Abstract
Fallow paddies experience natural flooding and draining water status due to rainfall and evaporation, which could induce considerable nitrous oxide (N2O) emissions and need to be studied specially. In this study, intact soil columns were collected from a fallow paddy field and the flooding-draining process was simulated in a microcosm experiment. The results showed that both N2O concentrations in the soil and N2O emission rates were negligible during flooding period, which were greatly elevated by draining the fallow paddy soil. The remarkable N2O concentrations in the soil and N2O emission/h during draining both had significant relationships with the Arch-amoA gene (P < 0.01) but not the Bac-amoA, narG, nirK, nirS, and nosZ genes, indicating that the ammonium-oxidizing archaea (AOA) might be the important players in soil N2O net production and emissions after draining. Moreover, we observed that N2O concentrations in the upper soil layers (0–10 cm) were not significantly different from that in the 10–20 cm layer under draining condition (P > 0.05). However, the number of AOA and the nitrification substrate (NH4+-N) in the 0–10 cm layer were significantly higher than in the 10–20 cm layer (P < 0.01), indicating N2O production in the 0–10 cm layer might be higher than the measured concentration and would contribute considerably to N2O emissions as shorter distance of gas diffusion to the soil surface.
Similar content being viewed by others
Introduction
Nitrous oxide (N2O), one of the important contributors to the radiative forcing by greenhouse gasses (GHGs), is expected to remain the largest emission throughout the 21st century and greatly contributes to stratospheric ozone destruction1. The latest reports by the world meteorological organization indicate that the global mean surface mole fractions for N2O were 328.9 ± 0.1 ppb in 2016, which is approximately 122% of the pre-industrial level2. Rice paddies are primary sources of atmospheric N2O, accounting for approximately 11% of global agricultural N2O emissions3. China has a large area of rice paddy fields, and the annual total planting area of rice was 30.9 million hectares in China, which was 19% of the global rice planting area in 20144. Furthermore, the total N2O emission from paddy fields in China is estimated to be 180 Gg yr−1 5.
In rice-based ecosystems, the midseason drainage and dry-wet alternation in the rice growing season usually cause obvious N2O emissions, whereas the continuously flooded fields emit less N2O into the atmosphere5,6,7. Therefore, the water content and related oxygen availability are considered key factors affecting soil N2O emissions. Several studies found that the optimum water condition for N2O production was 60–70% water-filled pore space (WFPS)8,9. Furthermore, N2O emissions exponentially increased with decreases in soil WFPS from 90% to 70%, whereas decreases were observed when the WFPS declined from 70% to 40%10. N2O production in the soil is mediated by microbial processes, mainly nitrification and denitrification11. Ammonia-oxidizing microorganisms (AOM), including ammonium-oxidizing archaea (encoded by Arch-amoA gene) and bacteria (encoded by Bac-amoA gene), play an important role in the nitrification of rice paddy soil and N2O production process12. Denitrification includes the reduction of nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), and N2O which are transformed by the narG and napA, nirS and nirK, qnorB and cnorB, and nosZ genes of denitrifiers, respectively13. By changing the soil aeration (O2) and redox state, soil moisture has a great effect on enzyme activities of nitrification and denitrification process, thus regulating soil N2O dynamics14,15. Numerous studies have investigated the relationship between N2O emission and the activities of functional nitrifiers and denitrifiers in paddy soil under different water conditions. Paddy soil in draining was shown to harbor significantly higher copy numbers of denitrifiers than those found in flooded soil, and functional genes of denitrifiers were closely linked to large N2O fluxes13,16,17. In addition, other studies have shown that the abundance of nitrifiers (Arch-amoA and Bac-amoA gene) in draining paddy soil was considerably more than that in flooded soil, and amoA gene abundance was significantly correlated to N2O emission rates12,18. These varying results imply that the importance of nitrifiers, denitrifiers, or both to N2O emission in draining paddy soil might differ between cases. However, most studies on the microbial regulation of N2O emissions from paddy soil have been carried out during the cropping season. Consequently, less attention has been payed to paddy soil in the fallow season only19, which is also an important period of rice paddy cultivation.
Traditionally, the fallow period of the double-rice fields is in the winter from November to April of the next year, and these fields are often submerged after heavy or prolonged rains20. Furthermore, without human management, the soil water conditions of fallow paddies mainly depend on the rainfall and atmospheric evaporation, resulting in fluctuations in soil moisture of fallow paddies. Such prolonged periods and great variation in soil water condition would induce considerable N2O emissions from fallow paddy fields. Previous studies reported that more than 33% of annual N2O emission occurred during the winter fallow season in Yintan, Jiangxi Province20, and earlier field observations revealed that annual N2O emissions as high as 60% to 90% occurred during the winter fallow season in Taoyuan, Hunan Province21, indicating the importance of N2O emission during that period. Nonetheless, studies on the microbial process and regulatory mechanisms of N2O production and emission under different water status in fallow paddy soil are scarce.
