Abstract
Survival of antibody-secreting plasma cells (PCs) is vital for sustained antibody production. However, it remains poorly understood how long-lived PCs (LLPCs) are generated and maintained. Here we report that ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) is preferentially upregulated in bone marrow LLPCs compared with their splenic short-lived counterparts (SLPCs). We studied ENPP1-deficient mice (Enpp1 −/−) to determine how the enzyme affects PC biology. Although Enpp1 −/− mice generated normal levels of germinal center B cells and plasmablasts in periphery, they produced significantly reduced numbers of LLPCs following immunization with T-dependent antigens or infection with plasmodium C. chabaudi. Bone marrow chimeric mice showed B cell intrinsic effect of ENPP1 selectively on generation of bone marrow as well as splenic LLPCs. Moreover, Enpp1 −/− PCs took up less glucose and had lower levels of glycolysis than those of wild-type controls. Thus, ENPP1 deficiency confers an energetic disadvantage to PCs for long-term survival and antibody production.
Similar content being viewed by others
Introduction
B cells undergo terminal differentiation upon stimulation with T-dependent or T-independent antigens. There are three fates of a stimulated B cell: differentiation into a memory B cell, a PC, or death by apoptosis. It has been demonstrated that PC can be generated by either extrafollicular or germinal center (GC) pathways in spleens and lymph nodes. While most PCs are thought to live only several days1,2,3,4, some manage to survive for long periods of time, sometimes for years, at particular anatomical sites such as the bone marrow (BM)5,6. These long-lived PCs (LLPCs) contribute to prolonged and sustained protection from re-infection (beneficial) or to long-term supply of self-damaging autoantibodies (pathogenic). Enhancing protective vaccine-induced LLPCs, to malaria, for example, and dampening pathogenic autoreactive LLPCs, such as those contributing to systemic lupus erythematosus, have been major hurdles in managing both diseases.
How LLPCs are generated and maintained in the BM is incompletely understood. It is thought that support for LLPC survival is mediated by cells in BM niches, including reticular stromal cells7,8, osteocytes9, megakaryocytes10, basophils11, and eosinophils12. These diverse cells provide crucial signals to LLPCs through direct cell-cell contact and/or the secretion of soluble factors such as IL-6 and APRIL7,13,14,15. Unlike long-lived hematopoietic stem cells (HSC), which also are resting cells and occupy similar BM niches, LLPCs are resting but metabolically active given the fact that a single PC can produce antibodies at up to 103 molecules per second16. How LLPCs are programed to be metabolically distinct from other B cell types has remained unknown until recently. Lam et al. elegantly demonstrated that LLPCs have an advantage over SLPCs in the use of glucose and mitochondrial import of pyruvate for survival and antibody production17. Because metabolic pathways are regulated by a diverse array of signals stimulated by environmental cues, it remains unclear what cell surface receptors and what signals are responsible for the metabolic programing of LLPC. In this report, we identified ENPP1 as a novel membrane enzyme receptor critical for LLPC survival by regulating glucose uptake and energy production.
ENPP1 was discovered in 1970 by Takahashi, Old and Boyse as a membrane alloantigen restricted primarily to plasmacytomas and normal plasma cells (PCs) that they termed plasma cell alloantigen 1 (PC-1)18. ENPP1 is expressed in many non-lymphoid tissues including cartilage, heart, kidney, liver, and salivary gland, and is highly expressed in chondrocytes, osteoblasts, and vascular smooth muscle cells19,20. The function of ENPP1 is multifaceted. First, ENPP1 has broad substrate specificity, including ATP, UTP, cAMP, and 2′3′-cGAMP21,22,23,24. The enzyme catalyzes 5′-phosphodiesterase bonds, mostly in ATP, to generate nucleoside 5′-monophosphates and inorganic pyrophosphate (PPi)21,24, the latter being an inhibitor of mineral crystallization during the process of bone formation. Consistent with this activity, mice with inactivating mutant alleles of Enpp1 25,26 or a genetically engineered null allele27 exhibit “stiff joints” and “tiptoe walking” due to excessive calcification of joints and paraspinal ligaments. Mutations of Enpp1 also cause blood vessel calcification in both mice25,28 and humans29,30,31,32. In addition, PPi is a stable high energy compound and can substitute for an ATP-derived energy supply at least in Entamoeba histolytica 33. Second, ENPP1 mediates nucleotide recycling by breaking down ATP to AMP, which is then converted to adenosine by 5′ nucleotidase21. Adenosine is then transported freely into cells for metabolism. Both ATP and adenosine are known to have immunoregulatory functions34. Third, ENPP1 is involved in adipocyte differentiation35 and plays a role in carbohydrate metabolism and insulin resistance (reviewed in36).
Although expression of ENPP1 on PCs was recognized almost five decades ago, little is known about the function of this molecule in PCs. In this report, we investigated the functions of ENPP1 in PC biology using Enpp1 −/− mice. Our data demonstrate that while ENPP1 is dispensable for normal B cell development, it is essential for the development and survival of LLPCs.
Results
Expression of ENPP1 gradually increases during B cell and PC maturation
Our previous analyses of ENPP1 expression on the surface of B lineage cells indicated that early and mature B cells express only low levels37. However, splenic GC B cells (GL7+PNA+) and PCs (B220dull/-CD138hi) exhibit markedly increased expression (37 and Fig. 1A). Interestingly, BM PCs expressed 2-fold more ENPP1 than their splenic counterparts (Fig. 1A). To confirm this finding, we analyzed Blimp1-YFP reporter mice (Blimp1 Yfp/+) in which plasmablasts (PBs) and mature PCs can be accurately distinguished by gating on B220+YFPint and B220dullYFPhi cells, respectively38,39. Fourteen days after immunization with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated keyhole limpet hemocyanin (NP-KLH) and alum, splenic YFPhi mature PCs expressed ~ 2-fold more ENPP1 than YFPint PBs while there was nearly another 2-fold increase in ENPP1 expression by YFPhi LLPCs in BM (Fig. 1B). Staining of human BM cells with a human-specific monoclonal anti-ENPP1 antibody40 also revealed high expression of ENPP1 on PCs compared with naïve B cells (Fig. 1C). These results thus demonstrated that high expression of ENPP1 is associated with LLPCs in BMs of both mice and humans.
