing stress. WIV and locomotor activity pattern were the same as control mice, result in offsetting the changes of voiding in the dark phase. However, control mice showed higher VF and lower Uvol/v only in the dark phase because of the housing stress during they are active, although the circadian rhythm created by the appropriate environment could leave urinations as regular pattern during they are rest/sleep.
Acute and temporal physiological stresses have been reported to activate the immune system. These stress signals can exert an anti-inflammatory effect and protect organs against ischemia by elevating plasma corticosterone levels27. However, 5 days of RS induced CMACD, as reported previously22. Even with the first RS, which corresponded to the acute stress phase, changes in voiding behavior such as higher VF and lower Uvol/v were observed (Fig. 1E and 1J). These results suggest that the bladder has lower stress tolerance than the kidney.
Uvol/v was previously reported to exhibit a circadian rhythm under the regulation of clock genes in the mouse bladder, which was enhanced in the sleep phase compared to that in the active phase2,7. The disruption of bladder capacity differences between day and night is speculated to be one cause of nocturia that is associated with circadian clock disorders in humans28,29,30. These changes were also observed in the present study (Figs 1H and 5H). Furthermore, we proved that RS is accompanied by CMACD in the bladder (Figs 2–4), whereas in PF-administered mice, the disrupted rhythm in Uvol/v and clock genes was restored (Figs 5H and 6). These results strengthen the relationship between nocturia and abnormalities in clock genes.
Urinary sensory-related molecules such as Piezo1, TRPV4, and Cx26, which create a circadian rhythm underlying the sensation of bladder fullness under the regulation of clock genes3,5,6, as well as clock genes, showed a circadian rhythm in the bladder mucosa of control mice. However, these rhythms were disrupted in RS mice (Fig. 2). These results indicate that RS mice suffer from CMACD in the bladder mucosa, underlying the sensation of bladder fullness, which might be one of the causes of nocturia in these mice25.
We also measured Per2 expression rhythms in both the ex vivo and in vivo bladder. The ex vivo bladder sustained circadian Per2 expression for several days as reported previously31, and a circadian rhythm was observed in control and RS mice. In addition, the amplitude in Per2 oscillation was significantly lower in RS mice (Figs 3 and 4). These results indicate that the circadian clock in the bladder was not completely disrupted, but rather partially damaged, by RS. In situations in which the regular clock function is completed disturbed, the circadian expression of clock genes should disappear. Furthermore, the function of clock genes remained lower in the bladder after the release from RS (Figs 3 and 4). It was suggested that changes in the circadian bladder function were not evoked temporally and immediately by stress-associated factors23; however, RS, for which effects accumulated gradually, is thought to affect the gene expression rhythm in the bladder by regulating clock genes.
Various stressors are suggested to affect urination in mice. Previously, it was shown that water avoidance stress leads to the development of pollakisuria, which is accompanied by the activation of immune cells in the bladder32. With social stress in mice, increased voiding was found to be accompanied not only by changes in local receptors in the bladder, but also by afferent nerve activity33. Moreover, physiological stress can cause an overactive bladder in humans34. Psychiatric stress, for which the causes are ambiguous, such as anxiety and post-traumatic-stress-disorder, can also increase nocturnal voiding35,36. These reports suggested that an unknown factor involving stress affects urination. In addition to stress-activated bladder function, CNS sensitivity, temperature, and irregular lifestyles can promote urination37,38. Interestingly, the circadian clocks in local organs are affected by these factors, often leading to various symptoms1,39. Therefore, RS-related voiding changes might be affected by various factors. We only suggest a relationship between stress and the circadian clock in the bladder as a possible cause of nocturia and have not investigated the detailed molecular changes occurring in response to RS that cause dyssynchronous clock gene expression, which exist in almost all cells; moreover, the precise circadian clock is not only determined by links between the CNS and peripheral nerves but also cell-to-cell circadian regulatory mechanisms7,40,41. Jet lag syndrome is caused by CMACD in the CNS, and results in an oscillatory shift in the circadian rhythm42. Therefore, RS was considered to induce CMACD in the bladder not only through the production of stress-associated hormones but also through neural network pathways. Further investigations are needed to reveal the mechanism underlying the relationship between the circadian clock and nocturia.
