Abstract
We aimed to evaluate the association between plasma epidermal growth factor (EGF) and the availability of dopamine transporter (DAT) measured from 123I-FP-CIT single-photon emission computed tomography in healthy controls in this study. Volume of interest template was applied to measure specific binding ratios (SBRs) of caudate nucleus, putamen, and striatum representing DAT availability as follows; SBR = (target– cerebellum)/cerebellum. Plasma EGF was negatively correlated with the availabilities of both caudate nucleus (r = −0.261, p = 0.019), and putamen (r = −0.341, p = 0.002). After dividing subjects according to Apo E genotyping, DAT availability of caudate nucleus of Apo e4 non-carriers (n = 60) showed the positive correlation with cerebrospinal fluid (CSF) α-synuclein (r = 0.264, p = 0.042). Plasma EGF was negatively correlated with DAT availabilities of Apo e4 non-carriers. Further studies are needed to clarify underlying mechanisms of this phenomenon.
Similar content being viewed by others
Introduction
Epidermal growth factor (EGF), 6 kDa protein made up of 53 amino acids, is found at high concentrations in bile, urine, milk, and prostate fluid, at medium concentrations in tears, follicular fluid, sperm, and seminal plasma, and at low concentrations in plasma, serum, and saliva1. EGF is known to involve in the development of the nervous system, stimulating proliferation, migration, differentiation of neuronal cells, enhancing survival, and inhibiting apoptosis2. Supplement of EGF to Parkinson disease (PD) model rat prevented the dopaminergic neurodegeneration3. Decreased level of EGF was found in striatum of patients with PD as compared with controls in postmortem study3. Several studies reported that low plasma EGF was correlated with cognitive decline in PD patients and the high conversion rate to Alzheimer’s disease (AD)4,5.
PD is a clinical syndrome showing bradykinesia, tremor, rigidity, and postural instability. It is characterized by the loss of dopaminergic neuron of the substantia nigra, and the presence of intraneuronal cytoplasmic inclusion6,7. The loss of dopaminergic neuron is parallel to the level of expression of the dopamine transporter (DAT) mRNA6. DAT is on the presynaptic dopaminergic nerve terminal and controls dopamine levels by active reuptake of dopamine from the synaptic cleft8. As 123I-FP-CIT reflects the striatal DAT density8, the availability of DAT measured from 123I-FP-CIT single-photon emission computed tomography (SPECT) can be used in evaluating the neurodegenerative disease9.
Although the effect of EGF in neurodegenerative disease is well documented in previous reports, the correlation of EGF with DAT in healthy controls has not been investigated yet. Therefore, we evaluated the association between plasma EGF and the availability of DAT measured from 123I-FP-CIT SPECT in healthy controls in this study.
Materials and Methods
Subjects
Data used in the preparation of this article were obtained from PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org 10. The study population consisted of 192 healthy controls with screening 123I-FP-CIT SPECT. According to PPMI criteria of healthy subjects, males or females with their age of 30 years or older at screening was included, and subjects with a neurological disorder, a first degree relative with idiopathic PD, Montreal Cognitive Assessment score of 26 or less, medications that might interfere with DAT SPECT scans, anticoagulants that might preclude safe completion of the lumbar puncture, or investigational drugs, and a condition that precludes the safe performance of routine lumbar puncture were excluded. Subjects without CSF biomarkers, plasma EGF, and Apo E genotyping were excluded. Medical history, cerebrospinal fluid (CSF), plasma EGF, and 123I-FP-CIT SPECT scans were downloaded. The PPMI study was approved by the local Institutional Review Boards of all participating sites (Institute for Neurodegenerative Disorders, University of Pennsylvania, University of California, Los Angeles, Coriell Institute for Medical Research, Clinical Trials Coordination Center, Laboratory of Neurogenetics; National Institute on Aging NIH, Institute for Neurodegenerative Disorders, Clinical Trials Statistical and Data Management Center, University of Iowa) and written informed consent was obtained from each subject at the time of enrollment for imaging data and clinical questionnaires. All methods were performed in accordance with the relevant guidelines and regulations.