PCs express high levels of ENPP1. (A) Spleen and BM cells from B6 mice immunized with NP-KLH/alum for 2 wks were stained with antibodies to ENPP1, B220, GL7, CD138, and PNA and analyzed by flow cytometry. Cells were gated for naïve (B220+GL7−CD138−), GC (GL7+PNA+) and PC cells (B220dull/−CD138+). The expression levels of ENPP1 are depicted as mean fluorescence intensity (MFI). Each symbol represents a mouse. (B) Spleen and BM cells from Blimp1Yfp/+ mice immunized with NP-KLH for 2 wks were analyzed by flow cytometry. The top panel indicates the gating schemes used for defining naïve (CD138−YFP−), PBs (CD138+YFPint) and PCs (CD138+YFPhi). FMO, fluorescence minus one control for the anti-ENPP1 antibody. The bar graph (bottom) is the absolute MFI of ENPP1 of indicated cell subsets. Error bars are of 4 mice. (C) BM cells from a human adult female donor were pre-enriched for PCs with anti-human CD138 magnetic beads and stained and analyzed by FACS. Cells were gated for naïve (CD20+CD10−CD38loCD19+IgM+), PCs (CD20−CD10−CD38+CD138+) and PCs (intracellular Ig) (CD20−CD38+/hiIntracellular Igκ/λ+), respectively. A non-paired two-tailed Student’s t-test was used. *p < 0.05, **p < 0.01, and ***p < 0.001.
ENPP1 is dispensable for development of B and T cells
The high expression of ENPP1 in LLPCs prompted us to examine the function of ENPP1 in PC development by studying ENPP1-deficient mice (Enpp1 −/−). While Enpp1 −/− mice have been extensively studied for skeletal, muscular and metabolic abnormalities27,28,35,41,42,43,44, we are unaware of studies focused on the immune system. First, we characterized the phenotypes and distributions of B and T cells in Enpp1 −/− mice by flow cytometry. We found that the development of B and T cells was grossly normal in Enpp1 −/− mice compared with Enpp1 +/+ littermates (designated wild-type or WT) (Figure S1A–C). Although the frequencies of BM pre-B and immature B cells were substantially higher in Enpp1 −/− mice than in WT controls, the frequencies and absolute numbers of B cell subsets in the periphery were comparable between Enpp1 −/− and WT mice (Figure S1). The mechanisms underlying the increased frequency of pre-B cells in ENPP1-deficient mice are currently unclear and warrant further investigation. Nevertheless, we conclude that ENPP1 is dispensable for B and T cell development in mice.
We next examined B cell proliferative responses to TLR ligands, including LPS and CpG oligodeoxynucleotides, or BCR ligation in vitro. Both Enpp1 −/− and WT B cells proliferated to comparable extents following stimulation (Figure S2A). Finally, we examined T-independent (TI) immune response by immunizing mice with NP-LPS and NP-Ficoll. TI antigen responses are characterized by fast generation of SLPCs with transient production of low affinity antibodies. Both Enpp1 −/− and WT mice generated equivalent antibody responses as assessed by NP-specific antibody levels in blood (Figure S2B and C). We therefore conclude that ENPP1 is dispensable for T-independent immune responses.
ENPP1 deficiency affects development of LLPCs in BM following T-dependent immune responses
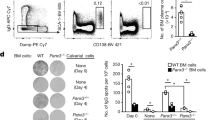
We next examined T-dependent antigen responses in Enpp1 −/− and WT mice by using a standard NP-KLH/alum immunization protocol for 2 wk. We first analyzed development of GCs and PCs by flow cytometry. The frequencies of GC B cells (GL7+PNA+) and switched IgG1+ GC B cells in spleens of WT and Enpp1 −/− mice were comparable (Fig. 2A). The frequencies of splenic B220dull/-CD138hi PCs were also comparable between Enpp1 −/− and WT mice (Fig. 2B). Next, we assessed dynamic generation of NP-specific splenic and BM PCs by sensitive ELISPOT assays over a course of 2 mo following immunization. As shown in Fig. 2C, the frequencies of NP-specific IgG1-secreting PCs were significantly lower in spleens of Enpp1 −/− mice than WT controls at 1 mo. At 2-mo, splenic PCs numbers had declined to base line levels for both groups of mice (Fig. 2C), consistent with the view that splenic PCs were primarily SLPCs. Interestingly, the frequencies of NP-specific IgG1+ PCs in the BM were significantly lower in Enpp1 −/− mice than in WT controls at both the 1- and 2-mo time points, perhaps initiating as early as 2-wk after immunization (Fig. 2C). Consistent with the changes in PC numbers, the levels of class-switched serum NP-specific antibodies were also lower in Enpp1 −/− mice than in WT controls (Fig. 2D). Taken together, these results indicate that during a T-dependent immune response, while the development of GCs was normal in Enpp1 −/− mice, the continuing production and/or persistence of BM PCs was impaired in the absence of ENPP1.
Enpp1−/− mice have normal GC responses but impaired antibody production following immunization with TD antigens. (A,B) WT and Enpp1−/− mice were immunized with NP-KLH/alum and were analyzed at 2 week by flow cytometry. The numbers are percentages of cells falling in each gate (dot plot histograms). Each symbol represents a mouse (right panels). (C) ELISPOT analysis of immunized mice as in (A) were performed at 0.5, 1 and 2 month following immunization. NP-specific IgG1+ PCs are shown. Error bars are of 6 mice. *p < 0.05. (D) The serum levels of NP-specific antibodies were measured by ELISA. Error bars are of 5–9 mice. *p < 0.05. (E) The mice indicated were infected with C. chabaudi for 8 days. Splenic and BM cells were stained and analyzed by flow cytometry. Each symbol represents a mouse. A non-paired two-tailed Student’s t-test was used. *p < 0.05. n.s., not significant.