In the present study, stress-affected bladder function was investigated using RS, which was applied at the same time point, namely the light phase, in mice, forcing the animals to wake up. This might affect the evaluation of nocturia. Although stress in the dark phase does not reportedly exaggerate CMACD22, RS in the dark phase might have different effects than that in the light phase, unrelated to circadian function in the bladder.
Higher-dose PF administration was reported to cause a loss in clock gene function in mice43. In addition, 100 mg/kg acute PF administration was reported to increase the mortality rate in these animals44. However, the intraperitoneal injection of 50 mg/kg PF, albeit only once, can successfully suppress allergic reactions in mice, and this was found to be under the regulation of clock genes, preventing effects on the SCN such as melatonin secretion and dark and light perception44,45. Local PF administration via inhalation was also reported to be effective without the development of any adverse effects46. Based on our results, intraoral PF administration resulted in improved nocturia even at lower doses, specifically 10 mg/kg. Although it was only partial, the effect of PF, reduced VF and increased Uvol/v in the light, was observed from day 3 of administration (Fig. 5G, 5H and 5J). It seems that setting the appropriate administration time according to changes in the circadian rhythm might have maximized the effects of the smaller dose, resulting in a potential circadian-modulating therapy for the treatment for nocturia, which is currently refractory47.
In conclusion, we indicated that RS affected circadian regulation system between the central clock in SCN and peripheral clocks, which induced CMACD in the mouse bladder and could be associated with nocturia. Thus, in modern society, coping with stress and other CMACDs might reduce nocturia. We believe that the present study suggests a novel aspect of nocturia and could lead to the development and further understanding of a new strategy for the treatment of nocturia.
Materials and Methods
Animals
Eight-to-twelve-week-old male C57BL/6 mice and age- and sex-matched C57BL/6 Pe2Luciferase knock in (Per2::Luc) mice were used for the following experiments. Mice were housed under 12-h light/dark conditions for 2 weeks before experiments with free access to food and water. All procedures were conducted in accordance with the Guiding Principles in the Care and Use of Animals in the Field of the Physiologic Society of Japan and the policies of the Institutional Animal Care and Use Committee. In addition, all experimental protocols were approved by the Animal Care Committee of the University of Yamanashi (Chuo, Yamanashi, Japan).
Application of restraint stress
Mice were subjected to RS for 2 h from ZT4 to ZT6, corresponding to the sleep phase and the time period at which circadian misalignment was most likely22; this was performed by enclosing the animals in a metal mesh of 12 × 12 cm (Supplementary Fig. 5). RS was applied for 5 days (from RS1 to RS5; Supplementary Fig. 2).
Metabolic cages
Voiding behaviors were compared between control and RS mice using metabolic cages. The precise urine collection and the artifacts elimination due to the drop of feces and morsels of food are capable by the use of mesh in the flooring of the cage2,48. The following parameters were evaluated: WIV (µL), Uvol (µL/24 h), Uvol/v (µL), and VF (number of times). After mice were acclimatized for 2 days in the cage, these parameters, including baseline levels, were recorded for 6 days (Supplementary Fig. 2) and compared between baseline, third day, and fifth day. Undisturbed voiding behavior was measured for 6 days using control mice. Urination during the light (sleep phase) was considered nocturia in mice. The definition of nocturia followed a previous report2.
PF670462 (PF) administration
A selective casein kinase Iε/δ inhibitor, PF670462 (PF; Tocris Bioscience, Ellisville, MO), was diluted with 100 μL of DW and administered intraorally (10 mg/kg) at the same time of RS loading after aesthesia using sevoflurane. This compound is known as a Per2 phosphorylation enzyme inhibitor that leads to the retention of nuclear Per2 and delays in the expression of clock genes comprising the circadian cycle43,44,45. Further, 100 μL of DW was administered to RS mice for 5 days as a control.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
RNA was extracted from mouse bladder mucosa every 6 h from ZT0 in control and RS mice and qRT-PCR assays were performed using the same primer sequences, reagents, and protocol as reported previously3,4. Representative clock genes including Per2, Bmal1, Rev-erbα, and urinary sensory-related molecules such as Piezo1, TRPV4, and Cx26 were measured. The mRNA level was calculated from the standard curve, which was run simultaneously with the sample tubes, and was normalized to Eif2a/Tbcc concentrations49.