Cerebrospinal fluid biomarkers and Plasma EGF
CSF biomarkers of Aβ1–42, tau protein phosphorylated at the threonine 181 position (p-Tau181), and total tau were analyzed with multiplex xMAP Luminex platform (Luminex Corp, Austin, TX, USA), and Innogenetics immunoassay kits (Innogenetics/Fujirebio, Ghent, Belgium). α-synuclein were analyzed using an enzyme-linked immunosorbent assay (Covance Research Products Inc., Denver, PA, USA). Plasma levels of EGF were measured by enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN, USA) according to manufacturer instructions. Samples were run in duplicate and data used for this study met quality control measures for technical performance.
Apo E Genotyping
Apo E genotyping was performed on DNA samples. Two non-synonymous single nucleotide polymorphisms, rs429358, and rs7412 were genotyped in each sample to distinguish between Apo e2, e3, and e4 alleles using TaqMan assays (Applied Biosystems, Foster City, CA, USA).
123I-FP–CIT SPECT
Protocol
123I-FP-CIT SPECT was performed during the screening visit for all subjects. SPECT scans were acquired 4 ± 0.5 hrs after injection of 111–185 MBq of 123I-FP-CIT. Subjects were pretreated with iodine solution or perchlorate prior to injection to block thyroid uptake. Raw data were acquired into a 128 × 128 matrix stepping each 3 or 4 degrees for the total projections. Raw projection data were reconstructed using iterative ordered subset expectation maximization with HERMES (Hermes Medical Solutions, Stockholm, Sweden). The reconstructed images were transferred to pmod (PMOD Technologies LLC, Zürich, Switzerland) for subsequent processing including attenuation correction.
Image analysis
Downloaded scans were loaded using pmod v3.6 (PMOD Technologies LLC, Zürich, Switzerland) with 123I-FP-CIT template11. Specific binding of 123I-FP-CIT regarding DAT was calculated using a region of interest analysis. A standard set of volume of interest (VOI) defining caudate nucleus, putamen, and striatum (caudate nucleus + putamen) based on the Automated Anatomical Labeling (AAL) atlas12. The cerebellum was chosen as a reference region. VOI template was applied to measure specific binding ratios (SBRs) of caudate nucleus, putamen, and striatum representing DAT availability as follows; SBR = (target– cerebellum)/cerebellum.
Statistical Analysis
Normality was examined using D’Agostino-Pearson omnibus test. Spearman correlation was used to measure the relationship of SBRs with CSF biomarkers, and plasma EGF. Mann-Whitney test was applied to compare SBRs, CSF biomarkers, and plasma EGF between Apo e4 non-carriers and carriers. Statistical analyses were performed using GraphPad Prism 7 for Mac OS X (GraphPad Software Inc, San Diego, CA, USA).
Results
Subjects’ characteristics
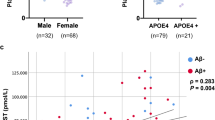
81 healthy subjects (49 male, 32 female) were included in this study. Mean age was 62.3 years. Mean BMI was 26.5 kg/m2. Twenty-one subjects were Apo e4 carriers (25.9%). DAT availabilities in caudate nucleus (r = −0.313, p = 0.004) showed a reduction with aging as expected. When subjects were divided according to Apo e4 genotyping, Aβ1–42 was higher in Apo e4 non-carriers than Apo e4 carriers. However, age, sex, BMI, α-synuclein, p-Tau181, total tau, and plasma EGF showed no significant differences between Apo e4 non-carriers and Apo e4 carriers. Subjects’ characteristics are summarized in Table 1.