To rule out the possibility that the compromised generation of BM LLPCs in Enpp1 −/− mice was restricted to responses to a single T-dependent antigen, we employed a malaria parasite infection model. Previous studies demonstrated that infection of mice with plasmodium Chabaudi (C.) chabaudi induced rapid and robust immune responses with high levels of PCs in spleen and BM45,46. Consistent with these reports, we observed that mice infected with C. chabaudi for 8 d produced ~10 times more splenic PCs than those immunized with NP-KLH (Fig. 2E vs. Figure 2B). Whereas the magnitude of inflammation (spleen weights) and the frequencies of splenic GCs, PBs, PCs and blood PCs were comparable between infected WT and Enpp1 −/− mice (Fig. 2E and Figure S3), the frequencies of BM PCs were significantly lower in Enpp1 −/− mice than in WT controls (Fig. 2E).
The effect of ENPP1 on BM PC development is B cell-intrinsic
To determine whether the impaired generation of BM PCs in Enpp1 −/− mice was due to cell-intrinsic or -extrinsic mechanisms, we generated chimera mice by reconstituting lethally irradiated CD45.1 B6 mice with BM HSCs (sorted as lineage-cKit+Sca-1+IL7R−) as we reported previously47. Eight weeks after transfer, most (>90%) splenocytes of the recipients exhibited the donor origin as assessed by CD45.1 staining. We immunized these mice with NP-KLH and alum and assessed GC responses by flow cytometry and ELISPOT assays. The frequencies of GCs were indistinguishable between recipients of WT HSCs (designated chimeric WT) and Enpp1 −/− HSCs (designated chimeric Enpp1 −/−) at 2 and 4 week following immunization (Fig. 3A), consistent with the results of immunized conventional mice (Fig. 2A). However, the frequencies of NP-specific PCs were significantly lower in the BM of chimeric Enpp1 −/− mice than chimeric WT controls. In spleens of recipient mice, the frequencies of NP-specific PCs also tended to be lower in chimeric Enpp1 −/− mice than WT controls (Fig. 3A). Interestingly, at 1 wk following a boost immunization, chimeric Enpp1 −/− mice generated more GCs than WT controls. In this case, the frequencies of splenic PCs were slightly increased but the numbers of BM PCs were significantly decreased in chimeric Enpp1 −/− mice compared with chimeric WT controls. At 12 wk following boost immunization, the frequencies of PCs were significantly lower in both spleen and BM of chimeric Enpp1 −/− mice than chimeric WT controls (Fig. 3B). These data indicate that long-term survival of PCs was impaired in Enpp1 −/− mice regardless of the location.
Decreased persistence of PCs in Enpp1−/− chimera mice. Chimeric mice were generated by reconstituting lethally irradiated CD45.1 (A and B) or Rag1−/− (C) mice with BM HSCs sort-purified from WT or Enpp1−/− mice. The HSC reconstituted mice were immunized with NP-KLH and alum once (A and C) or twice (B). (A–C) GC B cells were analyzed by flow cytometry as depicted in Fig. 2A. (A–C) NP-specific PCs in BM and spleen were enumerated by ELISPOT. Error bars are 3–7 mice per group. Data are representative of 2–4 independent experiments. A non-paired two-tailed Student’s t-test was used. *p < 0.05. n.s., not significant.
It has been reported that LLPCs are radiation resistant6 and that newly generated PCs need to expel pre-existing PCs before they can establish their own survival niches48. Therefore, it was possible that the immunization-induced ENPP1-deficient PCs were defective in displacing pre-existing, radiation-resistant PCs in CD45.1 B6 recipients (Fig. 3A and B). To examine this possibility, we generated chimeric mice using Rag1 −/− mice devoid of pre-existing BM PCs as recipients. The mice were examined 1 mo following immunization with NP-KLH and alum. In this case, similar to above results (Fig. 3A,B), chimeric Enpp1 −/− mice generated normal numbers of GCs but significantly lower numbers of PCs in both BM and spleen compared with controls (Fig. 3C). Taken together, these results support a B cell-intrinsic effect of ENPP1 on PC development and survival.
ENPP1 deficiency does not affect migration of PBs in vivo and in vitro
To determine whether the reduced frequency of BM PCs in Enpp1 −/− mice was due to an inability of ENPP1-deficient PBs to migrate into the BM, we first measured the frequencies of circulating PBs in mice immunized with NP-KLH or plasmodium-infected mice. PBs were detected by flow cytometry as B220+IgM−IgD−CD44+PNAloNP+ as previously reported49 (also refer to Fig. 4D). As shown in Fig. 4A, the frequencies of blood PBs peaked 9 d after immunization. There was no significant difference in the frequencies of blood PBs between chimeric Enpp1 −/− and WT mice (Fig. 4A). The frequencies of blood PBs in Enpp1 −/− and WT mice infected with C. chabaudi were also equivalent (Fig. 4B). Therefore, these data suggest that ENPP1 deficiency did not affect the output of PBs in peripheral blood of mice under both immunization and infection conditions.
ENPP1 deficiency does not affect migration of PBs. (A) Normal generation of PBs in Enpp1−/− chimera mice. Chimeric mice as in Fig. 3A were immunized with NP-KLH and alum. PBs (B220+IgMlo/−IgD−CD44+PNAloNP+) in blood at the indicated times following immunization were analyzed by flow cytometry. Error bars are of 5–10 mice per group. (B) PBs in blood of C. chabaudi infected mice as in Fig. 2E were analyzed by flow cytometry. Each symbol represents a mouse. (C) Expression of CXCR4 on splenic PBs were analyzed by flow cytometry. Representative data of 3 independent experiments are shown. (D) Splenic B cells from mice immunized with NP-KLH for 7 days were purified by negative selection and subjected to transwell assays in the presence of different concentrations of SDF-1. The cells migrating to the lower chamber were stained and analyzed by FACS. Top panel, gating strategy for defining the indicated B cell subsets. Lower panel, cell counts of triplicate assays. (E) A similar transwell assay as in (D) was performed with transwells that were pre-coated with VCAM1 or BSA. Error bars are of 3 mice. Data are representative of two independent experiments (D and E). A non-paired two-tailed Student’s t-test was used. n.s., not significant.