Therefore, in our study, soil samples were collected from a fallow paddy field in Huanghua, Hunan Province, and molecular methods were used to investigate the changes of nitrifiers and denitrifiers to flooding-draining practice in fallow paddy soil and the associated N2O emissions patterns. To reflect the soil properties and structure in situ, intact soil columns were used as experimental materials. Previous studies showed that intact soil responded with higher sensitivity to water changes than sieved soil did, and it also discharged more N2O22,23. Our objectives were to investigate (1) the patterns of N2O concentration and emission in fallow paddy soil under flooding and draining conditions, (2) the changes of nitrifying and denitrifying microbial communities to flooding and draining practices, and (3) the links between the N2O dynamics and changes in functional communities and the effects of soil water status. We hypothesized that the flooding and draining process would induce different patterns of N2O net production and emission in fallow paddy soil and different responses by nitrifiers and denitrifiers.
Results
Dynamics of N2O concentrations in soil and N2O emissions from soil surface
The patterns of N2O concentrations in each depth layer and N2O emissions during flooding period were considerably different from that during drainage period (Fig. 1). The N2O emissions under flooding conditions maintained stable and low rates (below 56.89 µg m−2 h−1). In addition, N2O uptake was monitored several times over the course of the flooding period, and the highest N2O negative emission was −16.20 µg m−2 h−1 at flooding day 38. In contrast, significantly elevated N2O emissions were recorded during drainage period. The N2O emissions were lower at the beginning of draining process until the N2O emission continuously increased from draining day 10. The peak N2O emission, which occurred on draining day 15, was 1204.81 µg m−2 h−1 and then the levels continued to decrease during the following drainage period.
Similar to the N2O emission pattern, the N2O concentrations in each depth layer during drainage period were also much higher than they were during flooding. Under flooding conditions, the N2O concentrations in the whole soil profile fluctuated at around 7.00 mg N2O m−3 within a narrow range, except on flooding day 1. Furthermore, there was no significant difference in the N2O concentration among four depth soil layers during this period (Table 1). During the drainage period, all four depth layers showed negligible N2O concentrations (<2.0 mg N2O m−3) until draining day 11. Then, the N2O concentrations in four depth layers increased continually to their peak values at different draining days. The N2O concentrations of the 0–5 cm layer peaked on draining day 15, 5–10 cm and 10–15 cm layers peaked on draining day 17, and the 15–20 cm layer peak value was on draining day 21. Furthermore, the peak N2O concentrations in the 0–5, 5–10, 10–15, and 15–20 cm depth layers were 31.14, 30.22, 35.16, and 41.92 mg N2O m−3, respectively, and the patterns of the four depth layers were not significantly different during the draining period (Table 1).
Properties of paddy soil columns during flooding and drying periods
An obvious variation was observed in the Eh of the entire soil column sample during the flooding and drainage periods (Fig. 1, Table 1). The soil Eh of the 0–20 cm layer rapidly fell during flooding process. The paddy soil draining caused a steep increased in the Eh with increasing draining time. However, the differences in Eh value varied significantly based on the drainage period in four depth layers (P < 0.001). The soil Eh of the 0–5 cm layer increased the fastest and was >540 mV at draining day 10, followed sequentially by the 5–10, 10–15, and 15–20 cm depth layers.
The variation in the soil water content in 0–5 cm layer was more obvious than that in the other three layers during both flooding and draining periods (Fig. 2a). First, the soil water content of the 0–5 cm depth layer was 92% WFPS after 12 h flooding, and it kept increasing to 103% WFPS in the following flooding period. In contrast, the water content of the other three depth layers remained at much lower levels. During draining, the water content of the paddy soil column of 0–5 cm layer immediately fell to 85% WFPS on draining day 1, and continuously decreased 22% over the following 17 days. In addition, the water contents of the 5–20 cm layers also decreased with increasing draining time, to 65% WFPS.