Ex vivo measurement of Per2 bioluminescence in the bladder
Male C57BL/6 Per2::Luc mice were used for this. The bladder was excised from these animals at ZT10, which is the peak time of Per2 expression3. The excised bladder was put in a 3.5-cm dish (Thermo Fisher Scientific, Waltham, CA) with phenol red-free Dulbecco’s Modified Eagle Medium (DMEM; WAKO, Tokyo, Japan) with 1% penicillin/streptomycin (P/S; Gibco®, Waltham, CA), 1% amphotericin B solution (AMPB, Sigma-Aldrich, St Louis, MO), and 0.2 μM Beetle Luciferin Potassium Salt (Promega, Madison, WI). Then, samples were immediately placed into a dish type luminometer (Kronos DioAB-2550; ATTO, Tokyo, Japan), and incubated under humidified conditions with 5% CO2 at 37 °C. The bioluminescence from excised bladder tissues was measured for 3 days at 10-min intervals. The Per2 expression rhythm was then compared between control and RS mice. To confirm the viability of bladder tissue, 15 μM of forskolin (Sigma-Aldrich) was added to the dish for 2 h and the medium was replaced, and measurements were restarted.
In vivo imaging of bladder-Per2 bioluminescence
The bladders from Per2::Luc mice were exposed, and the urine was discharged by puncturing the top of the bladder with a 27-G needle under anesthesia with isoflurane. A black plastic plate was then inserted between the skin and the exposed bladder to mask background bioluminescence (Supplementary Fig. 6). The mice were laid on their back, and 5 mg/kg of Beetle Luciferin Potassium Salt (Promega) was injected subcutaneously into the back near the neck; 5 min later, Per2-bioluminescence from the exposed bladder, which was marked with a region of interest (ROI), was measured every 6 h for 60 s from ZT6 using an in vivo imaging system (Perkin Elmer, Waltham, MA) for control, RS, and RS + PF670462-administered mice.
Statistical analyses
The experimental values were expressed as the means ± standard error (SE). The significance of differences between two groups was analyzed using the Mann-Whitney U-test. A one-way ANOVA with a Bonferroni’s test was used to compare differences among time points in each group. A two-way ANOVA with a Bonferroni’s test was used to compare differences at each time point between two groups. A P value less than 0.05 was considered significant.
Change history
08 November 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Okamura, H., Doi, M., Fustin, J. M., Yamaguchi, Y. & Matsuo, M. Mammalian circadian clock system: Molecular mechanisms for pharmaceutical and medical sciences. Adv Drug Deliv Rev 62, 876–884, https://doi.org/10.1016/j.addr.2010.06.004 (2010).
Ihara, T. et al. The Clock mutant mouse is a novel experimental model for nocturia and nocturnal polyuria. Neurourol. Urodyn. 36, 1034–1038, https://doi.org/10.1002/nau.23062 (2017).
Ihara, T. et al. Clock Genes Regulate the Circadian Expression of Piezo1, TRPV4, Connexin26, and VNUT in an Ex Vivo Mouse Bladder Mucosa. PLoS One 12, e0168234, https://doi.org/10.1371/journal.pone.0168234 (2017).
Ihara, T. et al. The Circadian expression of Piezo1, TRPV4, Connexin26, and VNUT, associated with the expression levels of the clock genes in mouse primary cultured urothelial cells. Neurourol. Urodyn., https://doi.org/10.1002/nau.23400 (2017).
Ihara, T. et al. The oscillation of intracellular Ca(2+) influx associated with the circadian expression of Piezo1 and TRPV4 in the bladder urothelium. Sci. Rep. 8, 5699, https://doi.org/10.1038/s41598-018-23115-w (2018).
Ihara, T. et al. The time-dependent variation of ATP release in mouse primary-cultured urothelial cells is regulated by the clock gene. Neurourol. Urodyn. 37, 2535–2543, https://doi.org/10.1002/nau.23793 (2018).
Negoro, H. et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commun 3, 809, https://doi.org/10.1038/ncomms1812 (2012).
Baron, K. G. & Reid, K. J. Circadian misalignment and health. Int. Rev. Psychiatry 26, 139–154, https://doi.org/10.3109/09540261.2014.911149 (2014).
Duffy, J. F., Zitting, K. M. & Chinoy, E. D. Aging and Circadian Rhythms. Sleep Med. Clin. 10, 423–434, https://doi.org/10.1016/j.jsmc.2015.08.002 (2015).