Homing of circulating PBs to the BM is mediated by well-characterized biological processes involving vascular adhesion and extravasation guided by CXCR4-CXCL12-mediated chemotaxis. Figure 4C shows that the levels of CXCR4 expressed by splenic PBs were equivalent between Enpp1 −/− and WT mice. Transwell migration assays revealed that comparable numbers of ENPP1-deficient and -sufficient B cells migrated toward SDF-1 (CXCL12) in a dose-dependent fashion (Fig. 4D). Pre-coating the transmembrane with VCAM1 did not affect transwell migration either (Fig. 4E). Finally, we analyzed expression of 34 genes involved in leukocyte trafficking in BM PCs by qPCR and flow cytometry. There were no significant differences in expression levels of these genes between WT and ENPP1-deficient PCs (Figure S4 and data not shown).
Finally, we measured the frequencies of Ki67+ BM PCs in WT and Enpp1 −/− mice immunized with NP-KLH/alum for 10 days. Ki67 is a nuclear protein associated with recent cell division and is expressed in newly formed PCs in the BM50. While the frequencies of Ki67+ PCs were equivalent in the spleens of WT and Enpp1 −/− mice, the frequencies of Ki67+ PCs were proportionally slightly increased in the BM of Enpp1 −/− mice compared with WT controls (Fig. S5). Taken together, these data suggest that ENPP1-deficient and –sufficient PBs and PCs are indistinguishable in homing capacity.
ENPP1-deficiency impairs glucose-mediated metabolic activity of PCs
Recently, Lam et al. reported that LLPCs take up more glucose than SLPCs and are more dependent on glucose for glycolysis and survival than SLPCs17. To determine whether ENPP1 deficiency would affect glucose uptake in PCs, we first examined the expression levels of glucose transporters in PCs. Among the 12-member glucose transporter (GLUT) family, GLUT1 was the dominant transporter expressed by PCs as determined by RNAseq analyses of an ENPP1-deficient plasmacytoma cell line (Fig. 5A). The high expression of GLUT1 in PCs was confirmed at the protein level by flow cytometric studies of BM and splenic PCs stained with an anti-GLUT1 antibody (Fig. 5B). Regardless of the location, both BM and splenic PCs expressed 2-fold higher levels of GLUT1 than other B and non-B cell subsets, suggesting a marked surge in demand for glucose for PC function. We next measured glucose uptake by PCs in vivo by injecting mice with 2NBDG, a fluorescent glucose analog, followed by flow cytometric analyses. Compared with WT controls, ENPP1-deficient mice exhibited significantly lower frequencies of 2NBDG+ PCs in both the BM and spleen (Fig. 5C). Consistent with a previous report17, BM PCs contained twice as many 2NBDGbri cells than splenic PCs (Fig. 5C). Because there was only about 25% of splenic PCs labeled with 2NBDG at a high level, this data is consistent with the view that the majority of splenic PCs are short-lived. In summary, our data indicate that the fewer 2NBDGbri PCs in Enpp1 −/− mice parallel results showing that ENPP1-deficent mice generated fewer LLPCs (Fig. 3).
Impaired glucose uptake and metabolism in PCs of Enpp1−/− mice. (A) Expression levels of glucose transporters in a mouse PCT cell line were analyzed by RNA-seq. RPKM, reads per kilobase of transcript per million mapped reads. (B) Expression levels of GLUT1 in B cell subsets of BM and spleen were measured by flow cytometry. MFI, mean fluorescence intensity. Error bars are of 3 mice in 2 independent experiments. (C) 2NBDG uptake in vivo by the indicated cell populations was quantitated by flow cytometry. The numbers are percentages of cells falling in each gate. Error bars of 4 mice per group. Data are representative of 2 independent experiments. A non-paired two-tailed Student’s t-test was used. *p < 0.05. (D) ENPP1-deficient PCs have reduced levels of glycolysis than wild-type PCs. Mitochondrial stress test to measure oxygen consumption rate (OCR, left panel) and glycolysis stress test to measure extracellular acidification rate (ECAR, right panel) of freshly isolated PCs from mice immunized with NP-KLH for 9 days. Error bars are technical triplicates. A non-paired two-tailed Student’s t-test was used. *p < 0.01. Data are representative of 2 independent experiments with similar results.
To further understand the role of ENPP1 in the regulation of metabolic activity essential for PC survival, we performed mitochondrial stress test to determine oxidative phosphorylation (measured as oxygen consumption rate (OCR)) and glycolysis stress test (measured as extracellular acidification rate (ECAR)) in purified PCs from immunized WT and Enpp1 −/− mice. For the mitochondrial stress test, cells were treated sequentially with oligomycin (an inhibitor of the ATP synthase), 2,4-Dinitrophenol (DNP, an uncoupler) and rotenone plus antimycin A (inhibitor of complex I and III of the respiratory chain respectively). For the glycolysis stress test, glucose-starved cells were treated sequentially with glucose, rotenone plus antimycin A and 2-deoxy-D-glucose (2-DG, an inhibitor of glycolysis)51. As shown in Fig. 5D, splenic PCs of Enpp1 −/− mice exhibited strongly reduced ECAR compared with WT controls even though Enpp1 −/− PCs displayed a modest but non-significant increase in maximal oxygen consumption after DNP treatment (Fig. 5D). LPS-stimulated B cells, which were mostly proliferating PBs, also exhibited similar patterns of metabolic changes with moderate reductions of ECAR in ENPP1-deficient cells (Figure S6). Together these results suggest that impaired glucose uptake in ENPP1-deficient PCs leads to a defect in glycolysis without a compensatory increase in either basal or maximal respiratory capacity. This loss of glycolytic capacity and no significant change in oxidative phosphorylation capacity likely renders ENPP1-deficient PCs at a great survival disadvantage in vivo.