Correlation of DAT availability with CSF biomarkers, and Plasma EGF
None of CSF biomarkers showed significant association with DAT availability (Table 2). However, plasma EGF was negatively correlated with the availabilities of both caudate nucleus (r = −0.261, p = 0.019), and putamen (r = −0.341, p = 0.002) (Fig. 1). After dividing subjects according to Apo E genotyping, DAT availability of caudate nucleus of Apo e4 non-carriers (n = 60) showed the positive correlation with α-synuclein (r = 0.264, p = 0.042), and that of putamen showed the trend with α-synuclein (r = 0.239, p = 0.066) (Fig. 2) (Table 3). Plasma EGF was negatively correlated with DAT availabilities of putamen (r = −0.368, p = 0.004) and striatum (r = −0.328, p = 0.011) of Apo e4 non-carriers.
Data availability
Data used in the preparation of this article were obtained from PPMI database (www.ppmi-info.org/data).
Discussion
In this study, plasma EGF was negatively correlated with striatal DAT availability. When subjects were categorized according to Apo E genotyping, negative correlation was observed in Apo e4 non-carriers. α-synuclein was positively correlated with DAT availability in Apo e4 non-carriers.
EGF is known to have protective effect in dopaminergic neuron13 and stimulate the uptake of dopamine14. Chen-Plotkin et al. reported that when the PD patients were divided by quartile according to plasma EGF values, the lowest quartile of the PD patients showed the highest conversion rate to Parkinson disease dementia and they demonstrated that plasma EGF was an independent variable predicting cognitive decline in PD patients4. Lim et al. also reported that low baseline plasma EGF predicted cognitive decline in PD patients and conversion from amnestic mild cognitive impairment (MCI) to AD5. Jiang et al. demonstrated that plasma EGF was decreased in the early stage of PD and there was no significant difference of plasma EGF between advanced PD and normal control15. In study regarding EGF and dopamine uptake in cultured rat astrocytes, they suggested the existence of Na + -dependent and Na + -independent dopamine uptake in cultured rat astrocytes and they concluded that EGF might stimulate the expression and translocation of the extraneuronal DAT16. In study assessing EGF-ErbB1 action on developing midbrain dopaminergic neuron, authors showed that EGF elevated DAT level in mesencephalic cultures and they reported that EGF receptor inhibitor reduced DAT level in the striatum, nucleus accumbens, and globus pallidum in neonatal rats17. EGF levels in both striatum and serum of patients with chronic schizophrenia were reduced comparing with those of normal controls in postmortem study18. Laakso et al. showed that striatal DAT availability measured by18F-CFT was reduced in patients with chronic schizophrenia as compared with normal controls19. Thus, we expected that the DAT availability would be decreased if the level of plasma EGF was decreased. Unlike our expectations, negative correlation between plasma EGF and DAT availability was observed in healthy subjects. There was no relevant study to explain this negative correlation.
α-synuclein, 14 kDa protein, consists of three domains with N-terminal lipid-binding α-helix, amyloid-binding central domain, and C-terminal acidic tail20,21, which has a function in suppression of apoptosis, regulation of glucose levels, modulation of calmodulin activity, chaperone activity, and regulation of dopamine biosynthesis20. Also, it has been known to be associated with neurodegenerative disease such as PD, dementia with Lewy bodies, MSA, and AD21. Especially in PD, α-synuclein interacts with tubulin, parkin, dopamine receptor, synphilin-1, phospholipase, and small ubiquitin related modifiers20. Previous studies showed that α-synuclein was not different between Apo e4 carriers and Apo e4 non-carriers in normal subjects, MCI, and AD, consistent with this study22,23. However, molecular linkage between Apo E and α-synuclein was demonstrated in one study using A30P and A53T transgenic mice. α-synuclein induces neuronal degeneration leading to Apo E deposition in spinal cords, astrocytes, and activated microglia of transgenic mice24. Astrocyte-secreted Apo E reduced α-synuclein uptake, and the effect was seen in Apo e4, followed by e3, and e225. Wersinger et al. reported that α-synuclein reduced DAT activity by recruitment of DAT from plasma membrane to cytoplasm in their in vitro study26. The decreased activity of DAT was caused by reduced dopamine uptake velocity, not by decreased DAT expression27. Kovacs et al. showed that the density of DAT, identified by immunochemistry, inversely correlated with the density of α-synuclein in the substantia nigra of patients with Lewy body disease and PD28. However, Bellucci et al. demonstrated that transgenic mice producing human α-synuclein had increased levels of striatal DAT29.