The initial inorganic nitrogen contents of the paddy soil samples were at low levels with mean values of 7.12 mg NH4+-N kg−1 and 1.96 mg NO3−-N kg−1 on flooding day 1 (Fig. 2b,c). The NH4+-N content in the four depth layers was consumed by approximately 44% over 13 flooding days, whereas the NO3−-N contents simultaneously decreased by 74%. Following the draining period, the paddy soil columns showed dramatically elevated NH4+-N contents in the topsoil layer with initial values of 9.97 and 7.27 mg kg−1 in 0–5 cm and 5–10 cm layers, respectively during the 12 h draining process. However, the ammonium concentrations in the 0–10 cm layer reduced continuously with a prolonged draining period, and the bottom soil layer exhibited steady ammonium concentrations during the 17 draining days. On the contrary, the NO3−-N contents in the soil were very low on draining day 1 at 0.13 mg kg−1. Then, the nitrate concentrations in the 0–10 cm layer increased significantly during the subsequent draining period (P < 0.001), while that in the lower layer increased slightly.
Quantitive changes of soil nitrifying and denitrifying microbes during flooding and drying
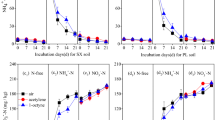
It is clear from the results that the abundance of ammonia oxidizers (Arch-amoA and Bac-amoA) and denitrifiers (narG, nirK, nirS and nosZ) showed similar distributions with soil depth over the entire flooding-draining process (Table 1). Specifically, the highest gene copy numbers were observed in 0–5 cm depth layer, sequentially followed by the 5–10, 10–15, and 15–20 cm layers (P < 0.01). However, the responses of these functional genes to water conditions caused by flooding or draining differed greatly (Fig. 3).
AOA and AOB can both oxidize ammonium. In the paddy soil, the numbers of Arch-amoA gene copies were one to two orders of magnitude higher than those of the Bac-amoA gene at all incubation times. Under flooding conditions, there was no significant change in the Arch-amoA and Bac-amoA abundance of each depth layer. Following draining, the Arch-amoA gene copies in the paddy soil column samples remained unchanged initially. Then, the Arch-amoA gene numbers in the 0–5 cm and 5–10 cm layers increased sharply with prolonged draining (P < 0.001), whereas the levels in the lower soil layer increased slightly. In total, the 0–5, 5–10, 10–15, and 15–20 cm layers showed 6.45, 7.11, 3.70, and 1.30 times higher numbers of gene copies respectively, during the 17-day draining period. In contrast to the Arch-amoA, the Bac-amoA gene abundance in the soil declined immediately as the draining period commenced. Furthermore, the decline was maintained at a relatively steady state during the following draining period.
The narG, nirK, nirS and nosZ genes are the key indicators of denitrification. The present results showed no significant changes occurred in narG and nosZ gene abundance in the soil during the flooding process, while the nirK and nirS gene copies in 0–5 cm layer were increased significantly with flooding time. During draining process, the narG and nirS gene numbers exhibited a sharp increase in the 0–10 cm soil layer immediately after draining for 12 h. Then, the narG and nirS gene copies in soil column fluctuated slightly during the 17 days of the draining incubation. The nirK gene abundances during draining process were significantly lower than in flooding status, and decreased continuously with draining time. Furthermore, the nosZ gene abundance in the soil did not differ between the draining and flooding periods.
Correlation analysis by Spearman test
Because of the significant N2O production and emission pattern under draining conditions, the correlations of soil variables and functional microorganisms with N2O in this process were analyzed only (Table 2). During the draining condition, the Arch-amoA gene involved in nitrification was the only population related to the N2O concentrations in the soil profile (P < 0.01), whereas the abundance of the other nitrifying and denitrifying populations (Bac-amoA, narG, nirK, nirS, and nosZ) were not correlated. The N2O concentrations in the soil profile were also positively linked to soil NO3−-N content and soil Eh (P < 0.01), while negatively correlated to soil WFPS (P < 0.05). As to the correlation of N2O emission from soil surface and soil functional microorganisms in each depth layer, the Arch-amoA gene in 0–20 cm layers was related to the N2O emission (P < 0.01), and also the narG, nirK, nirS, and nosZ gene copies in 15–20 cm layer were significantly correlated to N2O emission (P < 0.05). In addition, negative correlations were found between the N2O emission and the nitrification substrate of the soil NH4+-N content and soil WFPS in 0–10 cm depth layers (P < 0.01). And positive correlations were found between the N2O emission and the nitrification product of the soil NO3−-N in 0–15 cm soil layers, as well as the soil Eh in whole soil profile (P < 0.01). Moreover, the N2O emission had stronger correlations to soil properties (NO3−-N, NH4+-N, WFPS, and Eh) in the topsoil layers than it did in the lower depth layers during the draining period.