Discussion
The development of durable protective antibody responses to vaccines or infections is critically dependent on the generation of LLPCs in the BM. Although remarkable progress has been made in defining the environmental niches, cytokines, chemokines and transcriptional programs that characterize these cells (reviewed in52) it is only recently that metabolic factors conferring survival to LLPCs have been explored. Lam et al. (27) elegantly demonstrated that LLPCs are distinguished from SLPCs by their increased capacity to import and utilize glucose as a source of mitochondrial respiration, thereby promoting survival and high-level Ig secretion. Unanswered, however, was the question of how these unique attributes of LLPC are regulated.
Here we identify ENPP1 as a crucial regulator promoting survival of LLPC in the BM. While the frequencies of PCs in the spleens of ENPP1-deficient mice were only moderately reduced compared with ENPP1-sufficient controls in the early phase of a TD immune response, a significant reduction of LLPCs was found in the BM of Enpp1 −/− mice. This defect in BM LLPC survival/persistence was PC intrinsic as assessed by studying BM chimeric mice. Similar to the results of conventional mice, chimeric mice also produced comparable levels of GC B cells following immunization with NP-KLH. However, the frequencies of long-lived antigen-specific BM PCs were significantly lower in chimeric Enpp1 −/− mice than WT controls. Importantly, we found that PCs that reached the BM of Enpp1 −/− mice took up less glucose than BM PCs of WT controls. Considering the fact that BM PCs express two-fold more ENPP1 than splenic PCs (Fig. 1), we reasoned that ENPP1 allows BM LLPCs to consume more glucose to better facilitate higher amount of antibody production and longer time of survival. This view is consistent with the finding that LLPCs rely more heavily on glucose for glycolysis and antibody production than splenic SLPCs17. Indeed, ENPP1-deficient PCs exhibited greater reduction in ECAR (Fig. 5), a parameter indicating an impaired glycolysis pathway, which would be expected to lead to reduced levels of energy production thereby poor survival of PCs in vivo.
One important question related to the decreased numbers of BM PCs in Enpp1 −/− mice is whether the ENPP1-deficient PBs are capable of homing to the BM in the first place. Our analyses indicated that the frequencies of PBs in peripheral blood of Enpp1 −/− mice were within the normal range following immunization with NP-KLH or infection with C. chabaudi. Homing of PBs to the BM is mediated by the chemokine CXCL12 and the receptor CXCR4 on PC53 as well as adhesion molecules including integrins54. Our analyses indicated that expression of CXCR4 on PBs and PCs was comparable between Enpp1 −/− and WT mice. The chemotactic response of PBs to CXCL12 in vitro was also comparable between these mice. In addition, we analyzed 34 adhesion molecules, including a panel of 10 integrin molecules known to be important for lymphocyte trafficking, and found no differences in expression between PCs of Enpp1 −/− and WT mice. The expression levels of other adhesion molecules including VCAM1, CD44 and CD144 were also normal on PCs of Enpp1 −/− mice as detected by flow cytometry (data not shown). Considering the results that ENPP1-sufficient and -deficient B cells including PBs were indistinguishable in transwell migration assays (Fig. 4), it is unlikely that the homing of ENPP1-deficient PBs to the BM was impaired.
The functions of ENPP1 have been broadly studied in the fields of bone formation and type 2 diabetes. Previous studies revealed that osteoblasts and chondrocytes express high levels of ENPP120,24,55. ENPP1 plays a negative regulatory role in bone mineralization mediated by PPi, a product from ATP hydrolysis mediated by ENPP156. While direct evidence is still lacking, it is possible that high expression of ENPP1 on PCs may suppress new bone formation to preserve survival niches. In addition to the catalytic activity of ENPP1, ENPP1 has been found to modulate insulin receptor activity and glucose homeostasis (reviewed in57). By directly interacting with the alpha chain of the insulin receptor, ENPP1 downregulates the insulin signaling pathway causing insulin resistance58,59. Increased ENPP1 expression in the liver of ENPP1 transgenic mice correlates with insulin resistance and glucose intolerance60. These data suggest that ENPP1 is critical for insulin-stimulated glucose metabolism. While the responsiveness of PCs to insulin stimulation remains unclear, our findings that ENPP1-deficient PCs fail to gain the LLPC phenotype by consuming more glucose for antibody production and survival highlight the possibility that ENPP1 is a critical regulator for glucose-dependent metabolic processes in PCs.
The dominant substrate of ENPP1 is ATP. ATP is released from cells undergoing stress, inflammatory stimulation, hypoxia or death61. Extracellular ATP is an important mediator of inflammation with broad effects on cellular functions62. Given that the BM microenvironment is enriched for developing cells with a high frequency of apoptotic events, rapid degradation of extracellular ATP would be expected to minimize the negative effect of ATP on LLPCs. Consistent with this view, preliminary experiments suggest that ENPP1 suppresses exogenous ATP-induced oxygen consumption but enhances glycolysis in LPS-induced PBs (data not shown). In addition, hydrolysis of ATP by ENPP1 also produces PPi, a high-energy substitute for ATP that can be transported freely crossing cell membranes. PPi has been found to be beneficial for sperm survival and to increase in vitro fertilization rates63. Therefore, the beneficial effect of ENPP1 on PC survival could be multifaceted.
Controlling plasma cell-related diseases such as multiple myeloma has been very difficult, due in part to a paucity of good target molecules on the cell surface. Our findings suggest that ENPP1 could be an attractive therapeutic target for intervention in PC-related diseases if PC specificity could be achieved. In addition, in view of the importance of LLPC for enduring antibody-mediated immunity to vaccines and infections, further understandings of the signals promoting PC longevity could prove to be informative for rational vaccine design.