Hood, S. & Amir, S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 127, 437–446, https://doi.org/10.1172/jci90328 (2017).
Yamazaki, S. et al. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. USA 99, 10801–10806, https://doi.org/10.1073/pnas.152318499 (2002).
Valentinuzzi, V. S., Scarbrough, K., Takahashi, J. S. & Turek, F. W. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 273, R1957–1964 (1997).
Cedernaes, J. et al. Acute Sleep Loss Induces Tissue-Specific Epigenetic and Transcriptional Alterations to Circadian Clock Genes in Men. J. Clin. Endocrinol. Metab. 100, E1255–1261, https://doi.org/10.1210/jc.2015-2284 (2015).
Cajochen, C. et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. Journal of applied physiology (Bethesda, Md.: 1985) 110, 1432–1438, https://doi.org/10.1152/japplphysiol.00165.2011 (2011).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 106, 4453–4458, https://doi.org/10.1073/pnas.0808180106 (2009).
Du, H. B., Bin, K. Y., Liu, W. H. & Yang, F. S. Shift work, night work, and the risk of prostate cancer: A meta-analysis based on 9 cohort studies. Medicine (Baltimore) 96, e8537, https://doi.org/10.1097/md.0000000000008537 (2017).
Saulle, R., Bernardi, M., Chiarini, M., Backhaus, I. & La Torre, G. Shift work, overweight and obesity in health professionals: a systematic review and meta-analysis. Clin. Ter. 169, e189–e197, https://doi.org/10.7417/t.2018.2077 (2018).
Angerer, P., Schmook, R., Elfantel, I. & Li, J. Night Work and the Risk of Depression. Deutsches Arzteblatt international 114, 404–411, https://doi.org/10.3238/arztebl.2017.0404 (2017).
Eckel-Mahan, K. L. et al. Reprogramming of the circadian clock by nutritional challenge. Cell 155, 1464–1478, https://doi.org/10.1016/j.cell.2013.11.034 (2013).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529, https://doi.org/10.1016/j.cell.2014.09.048 (2014).
Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell host & microbe 17, 681–689, https://doi.org/10.1016/j.chom.2015.03.006 (2015).
Tahara, Y. et al. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci. Rep. 5, 11417, https://doi.org/10.1038/srep11417 (2015).
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409, https://doi.org/10.1038/nrn2647 (2009).
Makino, S., Smith, M. A. & Gold, P. W. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136, 3299–3309, https://doi.org/10.1210/endo.136.8.7628364 (1995).
Gulur, D. M., Mevcha, A. M. & Drake, M. J. Nocturia as a manifestation of systemic disease. BJU Int. 107, 702–713, https://doi.org/10.1111/j.1464-410X.2010.09763.x (2011).
Kalliokoski, O. et al. Mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. PLoS One 8, e58460, https://doi.org/10.1371/journal.pone.0058460 (2013).
Abe, C. et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20, 700–707, https://doi.org/10.1038/nn.4526 (2017).
Kira, S. et al. Lack of Change in the Adaptation Ability of the Bladder for the Urine Production Rate in Aged Men with Nocturia. Urol. Int. 100, 445–449, https://doi.org/10.1159/000488002 (2018).
Kim, J. W. Effect of Shift Work on Nocturia. Urology 87, 153–160, https://doi.org/10.1016/j.urology.2015.07.047 (2015).
Goessaert, A. S., Krott, L., Walle, J. V. & Everaert, K. Exploring nocturia: gender, age, and causes. Neurourol. Urodyn. 34, 561–565, https://doi.org/10.1002/nau.22638 (2015).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Smith, A. L. et al. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78, 967.e961–967, https://doi.org/10.1016/j.urology.2011.06.041 (2011).
Mingin, G. C. et al. Social stress in mice induces urinary bladder overactivity and increases TRPV1 channel-dependent afferent nerve activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R629–638, https://doi.org/10.1152/ajpregu.00013.2015 (2015).
Lai, H., Gardner, V., Vetter, J. & Andriole, G. L. Correlation between psychological stress levels and the severity of overactive bladder symptoms. BMC Urol. 15, 14, https://doi.org/10.1186/s12894-015-0009-6 (2015).
Golabek, T. et al. Lower urinary tract symptoms, nocturia and overactive bladder in patients with depression and anxiety. Psychiatr. Pol. 50, 417–430, https://doi.org/10.12740/PP/OnlineFirst/59162 (2016).