Discussion
In the rice-growing season, paddy fields are often subjected to anthropogenic submergence and drainage several times to maximum the rice yields, while the traditional dry soil conditions of the fallow paddies would be acutely susceptible to heavy or prolonged rain and natural evaporation6,20. Different water conditions affect the biogeochemistry of paddy soils with respect to N2O production and emission24. Many studies have found that the most N2O was emitted from the paddy soils subjected to flooding-drying practice and the least N2O would degas from continuously flooded paddy soil either during cropping or fallow seasons6,13,17,21. In our study, we clearly detected very low levels of N2O production in each depth layer and N2O emission rates during the flooding period whereas the levels were significantly elevated in this fallow paddy soils after draining (Fig. 2). The average value of the N2O emission during draining period was 23.06 times higher than it was during the flooding period, and the peak N2O emission was 1204.81 µg m−2 h−1 after 15 days draining by evaporation.
When the submerged soil column was draining, O2 was able to infiltrate the soil matrix with water evaporation, and the redox state of the paddy soil gradually tended from reduction to oxidation reaction16. In our study, the soil WFPS ranged from 85% to 60% and the Eh value increased from −136.54 mV to 586.92 mV during the 17 days of the draining period (Figs 2, 3a). During this transition process, the water condition (60–70% WFPS) was conducive to N2O production8,9, and both nitrification and denitrification should contribute to the N2O production and induce large N2O emissions25. However, the soil NO3−-N content in this study was only 0.13 mg kg−1 at the beginning of the draining period (Fig. 3b), and this low availability of the NO3− substrate severely impeded the denitrifiers activity as well as N2O emission26. The nitrification substrate in the upper soil layers was 13.35 mg NH4+-N kg−1 (Fig. 3c), which was relatively sufficient for nitrifiers activity and would be activated by continuous water evaporation and O2 infiltration. In addition, this point was proven by the observation that the NH4+-N content in the soil was gradually depleted after draining, which was accompanied by a significant increase in the nitrification product (NO3−-N). The correlation analysis also showed that the N2O net production and emissions were more relevant to functional genes of the nitrifiers than those of the denitrifiers (Table 3). Therefore, we could speculate that nitrification might be the more dominant process in N2O net production and emission in this draining paddy soil.
Ammonium-oxidizing microorganisms (AOM) play vital roles in catalyzing the first step of nitrification, consists of AOB27 and AOA28. In our study, the amoA gene copies of AOA in the paddy soil columns were one to two orders of magnitude higher than those of the AOB during the entire draining process (P < 0.01), demonstrating that the AOA were more abundant nitrifiers in this paddy soil column. However, the numerical dominance also indicates a functional advantage to what extent and which kind of population is responsible for nitrification or N2O emissions are still open questions. Although both bacteria and archaea have ammonium oxidizing functions, their ecological niches are very different, and many crucial intermediates in their ammonium oxidation are not common29. These discrepancies result in the inequivalent contributions of AOA and AOB to N2O emissions. In our study, the amoA gene abundance of AOA and AOB showed different responses to the draining period. The N2O concentrations and N2O emissions were intensively correlated to Arch-amoA abundance in the entire soil column (both showed R = 0.88, P < 0.01), whereas no significant relationship was observed between N2O concentrations/emissions and Bac-amoA abundance (Table 3), indicating the more important role of archaea in the process of ammonia oxidation during this draining period. Previous studies have demonstrated that under extremely low concentrations of ammonium substrate, AOA played important roles in the N cycles30. For example, the abundance of AOA was much more than AOB at very low ammonium concentration in the open ocean (≤10 µM), and AOA abundance also exhibited more intense correlation to nitrification rates31,32,33. A preference of AOA for low NH4+ concentrations has also been reported from several environmental studies, especially for soil environments30,34,35. The NH4+ and NO3− concentration in this paddy soil sample were very low at the beginning of the draining process at only 8.63 mg NH4+-N kg−1 soil and 0.13 NO3−-N kg−1 soil on average (Fig. 3b,c). Such a low availability of inorganic nitrogen might restrict the AOB activities, while AOA were highly adapted to this energy-stressed environments in contrast to bacteria36. Moreover, it has been previously hypothesized that the oxygen or carbon dioxide in the exudates of paddy soil affected AOA more than they did AOB37. Therefore, the significant increase in Arch-amoA abundance rather than Bac-amoA abundance in our study might be attributed to the higher affinity of AOA for oxygen increase in the process of draining soil.