Materials and Methods
Mice and cells
C57BL/6 J (B6) (CD45.2 and CD45.1) and Rag1 −/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Enpp1 −/− mice were described previously27. Blimp-1 YFP reporter mice were obtained from Dr. Eric Meffre at Yale University. All mice were housed under pathogen-free conditions. All animal studies were performed under protocols of LIG-16 approved by the NIAID IACUC and all experiments were done in accordance with the NIH guidelines and regulations.
To generate chimeric mice, CD45.1 and Rag1 −/− mice were irradiated at 1,100 (with a short interval) and 500 rad, respectively, one day before i.v. injection with 0.6–2 × 104 sort-purified HSCs (Lin-Sca1+c-Kit+IL7R−) from WT or Enpp1 −/− mice, as previously reported47. The efficiency of reconstitution was verified by FACS analyses of blood for frequencies of B and T cells shortly before mice were immunized 8 wks later.
Human bone marrow cells were purchased from Allcells (Alameda, CA) and processed for antibody staining and flow cytometry analysis as described below.
Immunization and ELISA
Mice were immunized i.p. with NP-LPS (50 μg), NP-Ficoll (20 μg), NP23-KLH (50 μg in alum) (Biosearch Technologies, Novato, CA). At different time points after immunization, the mice were bled and the serum antibodies were measured by ELISA. Briefly, pre-coated plates with NP26-BSA (Biosearch Technology) were incubated with diluted serum samples and developed with HRP-conjugated mouse isotype-specific Ab (Southern Biotech) and substrate o-phenylenediamine dihydrochloride (Sigma-Aldrich). The plates were read at 450 nm using an ELISA plate reader.
Flow cytometry (FACS)
Single cell suspensions from thymus, BM, spleen and peritoneum of mice were prepared and stained with fluorochrome-labeled mAbs using standard procedures. A cell viability dye, 7AAD or Fixable Violet dye (Invitrogen), was routinely included. All Abs, except as indicated, were purchased from BD-Biosciences (San Diego, CA). The anti-human ENPP1 (clone 2D7, provided by Dr. James Goding, Monash University, Victoria, Australia) and mouse ENPP1 (clone YE1/19.1, provided by Dr. Fumio Takei, Terry Fox Laboratory, Canada) were labeled with allophycocyanin (APC)37. Cells were analyzed using a FACSCalibur, LSR II (BD Biosciences) or sorted on a FACS Aria sorter (BD Biosciences).
Quantitative Real-time RT-PCR (qPCR)
Total RNA from sorted follicular (FO) (B220+CD23+CD21int), GC (PNA+GL-7+) and PCs (B220dull/-CD138bri) was reverse-transcribed with SuperScriptTM II enzymes (Invitrogen, Carlsbad, CA). The cDNA was subjected to real-time PCR analysis using an ABI Prism 7900HT system and the SYBR Green PCR Master Mix reagents (Applied Biosystems). PCR primer sequences were: Enpp1 forward, 5′-TTAATGTGTGACTTACTGGGTTTGATC-3′; Enpp1 reverse, 5′-GGTTGAGGCTGCCATGACTT-3′. All other primers were described previously64.
In vitro treatment
Splenic B cells were purified by negative selection using magnetic-activated Dynal beads (Invitrogen, Carlsbad, CA) and labeled with CFSE using a standard protocol. Cells (1–2 × 106/ml) were cultured in 24-well plates for different periods of time with 20 μg/ml of LPS (Sigma-Aldrich), 1 μg/ml of CpG ODN 1826 (Invivogene, San Diego, CA), or 10 μg/ml of F(ab’)2 anti-μ Ab (Jackson ImmunoResearch Laboratory) plus 1 μg/ml of anti-CD40 Ab (Southern Biotech). The cells were stained with 7AAD and annexin V and analyzed by FACS.
ELISPOT assays
Spleen and BM PCs were quantified by NP-specific Ig ELISPOT assays. Briefly, aliquots of 1.25–5.0 × 105 spleen and BM cells were plated in triplicate in NP26-BSA pre-coated 96-well PVDF membrane plates (Millipore) and were incubated overnight at 37 °C in 5% CO2. The plates were washed with PBS containing 0.05% Tween-20 and incubated with HRP-conjugated anti-mouse IgM or IgG1 (Jackson ImmunoResearch Laboratory), followed by reaction with FAST 5-bromo-4-chlor-3-indolyl phosphate/NBT chromogen substrate (Sigma-Aldrich). The plates were scanned with a CTL-ImmunoSpot® S5 Core Analyzer (Cellular Technology) and analyzed by ImmunoSpot® Software 4.0 (Cellular Technology).
Glucose uptake and Metabolic analyses
For in vivo 2NBDG labeling, mice were injected i.v. with 100 μg 2NBDG (Cayman Chemicals) in PBS and euthanized 15 minutes later. BM and splenic cells were isolated, stained and analyzed by flow cytometry.
For extracellular flux assays, we used a standardized protocol to measure OCR and ECAR by a Seahorse XF 96 analyzer described in great detail recently51. Ex vivo PCs were purified from 22 mice immunized with NP-KLH and alum for 9 days using a CD138+ plasma cell isolation kit (Miltenyi Biotech). 8 × 105 cells were plated in 96-well Cell-Tack (Corning)-coated Seahorse plates and analyzed.