Apo E has three isoforms which are known to Apo e2, Apo e3, and Apo e430. Among them, Apo e4 is known to be the greatest risk factor for AD, followed by Apo e3, contrary to protective effect of Apo e231. Apo E has a major role in metabolism of Aβ, which is abundant in brain of Apo e4 carriers than Apo e4 non-carriers31. In this study, CSF Aβ1–42 was higher in Apo e4 non-carriers than Apo e4 carriers. Consistent with this study, Prince et al. showed decreased amount of CSF Aβ in Apo e4 carriers in both AD and normal control groups32. α-synuclein attenuated the effect of EGF by showing decreased luciferase activity in a study by Iwata et al.33. Therefore, Apo e4 non-carriers may have higher CSF Aβ1–42, CSF α-synuclein, and CSF α-synuclein leading to both lower plasma EGF and higher DAT availability.
This is the first study that investigated the association between Apo E genotyping, Aβ1–42, α-synuclein, plasma EGF, and DAT availability in healthy controls. However there are several limitations in this study. First, subjects included in this study was collected from PPMI database. Second, the number of subjects was small in Apo e4 carriers. Third, as PPMI database was collected from multiple institutes, the difference in image acquisition may affect the results.
In conclusion, plasma EGF was negatively correlated with DAT availabilities of Apo e4 non-carriers. DAT availability was positively correlated with α-synuclein in Apo e4 non-carriers. Further studies are needed to clarify underlying mechanisms of this phenomenon.
References
Wee, P. & Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers ( Basel ) 9, https://doi.org/10.3390/cancers9050052 (2017).
Xian, C. J. & Zhou, X. F. Roles of transforming growth factor-alpha and related molecules in the nervous system. Mol Neurobiol 20, 157–183, https://doi.org/10.1007/BF02742440 (1999).
Iwakura, Y. et al. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. J Neurochem 93, 974–983, https://doi.org/10.1111/j.1471-4159.2005.03073.x (2005).
Chen-Plotkin, A. S. et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol 69, 655–663, https://doi.org/10.1002/ana.22271 (2011).
Lim, N. S. et al. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann Clin Transl Neurol 3, 346–355, https://doi.org/10.1002/acn3.299 (2016).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Dickson, D. W. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2, https://doi.org/10.1101/cshperspect.a009258 (2012).
Park, E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J Nucl Med Technol 40, 222–228, https://doi.org/10.2967/jnmt.112.111617 (2012).
Thomas, A. J. et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88, 276–283, https://doi.org/10.1212/WNL.0000000000003512 (2017).
Parkinson Progression Marker, I. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 95, 629–635, https://doi.org/10.1016/j.pneurobio.2011.09.005 (2011).
Garcia-Gomez, F. J. et al. Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane. Rev Esp Med Nucl Imagen Mol 32, 350–356, https://doi.org/10.1016/j.remn.2013.02.009 (2013).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289, https://doi.org/10.1006/nimg.2001.0978 (2002).
Casper, D. & Blum, M. Epidermal growth factor and basic fibroblast growth factor protect dopaminergic neurons from glutamate toxicity in culture. J Neurochem 65, 1016–1026 (1995).
Hadjiconstantinou, M., Fitkin, J. G., Dalia, A. & Neff, N. H. Epidermal growth factor enhances striatal dopaminergic parameters in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse. J Neurochem 57, 479–482 (1991).
Jiang, Q. W. et al. Plasma epidermal growth factor decreased in the early stage of Parkinson’s disease. Aging Dis 6, 168–173, https://doi.org/10.14336/AD.2014.0925 (2015).