In addition, significant differences in functional genes abundance (especially Arch-amoA), as well as NH4+ and NO3− concentration were observed along the vertical soil profile (P < 0.01), with a gradual decrease from the 0–5 cm layer to the 5–10, 10–15, and 15–20 cm layers (Figs 3, 4). Compared to the subsoil, the surface soil usually contains more soil nutrients and harbors a higher density of microorganisms14,38,39. As a result, the N2O production in the surface soil should be higher than that in the subsoil. However, the N2O concentrations in the 0–10 cm depth layer was not significantly different from that in the 10–20 cm depth layer during the draining period (P > 0.05) (Fig. 2). This abnormal phenomenon might have its attributable reasons. The N2O concentration detected in each depth layer was a collective result of the N2O production, migration, and consumption of this layer40. With water evaporation from soil matrix, the water content in the 0–10 cm layer decreased rapidly because of soil draining, whereas no obvious change occurred in the 10–20 cm layer. The N2O concentrations in the 0–5 cm depth layer showed the highest increment during draining day 10 and peaked first, followed by levels in the 5–10, 10–15, and 15–20 cm layers in that order. Furthermore, aeration of the upper soil layers was higher and distance of gas diffusion to the soil surface was shorter than that of the lower layers, which would facilitate the gaseous diffusion41. Therefore, we could speculate that the N2O produced in the upper soil layers likely contributed more to the N2O emissions than that in the lower soil layers did, resulting in more N2O accumulation in lower soil layers.
(Wei) Schematic diagram of the pot for gas sampling. (1) silicon tubes, (2) stainless steel tube, (3) three-way stopcock, (4) gas sampling static chamber, (5) water. A layer was 0–5 cm depth layer; B layer was 5–10 cm depth layer; C layer was 10–15 cm depth layer; and D layer was 15–20 cm depth layer.
Materials and Methods
The sampling field was a double-cropping rice paddy over 100 years old, located in Huanghua town, Hunan Province, China (28°14′08″N; 113°13′05″E). This paddy soil is developed from Quaternary red clay and has been classified as loamy clay (Hydragric Anthrosols)42. The soil samples for basic property determination were collected using a five-point sampling method on February 20, 2014, which was still the winter fallow period. Soil properties at the point of the sampling were pH (H2O) 5.11; NH4+-N, 2.57 mg kg−1; NO3−-N, 2.73 mg kg−1; and organic matter content, 30.50 g kg−1.
Intact soil cores collection and device installation
The intact soil cores (15 cm diameter, 0–20 cm depth) were collected from the sampling paddy field by self-made device, which have been used in a previous study39. Intact soil cores were collected using PVC cylinders (15 cm diameter, 25 cm high), and one hole (1.6 cm diameter each) through cylinder wall was made at positions of 2.5, 7.5, 12.5 and 17.5 cm from the bottom of cylinder, respectively. In the field, sampling points were selected randomly, then plant residues above the soil surface were manually removed. Soil cores were dug by fitting into the PVC cylinders and trimming off outer soil with a spade. After the soil cores (0–20 cm) were removed from the field, the bottom was covered with a plate (PVC plate, 18 cm diameter) and the cylinder was wrapped with film to prevent water loss. In total, 24 intact soil cores were prepared.
In laboratory, the bottom plates were sealed to each cylinder with glue. After that, a horizontal tunnel was made across the soil cores with a stainless tube (1.6 cm diameter) through each hole in the PVC cylinder. Then a silicon tube (14 cm long, 1.2 cm internal diameter, 0.2 cm wall thickness) closed with silicone septa at both ends, was inserted into each tunnel of the soil cores. One end of the stainless steel tube (3 cm long, 2 mm diameter) was inserted into the silicon tube through the septa and the other end was connected to a three-way stopcock outside the cylinder (Fig. 4). At last the holes in PVC wall were sealed with glue and the space between the soil columns and PVC cylinders was filled with soil slurry.
Soil incubation
The entire incubation consisted of flooding period (1–38 days) and draining period (1–30 days). Three soil cores were selected randomly for gas sampling, three for redox potential (Eh) monitoring, and 18 for soil sampling at six time points. Thus, each treatment had three experimental replicates. Then, 500 mL double distilled water was added to each soil core from the soil surface, and 2 cm of the free water layer was maintained throughout the flooding period. After the flooding incubation, the free water above the soil surface was removed using a syringe, and the soil cores were drained by air evaporation for approximately 30 days. The pots were constantly incubated at 28 °C.