Generation of ENPP1-deficient plasmacytoma
Pristane-induced plasmacytoma was generated in Enpp1 −/− Myc/Bclxl transgenic (Tg) mice that were generated by several rounds of breeding of Enpp1 +/− with Em-BCLxL Tg65 and heterozygous C.iMycCα 66 mice. Mice were injected with 0.4 ml of pristane and monitored for signs of ascites. Plasma cell tumors were diagnosed using Wright-Giemsa–stained cytofuge preparations of ascites. When mice carried more than 100 tumor cells per field, they were euthanized and peritoneal neoplastic tissues, ascites and spleens were collected, cell suspensions were prepared and cultured with complete RPMI 1640 medium in the presence of IL6 till cell lines were established. The phenotype of the cell lines was assessed by flow cytometry. One cell line (10659) used in this study was B220−, CD19−, CD138+, IgMlo/−, Igκlo, IgD−, CD5−, CD23−, CD21−, integrin αV+, β1+, β3+, and CD11blo.
Statistical analysis
Data were analyzed using 2-tailed Student’s t test (2-tailed). P < 0.05 is regarded as statistically significant.
References
Jacob, J., Kassir, R. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 173, 1165–1175 (1991).
Smith, K. G., Weiss, U., Rajewsky, K., Nossal, G. J. & Tarlinton, D. M. Bcl-2 increases memory B cell recruitment but does not perturb selection in germinal centers. Immunity 1, 803–813 (1994).
Sze, D. M., Toellner, K. M., Garcia de Vinuesa, C., Taylor, D. R. & MacLennan, I. C. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med 192, 813–821 (2000).
Ho, F., Lortan, J. E., MacLennan, I. C. & Khan, M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol 16, 1297–1301 (1986).
Manz, R. A. & Radbruch, A. Plasma cells for a lifetime? Eur J Immunol 32, 923–927 (2002).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Minges Wols, H. A., Underhill, G. H., Kansas, G. S. & Witte, P. L. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol 169, 4213–4221 (2002).
Tokoyoda, K., Egawa, T., Sugiyama, T., Choi, B. I. & Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20, 707–718 (2004).
Geffroy-Luseau, A., Jego, G., Bataille, R., Campion, L. & Pellat-Deceunynck, C. Osteoclasts support the survival of human plasma cells in vitro. Int Immunol 20, 775–782 (2008).
Winter, O. et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 116, 1867–1875 (2010).
Rodriguez Gomez, M. et al. Basophils support the survival of plasma cells in mice. J Immunol 185, 7180–7185 (2010).
Chu, V. T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 12, 151–159 (2011).
Belnoue, E. et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111, 2755–2764 (2008).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 171, 1684–1690 (2003).
O’Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199, 91–98 (2004).
Masciarelli, S. & Sitia, R. Building and operating an antibody factory: redox control during B to plasma cell terminal differentiation. Biochim Biophys Acta 1783, 578–588 (2008).
Lam, W. Y. et al. Mitochondrial Pyruvate Import Promotes Long-Term Survival of Antibody-Secreting Plasma Cells. Immunity 45, 60–73 (2016).
Takahashi, T., Old, L. J. & Boyse, E. A. Surface alloantigens of plasma cells. J Exp Med 131, 1325–1341 (1970).
Harahap, A. R. & Goding, J. W. Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol 141, 2317–2320 (1988).
Nitschke, Y., Weissen-Plenz, G., Terkeltaub, R. & Rutsch, F. Npp1 promotes atherosclerosis in ApoE knockout mice. J Cell Mol Med 15, 2273–2283 (2011).
Goding, J. W., Grobben, B. & Slegers, H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta 1638, 1–19 (2003).
Li, L. et al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol 10, 1043–1048 (2014).
Namasivayam, V., Lee, S. Y. & Muller, C. E. The promiscuous ectonucleotidase NPP1: molecular insights into substrate binding and hydrolysis. Biochim Biophys Acta 1861, 603–614 (2017).
Terkeltaub, R. A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol 281, C1–C11 (2001).
Babij, P. et al. New variants in the Enpp1 and Ptpn6 genes cause low BMD, crystal-related arthropathy, and vascular calcification. J Bone Miner Res 24, 1552–1564 (2009).
Okawa, A. et al. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet 19, 271–273 (1998).
Sali, A., Favaloro, J.M., Terkeltaub, R., Goding J.W. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein-1 (PC-1) produces abnormal calcification of periarticular tissues. 267–282 (Shaker Publishing BV, 1999).
Johnson, K., Polewski, M., van Etten, D. & Terkeltaub, R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol 25, 686–691 (2005).
Crade, M., Lewis, D. F. & Nageotte, M. P. In utero appearance of idiopathic infantile arterial calcification: ultrasound study of a 28-week fetus. Ultrasound Obstet Gynecol 1, 284–285 (1991).
Reitter, A. et al. Fetal Hydrops, Hyperechogenic Arteries and Pathological Doppler Findings at 29 Weeks: Prenatal Presentation of Generalized Arterial Calcification of Infancy - A Novel Mutation in ENPP1. Fetal Diagn Ther 25, 264–268 (2009).
Rutsch, F. et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34, 379–381 (2003).
Samon, L. M., Ash, K. M. & Murdison, K. A. Aorto-pulmonary calcification: an unusual manifestation of idiopathic calcification of infancy evident antenatally. Obstet Gynecol 85, 863–865 (1995).
Chi, A. & Kemp, R. G. The primordial high energy compound: ATP or inorganic pyrophosphate? J Biol Chem 275, 35677–35679 (2000).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Liang, J., Fu, M., Ciociola, E., Chandalia, M. & Abate, N. Role of ENPP1 on adipocyte maturation. PLoS ONE 2, e882 (2007).
Bacci, S., De Cosmo, S., Prudente, S. & Trischitta, V. ENPP1 gene, insulin resistance and related clinical outcomes. Curr Opin Clin Nutr Metab Care 10, 403–409 (2007).
Abbasi, S. et al. Characterization of monoclonal antibodies to the plasma cell alloantigen ENPP1. Hybridoma (Larchmt) 30, 11–17 (2011).
Kallies, A. et al. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 200, 967–977 (2004).