Inazu, M. et al. Regulation of dopamine uptake by basic fibroblast growth factor and epidermal growth factor in cultured rat astrocytes. Neurosci Res 34, 235–244 (1999).
Iwakura, Y. et al. Qualitative and quantitative re-evaluation of epidermal growth factor-ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: target-derived neurotrophic signaling (Part 1). J Neurochem 118, 45–56, https://doi.org/10.1111/j.1471-4159.2011.07287.x (2011).
Futamura, T. et al. Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 7, 673–682, https://doi.org/10.1038/sj.mp.4001081 (2002).
Laakso, A. et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res 52, 115–120 (2001).
Emamzadeh, F. N. Alpha-synuclein structure, functions, and interactions. J Res Med Sci 21, 29, https://doi.org/10.4103/1735-1995.181989 (2016).
Wong, Y. C. & Krainc, D. Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23, 1–13, https://doi.org/10.1038/nm.4269 (2017).
Berge, G. et al. Alpha-synuclein measured in cerebrospinal fluid from patients with Alzheimer’s disease, mild cognitive impairment, or healthy controls: a two year follow-up study. BMC Neurol 16, 180, https://doi.org/10.1186/s12883-016-0706-0 (2016).
Korff, A. et al. Alpha-Synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 36, 679–688, https://doi.org/10.3233/JAD-130458 (2013).
Gallardo, G., Schluter, O. M. & Sudhof, T. C. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci 11, 301–308, https://doi.org/10.1038/nn2058 (2008).
Nielsen, H. M. et al. Apolipoprotein e affects neuronal alpha-synuclein uptake in an isoform-dependent manner. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11, P231, https://doi.org/10.1016/j.jalz.2015.07.270.
Wersinger, C., Prou, D., Vernier, P. & Sidhu, A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J 17, 2151–2153, https://doi.org/10.1096/fj.03-0152fje (2003).
Sidhu, A., Wersinger, C. & Vernier, P. Alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson’s disease. FEBS Lett 565, 1–5, https://doi.org/10.1016/j.febslet.2004.03.063 (2004).
Kovacs, G. G., Milenkovic, I. J., Preusser, M. & Budka, H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov Disord 23, 1608–1612, https://doi.org/10.1002/mds.22207 (2008).
Bellucci, A. et al. Redistribution of DAT/alpha-synuclein complexes visualized by “in situ” proximity ligation assay in transgenic mice modelling early Parkinson’s disease. PLoS One 6, e27959, https://doi.org/10.1371/journal.pone.0027959 (2011).
Kim, J., Basak, J. M. & Holtzman, D. M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303, https://doi.org/10.1016/j.neuron.2009.06.026 (2009).
Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H. & Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118, https://doi.org/10.1038/nrneurol.2012.263 (2013).
Prince, J. A., Zetterberg, H., Andreasen, N., Marcusson, J. & Blennow, K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology 62, 2116–2118 (2004).
Iwata, A., Miura, S., Kanazawa, I., Sawada, M. & Nukina, N. alpha-Synuclein forms a complex with transcription factor Elk-1. J Neurochem 77, 239–252 (2001).
Acknowledgements
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including abbVie, Avid, Biogen, Bristol-Myers Squibb, COVANCE, GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Lilly, Merck, MesoScaleDiscovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, TEVA, and UCB. This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2017R1D1A1B03033235).
Author information
Authors and Affiliations
Contributions
Kyoungjune Pak, Seunghyeon Shin: study design, image analysis, write the manuscript So Jung Kim, Keunyoung Kim, Bum Soo Kim, Seong Jang Kim: data analysis In Joo Kim: study design, image analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pak, K., Shin, S., Kim, S.J. et al. Correlation of Plasma EGF with Striatal Dopamine Transporter Availability in Healthy Subjects. Sci Rep 7, 13261 (2017). https://doi.org/10.1038/s41598-017-13771-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13771-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.