Gas sampling and determination
Gas samples were collected on flooding day 1, 3, 5, 7, 9, 13, 17, 21, 25, 30, 34, 38, and draining day 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 30. Using a static chamber method, two gas samples (30 mL) were first collected at 9:00 a.m. and 10:00 a.m. on each sampling day. And then the gas samples (5 mL) were collected from the silicon tubes in 0–5 cm, 5–10 cm, 10–15 cm, and 15–20 cm depth layer (Fig. 1), and an equal volume of helium was injected back into the tubes afterwards. The N2O concentrations of gas samples were determined using a gas chromatograph (Agilent 7890 A, Agilent Technologies, Santa Clara, CA, USA) fitted with an electron capture detector (ECD) for N2O analyses at 350 °C. In addition, the N2O emission/h (the N2O cumulative emission per hour) was calculated (expressed in µg N2O m−2 h−1) referred to the formula established by previous study39. The N2O concentrations (net prodcution) in each soil layer were expressed as milligram N2O per cubic meter (mg N2O m−3).
Soil sampling and measurements
Soil samples were collected on flooding day 1, 7, 13, and draining day 1, 11, 17, representing the initial, increasing, and peak phases of N2O emission during the draining period (based on the pre-experiment) and their corresponding time in the flooding period. The soil sampler consisted of two, longitudinally split stainless steel tubes (25 cm long, 4 cm inside diameter), connected to each other by rubber belts. Prior to sampling the flooding soils, the surface water layer was first removed, and then the sampler was inserted vertically into the soil column to obtain a 20-cm-long soil sample. Then, each soil sample was immediately divided into four parts as follows: 0–5, 5–10, 10–15, and 15–20 cm depth, respectively. This procedure was repeated three times with each soil column, and then two portions of the mixed soil samples were divided, and one was stored at −80 °C for further molecular analysis while the other portion was stored at 4 °C for chemical property analysis.
The soil water content was analyzed using the oven-drying method, and all results were converted to water-filled pore space (WFPS) units. The soil substrate availability of nitrate-N (NO3−-N) and ammonia-N (NH4+-N) was determined by extracting the samples with 2 M potassium chloride (KCl) solution, followed by analysis using a continuous-flow injection analyzer (FIAstar 5000, Foss Corporation, Hillerod, Denmark). The soil Eh value was monitored using redox electrodes (FJA-3, Nanjing, China), which were inserted vertically at depths of 2.5, 7.5, 12.5 and 17.5 cm before flooding treatment.
DNA extraction
Soil microbial DNA was extracted from 0.3 g soil and stored at −80 °C using a previously described method43. The DNA quality and concentration were measured using a NanoDrop NA-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Each soil sample was extracted three times, pooled, and then stored at −80 °C for further analysis.
Quantitative polymerase chain reaction (qPCR)
The forward (F) and reverse (R) primer pairs amoA-23F/amoA-616R, amoA-1F/amoA-2R, narG-571F/narG-773R, nirK-876F/nirK-1055R, nirS-cd3aF/nirS-R3cd, and nosZ-1126F/nosZ-1381R were used to quantify the ammonia monooxygenase gene of archaea (Arch-amoA) and bacteria (Bac-amoA), nitrate reductase gene (narG), nitrite reductase gene (nirK and nirS), and nitrous oxide reductase gene (nosZ). The primer sequences and thermal conditions used for the analysis are listed in Table 3. The quantitative polymerase chain reaction (qPCR) assay was performed using an ABI 7900HT (Applied Biosystems, Foster City, CA, USA). The 10 μL reaction mixtures consisted of 5 μL 2× SYBR green mix II (Takara Biotechnology Co. Ltd., Dalian, China), 0.2 μL 50× Rox Reference Dye (Takara Biotechnology Co. Ltd., Dalian, China), 0.3 μL (0.4 μmol L−1) each of the F and R primers, and 5 ng sample DNA template. Standard curves were constructed using a series of 10-fold dilutions of linearized plasmids containing the target gene. Three parallel PCR replicates of all samples were performed on each plate. A melting curve analysis was conducted following the assay to verify the specificity of the amplification product. The PCR efficiency was in the range of 90–110%.
Data treatment and statistical analysis
Given that the depth within cores and the core values over time are not independent, the repeated measures analysis of variance was employed to compare the effects of soil depth and incubation time (flooding time and draining time respectively) on N2O, physical and chemical properties, and functional gene abundances (Table 2). The variance of functional gene abundances in each depth layer over incubation time were analyzed using one-way ANOVA method, and least significant difference (LSD) test was used to evaluate the effects of the above ANOVA tests. Due to the nonnormal distribution of the soil NO3− concentrations after draining (including the transformed data), spearman’s correlation was used to analyze the relationship of soil variables and N2O concentrations and emissions after draining. A P < 0.05 was considered significant, and all the statistical analyses were performed using the statistical package for the social sciences (SPSS) 13.0 (SPSS Inc., USA).