Rutishauser, R. L. et al. Transcriptional repressor Blimp-1 promotes CD8( + ) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Yoon, J. et al. Plasma cell alloantigen ENPP1 is expressed by a subset of human B cells with potential regulatory functions. Immunol Cell Biol 94, 719–728 (2016).
Hajjawi, M. O. et al. Mineralisation of collagen rich soft tissues and osteocyte lacunae in Enpp1(−/−) mice. Bone 69, 139–147 (2014).
Huesa, C. et al. Deficiency of the bone mineralization inhibitor NPP1 protects mice against obesity and diabetes. Dis Model Mech 7, 1341–1350 (2014).
Nam, H. K., Liu, J., Li, Y., Kragor, A. & Hatch, N. E. Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation. J Biol Chem 286, 39059–39071 (2011).
Villa-Bellosta, R., Wang, X., Millan, J. L., Dubyak, G. R. & O’Neill, W. C. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol 301, H61–68 (2011).
Achtman, A. H., Khan, M., MacLennan, I. C. & Langhorne, J. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J Immunol 171, 317–324 (2003).
Nduati, E. W. et al. Distinct kinetics of memory B-cell and plasma-cell responses in peripheral blood following a blood-stage Plasmodium chabaudi infection in mice. PLoS One 5, e15007 (2010).
Wang, H. et al. IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood 112, 4028–4038 (2008).
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6, 741–750 (2006).
O’Connor, B. P., Cascalho, M. & Noelle, R. J. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J Exp Med 195, 737–745 (2002).
Halliley, J. L. et al. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 43, 132–145 (2015).
Traba, J., Miozzo, P., Akkaya, B., Pierce, S. K. & Akkaya, M. An Optimized Protocol to Analyze Glycolysis and Mitochondrial Respiration in Lymphocytes. J Vis Exp, https://doi.org/10.3791/54918 (2016).
Corcoran, L. M. & Nutt, S. L. Long-Lived Plasma Cells Have a Sweet Tooth. Immunity 45, 3–5 (2016).
Hargreaves, D. C. et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 194, 45–56 (2001).
Cyster, J. G. Homing of antibody secreting cells. Immunol Rev 194, 48–60 (2003).
Johnson, K. & Terkeltaub, R. Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage. Front Biosci 10, 988–997 (2005).
Terkeltaub, R., Rosenbach, M., Fong, F. & Goding, J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Relevance to calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum 37, 934–941 (1994).
Prudente, S. & Trischitta, V. Editorial: The pleiotropic effect of the ENPP1 (PC-1) gene on insulin resistance, obesity, and type 2 diabetes. J Clin Endocrinol Metab 91, 4767–4768 (2006).
Frittitta, L. et al. Elevated PC-1 content in cultured skin fibroblasts correlates with decreased in vivo and in vitro insulin action in nondiabetic subjects: evidence that PC-1 may be an intrinsic factor in impaired insulin receptor signaling. Diabetes 47, 1095–1100 (1998).
Maddux, B. A. & Goldfine, I. D. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes 49, 13–19 (2000).
Maddux, B. A. et al. Overexpression of the insulin receptor inhibitor PC-1/ENPP1 induces insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab 290, E746–749 (2006).
Lazarowski, E. R., Boucher, R. C. & Harden, T. K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64, 785–795 (2003).
Cauwels, A., Rogge, E., Vandendriessche, B., Shiva, S. & Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 5, e1102 (2014).
Yi, Y. J., Sutovsky, M., Kennedy, C. & Sutovsky, P. Identification of the inorganic pyrophosphate metabolizing, ATP substituting pathway in mammalian spermatozoa. PLoS One 7, e34524 (2012).
Lee, C. H. et al. Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J Exp Med 203, 63–72 (2006).
Grillot, D. A. et al. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J Exp Med 183, 381–391 (1996).
Rutsch, S. et al. IL-6 and MYC collaborate in plasma cell tumor formation in mice. Blood 115, 1746–1754 (2010).
Acknowledgements
We are indebted to Drs. Fumio Takei at Terry Fox Laboratory (Vancouver, Canada) and James Goding at Monash University (Victoria, Australia) for providing us with anti-ENPP1 antibodies. We thank Dr. Robert Terkeltaub at University of California at San Diego for ENPP1 knockout mice, Drs. Eric Meffre at Yale University and Amanda Poholek at NIH for Blimp-1 YFP reporter mice. We thank Larry Lantz at NIAID for technical assistance and Alfonso Macias for managing our mouse colony. This work was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (H.W., I.G., S.J., S.C., D.S., C.Q., Y.G., J.S., S.Y.K., S.A., Z.A., J.Y., A.L.K., PD, and H.C.M.), National Cancer Institute (W.D.) and National Heart, Lung, and Blood Institute (J.T. and M.N.S.).
Author information
Authors and Affiliations
Contributions
H.W., I.G., J.T. and H.C.M. designed the experiments. H.W., I.G., J.T., S.J., S.C., D.S., C.Q., Y.G., J.S., S.Y.K., S.A., Z.A., J.Y., W.D. and A.L.K. performed the experiments and analyzed the data. M.N.S., P.D., and H.C.M. analyzed the data. H.W., J.T. and H.C.M. wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Gonzalez-Garcia, I., Traba, J. et al. ATP-degrading ENPP1 is required for survival (or persistence) of long-lived plasma cells. Sci Rep 7, 17867 (2017). https://doi.org/10.1038/s41598-017-18028-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18028-z
This article is cited by
-
Discovery of VH domains that allosterically inhibit ENPP1
Nature Chemical Biology (2024)
-
An atlas of the bone marrow bone proteome in patients with dysproteinemias
Blood Cancer Journal (2023)
-
Krüppel-like factor 2 controls IgA plasma cell compartmentalization and IgA responses
Mucosal Immunology (2022)
-
FOXM1 regulates glycolysis and energy production in multiple myeloma
Oncogene (2022)
-
Peripheral antibody concentrations are associated with highly differentiated T cells and inflammatory processes in the human bone marrow
Immunity & Ageing (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.