Eidlitz-Markus, T., Shuper, A. & Amir, J. Secondary enuresis: post-traumatic stress disorder in children after car accidents. Isr. Med. Assoc. J. 2, 135–137 (2000).
de Groat, W. C., Griffiths, D. & Yoshimura, N. Neural control of the lower urinary tract. Compr Physiol 5, 327–396, https://doi.org/10.1002/cphy.c130056 (2015).
Ishizuka, O., Imamura, T. & Nishizawa, O. Cold Stress and Urinary Frequency. Low Urin Tract Symptoms 4(Suppl 1), 67–74, https://doi.org/10.1111/j.1757-5672.2011.00127.x (2012).
Chappuis, S. et al. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab 2, 184–193, https://doi.org/10.1016/j.molmet.2013.05.002 (2013).
White, R. S. et al. Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front. Pharmacol. 5, 293, https://doi.org/10.3389/fphar.2014.00293 (2014).
Sui, G. et al. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am. J. Physiol. Renal Physiol. 306, F286–298, https://doi.org/10.1152/ajprenal.00291.2013 (2014).
Yamaguchi, Y. et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 342, 85–90, https://doi.org/10.1126/science.1238599 (2013).
Meng, Q. J. et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl. Acad. Sci. USA 107, 15240–15245, https://doi.org/10.1073/pnas.1005101107 (2010).
Kennaway, D. J. et al. Acute inhibition of casein kinase 1delta/epsilon rapidly delays peripheral clock gene rhythms. Mol. Cell. Biochem. 398, 195–206, https://doi.org/10.1007/s11010-014-2219-8 (2015).
Nakamura, Y. et al. Inhibition of IgE-mediated allergic reactions by pharmacologically targeting the circadian clock. J. Allergy Clin. Immunol. 137, 1226–1235, https://doi.org/10.1016/j.jaci.2015.08.052 (2016).
Keenan, C. R. et al. Casein Kinase 1delta/epsilon Inhibitor, PF670462 Attenuates the Fibrogenic Effects of Transforming Growth Factor-beta in Pulmonary Fibrosis. Front. Pharmacol. 9, 738, https://doi.org/10.3389/fphar.2018.00738 (2018).
Oelke, M., Adler, E., Marschall-Kehrel, D., Herrmann, T. R. & Berges, R. Nocturia: state of the art and critical analysis of current assessment and treatment strategies. World J. Urol. 32, 1109–1117, https://doi.org/10.1007/s00345-014-1396-0 (2014).
Yoshiyama, M. et al. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex during the micturition cycle. Am. J. Physiol. Renal Physiol. 308, F1128–1134, https://doi.org/10.1152/ajprenal.00016.2015 (2015).
Kosir, R. et al. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol. Biol. 11, 60, https://doi.org/10.1186/1471-2199-11-60 (2010).
Acknowledgements
This work was supported financially by Suzuki Urinary Medicine Promotion Foundation, JSPS KAKENHI Grant Number 19K18579, and Astellas Pharma Inc.
Author information
Authors and Affiliations
Contributions
M.Y., M.T., Y.N., A.N. and S. Koizumi designed the experiments and supervised the studies. T.I., Y.N., S.T. and M.K. performed the main experiments. S.T., M.K., S. Kira, H.N., N.S., M.K., E.S. and Y.S. prepared experimental samples and animals. T.I., T.M., M.T., Y.D., E.S., Y.S. and M.Y. wrote the main manuscript text and figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ihara, T., Nakamura, Y., Mitsui, T. et al. Intermittent restraint stress induces circadian misalignment in the mouse bladder, leading to nocturia. Sci Rep 9, 10069 (2019). https://doi.org/10.1038/s41598-019-46517-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46517-w
This article is cited by
-
Mechanisms of oxidative stress in interstitial cystitis/bladder pain syndrome
Nature Reviews Urology (2024)
-
Glucocorticoids coordinate the bladder peripheral clock and diurnal micturition pattern in mice
Communications Biology (2023)
-
Effects of fatty acid metabolites on nocturia
Scientific Reports (2022)
-
Time-of-day dependent changes in guinea pig bladder afferent mechano-sensitivity
Scientific Reports (2021)
-
Bladder outlet obstruction disrupts circadian bladder function in mice
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.