References
Ravishankara, A. R., Daniel, J. S. & Portmann, R. W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science. 326, 123–125 (2009).
WMO. The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2016. Greenhouse Gas Bulletin, 1–7, https://library.wmo.int/opac/doc_num.php?explnum_id=4022 (2017)
USEPA. Global anthropogenic non-CO2 greenhouse gas emissions: 1990–2020. United States Environmental Protection Agency, Washington, D. C., USA (2006).
FAO. Statistical Database of the Food and Agricultural Organization of the United Nations FAO-STAT, http://www.fao.org/faostat/en/#data/GR (2014)
Zhang, W., Yu, Y. Q., Li, T. T., Sun, W. J. & Huang, Y. Net Greenhouse Gas Balance in China’s Croplands over the Last Three Decades and Its Mitigation Potential. Environ. Sci. Technol. 48, 2589–2597 (2014).
Haque, M. M., Kim, S. Y., Ali, M. A. & Kim, P. J. Contribution of greenhouse gas emissions during cropping and fallow seasons on total global warming potential in mono-rice paddy soils. Plant Soil. 387, 251–264 (2014).
Ma, J., Ji, Y., Zhang, G., Xu, H. & Yagi, K. Timing of midseason aeration to reduce CH4 and N2O emissions from double rice cultivation in China. Soil Sci. Plant Nutr. 59, 35–45 (2013).
Vilain, G., Garnier, J., Tallec, G. & Cellier, P. Effect of slope position and land use on nitrous oxide (N2O) emissions (Seine Basin, France). Agri. Forest Meteorol. 150, 1192–1202 (2010).
Chen, Z. et al. Increased N2O emissions during soil drying after waterlogging and spring thaw in a record wet year. Soil Biol. Biochem. 101, 152–164 (2016).
Castellano, M. J. et al. Hydrological and biogeochemical controls on the timing and magnitude of nitrous oxide flux across an agricultural landscape. Global Change Biol. 16, 2711–2720 (2010).
Saggar, S. et al. Denitrification and N2O:N2 production in temperate grasslands: processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 465, 173–195 (2013).
Fujii, C. et al. Succession and community composition of ammonia-oxidizing archaea and bacteria in bulk soil of a Japanese paddy field. Soil Sci. Plant Nutr. 56, 212–219 (2010).
Liu, J. et al. Denitrifying communities differentially respond to flooding drying cycles in paddy soils. Appl. Soil Ecol. 62, 155–162 (2012).
Ruser, R. et al. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 38, 263–274 (2006).
Braker, G. & Conrad, R. Diversity, structure, and size of N(2)O-producing microbial communities in soils–what matters for their functioning? Adv. Appl. Microbiol. 75, 33–70 (2011).
Riya, S., Takeuchi, Y., Zhou, S., Terada, A. & Hosomi, M. Nitrous oxide production and mRNA expression analysis of nitrifying and denitrifying bacterial genes under floodwater disappearance and fertilizer application. Environ. Sci. Pollut. R. 24, 15852–15859 (2017).
Yang, H. et al. Responses of nitrifying and denitrifying bacteria to flooding-drying cycles in flooded rice soil. Appl. Soil Ecol. 103, 101–109 (2016).
Ke, X., Lu, W. & Conrad, R. High Oxygen Concentration Increases the Abundance and Activity of Bacterial Rather than Archaeal Nitrifiers in Rice Field Soil. Microb. Ecol. 70, 961–970 (2015).
Fitzgerald, G. J., Scow, K. M. & Hill, J. E. Fallow season straw and water management effects on methane emissions in California rice. Global Biogeochem. Cy. 14, 767–776 (2000).
Zhang, G. et al. Drainage and tillage practices in the winter fallow season mitigate CH4 and N2O emissions from a double-rice field in China. Atmos. Chem. Phys. 16, 11853–11866 (2016).
Shang, Q. et al. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: a 3-year field measurement in long-term fertilizer experiments. Global Change Biol. 17, 2196–2210 (2011).
Li, X., Xia, L. & Yan, X. Application of membrane inlet mass spectrometry to directly quantify denitrification in flooded rice paddy soil. Biol. Fert. Soils. 50, 891–900 (2014).
Uchida, Y., Wang, Y., Akiyama, H., Nakajima, Y. & Hayatsu, M. Expression of denitrification genes in response to a waterlogging event in a Fluvisol and its relationship with large nitrous oxide pulses. FEMS Microbiol. Ecol. 88, 407–423 (2014).
Berger, S., Jang, I., Seo, J., Kang, H. & Gebauer, G. A record of N2O and CH4 emissions and underlying soil processes of Korean rice paddies as affected by different water management practices. Biogeochemistry. 115, 317–332 (2013).
Mathieu, O. et al. Quantifying the contribution of nitrification and denitrification to the nitrous oxide flux using 15N tracers. Environ. Pollut. 144, 933–940 (2006).
Wang, R. et al. Effects of nitrate concentration on the denitrification potential of a calcic cambisol and its fractions of N2, N2O and NO. Plant Soil. 363, 175–189 (2012).
Purkhold, U. et al. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl. Environ. Microb. 66, 5368–5382 (2000).
Pester, M., Schleper, C. & Wagner, M. The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr. Opin. Microbiol. 14, 300–306 (2011).
Azziz, G., Trasante, T., Monza, J. & Irisarri, P. The effect of soil type, rice cultivar and water management on ammonia-oxidizing archaea and bacteria populations. Appl. Soil Ecol. 100, 8–17 (2016).
Verhamme, D. T., Prosser, J. I. & Nicol, G. W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 5, 1067–1071 (2011).
Beman, J. M., Popp, B. N. & Francis, C. A. Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J. 2, 429–441 (2008).
Martens-Habbena, W., Berube, P. M., Urakawa, H., de la Torre, J. R. & Stahl, D. A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 461, 976–979 (2009).
Wuchter, C. et al. Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103, 12317–12322 (2006).
Pratscher, J., Dumont, M. G. & Conrad, R. Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc. Natl. Acad. Sci. USA 108, 4170–4175 (2011).
Di, H. J. et al. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2, 621–624 (2009).
Valentine, D. L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nature reviews. Microbiology. 5, 316–323 (2007).
Chen, X. P., Zhu, Y. G., Xia, Y., Shen, J. P. & He, J. Z. Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ. Microbiol. 10, 1978–1987 (2008).
Gao, X., Rajendran, N., Tenuta, M., Dunmola, A. & Burton, D. L. Greenhouse Gas Accumulation in the Soil Profile is not Always Related to Surface Emissions in a Prairie Pothole Agricultural Landscape. Soil Sci. Soc. Am. J. 78, 805 (2014).
Wang, L. et al. Stimulatory effect of exogenous nitrate on soil denitrifiers and denitrifying activities in submerged paddy soil. Geoderma. 286, 64–72 (2017).
Chapuis-Lardy, L., Wrage, N., Metay, A., Chotte, J.-L. & Bernoux, M. Soils, a sink for N2O? A review. Global Change Biol. 13, 1–17 (2007).
Clough, T. J., Sherlock, R. R. & Rolston, D. E. A Review of the Movement and Fate of N2O in the Subsoil. Nutr. Cycl. Agroecosyst. 72, 3–11 (2005).
Soil Survey Staff. Keys to Soil Taxonomy, 11th ed. USDA-Natural Resources Conservation Service, Washington, DC (2010).
Chen, Z. et al. Impact of long-term fertilization on the composition of denitrifier communities based on nitrite reductase analyses in a paddy soil. Microb. Ecol. 60, 850–861 (2010).
Rotthauwe, J. H., Witzel, K. P. & Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol. 63, 4704–4712 (1997).
Sahan, E. & Muyzer, G. Diversity and spatio-temporal distribution of ammonia-oxidizing archaea and bacteria in sediments of the Westerschelde estuary. FEMS Microbiol Rev. 64, 175–186 (2008).
Henry, S. et al. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Meth. 59, 327–335 (2004).
Throback, I. N., Enwall, K., Jarvis, A. & Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol. 49, 401–417 (2004).
Chen, Z. et al. Differentiated response of denitrifying communities to fertilization regime in paddy soil. Microb. Ecol. 63, 446–459 (2012).
Acknowledgements
This work was supported by Grants from the China Postdoctoral Science Foundation (2017M622386) and the National Science Foundation of China (41807043, 41330856). We would like to thank Editage [www.editage.cn] for English language editing.
Author information
Authors and Affiliations
Contributions
Ling Wang and Wenxue Wei conceived and designed the experiments; Ling Wang, Kun Li performed the experiments; Ling Wang, Kun Li and Rong Sheng analyzed the data; Ling Wang, Zhaohua Li and Wenxue Wei wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Li, K., Sheng, R. et al. Remarkable N2O emissions by draining fallow paddy soil and close link to the ammonium-oxidizing archaea communities. Sci Rep 9, 2550 (2019). https://doi.org/10.1038/s41598-019-39465-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39465-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.