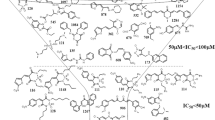
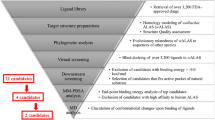
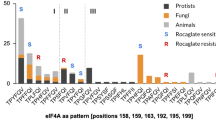
Abstract
Diseases caused by parasitic flatworms impart a considerable healthcare burden worldwide. Many of these diseases—for example, the parasitic blood fluke infection schistosomiasis—are treated with the drug praziquantel (PZQ). However, PZQ is ineffective against disease caused by liver flukes from the genus Fasciola because of a single amino acid change within the target of PZQ, a transient receptor potential ion channel in the melastatin family (TRPMPZQ), in Fasciola species. Here, we identify benzamidoquinazolinone analogs that are active against Fasciola TRPMPZQ. Structure–activity studies define an optimized ligand (BZQ) that caused protracted paralysis and tegumental damage to these liver flukes. BZQ also retained activity against Schistosoma mansoni comparable to PZQ and was active against TRPMPZQ orthologs in all profiled species of parasitic fluke. This broad-spectrum activity manifests as BZQ adopts a pose within the binding pocket of TRPMPZQ that is dependent on a ubiquitously conserved residue. BZQ therefore acts as a universal activator of trematode TRPMPZQ and a first-in-class, broad-spectrum flukicide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the main article, Extended Data, Supplementary Materials and the accompanying source data for each figure. Source data are provided with this paper.
References
Andrews, P. et al. Praziquantel. Med. Res. Rev. 3, 147–200 (1983).
Waechtler, A. et al. Praziquantel—50 years of research. ChemMedChem 18, e202300154 (2023).
Spangenberg, T. Alternatives to praziquantel for the prevention and control of schistosomiasis. ACS Infect. Dis. 7, 939–942 (2021).
Farid, Z. et al. Praziquantel and Fasciola hepatica infection. Trans. R. Soc. Trop. Med. Hyg. 83, 813 (1989).
Arafa, W. M. et al. Comparing an in vivo egg reduction test and in vitro egg hatching assay for different anthelmintics against Fasciola species, in cattle. Vet. Parasitol. 214, 152–158 (2015).
Mehmood, K. et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Micro. Pathog. 109, 253–262 (2017).
Beesley, N. J. et al. A major locus confers triclabendazole resistance in Fasciola hepatica and shows dominant inheritance. PLoS Pathog. 19, e1011081 (2023).
Fairweather, I. et al. Drug resistance in liver flukes. Int J. Parasitol. Drugs Drug Resist. 12, 39–59 (2020).
Kelley, J. M. et al. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 32, 458–469 (2016).
McVeigh, P. et al. Reasons to be nervous about flukicide discovery. Trends Parasitol. 34, 184–196 (2018).
Selzer, P. M. & Epe, C. Antiparasitics in animal health: quo vadis? Trends Parasitol. 37, 77–89 (2021).
Park, S. K. et al. The anthelmintic drug praziquantel activates a schistosome transient receptor potential channel. J. Biol. Chem. 294, 18873–18880 (2019).
Park, S. K. & Marchant, J. S. The journey to discovering a flatworm target of praziquantel: a long TRP. Trends Parasitol. 36, 182–194 (2020).
Harder, A. Activation of transient receptor potential channel Sm.(Schistosoma mansoni)TRPMPZQ by PZQ, enhanced Ca++ influx, spastic paralysis, and tegumental disrupture—the deadly cascade in parasitic schistosomes, other trematodes, and cestodes. Parasitol. Res. 119, 2371–2382 (2020).
Park, S. K. et al. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci. Transl. Med. 13, eabj5832 (2021).
Rohr, C. M. et al. Natural variation in the binding pocket of a parasitic flatworm TRPM channel resolves the basis for praziquantel sensitivity. Proc. Natl Acad. Sci. USA 120, e2217732120 (2023).
Chulkov, E. G. et al. Identification of novel modulators of a schistosome transient receptor potential channel targeted by praziquantel. PLoS Negl. Trop. Dis. 15, e0009898 (2021).
Fairweather, I. & Boray, J. C. Fasciolicides: efficacy, actions, resistance and its management. Vet. J. 158, 81–112 (1999).
Setshedi, K. J. et al. 2-Aroyl quinazolinone: synthesis and in vitro anti-parasitic activity. Chem. Biol. Drug Des. 102, 763–772 (2023).
Ashraf-Uz-Zaman, M. et al. Quinazolinone compounds have potent antiviral activity against Zika and dengue virus. J. Med. Chem. 66, 10746–10760 (2023).
Pan, M. et al. Asymmetric synthesis of N–N axially chiral compounds by phase-transfer-catalyzed alkylations. Org. Lett. 24, 374–378 (2022).
Lin, W. et al. Asymmetric synthesis of N–N axially chiral compounds via organocatalytic atroposelective N-acylation. Chem. Sci. 13, 141–148 (2022).
Yin, Y. & Lee, S. Y. Current view of ligand and lipid recognition by the menthol receptor TRPM8. Trends Biochem. Sci. 45, 806–819 (2020).
Chulkov, E. G., Rohr, C. M. & Marchant, J. S. Praziquantel activates a native cation current in Schistosoma mansoni. Front. Parasitol. 2, 1285177 (2023).
Karnovsky, M. J. A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 27, 137–138A (1965).
Glauert, A. M. & Lewis, P. R. Biological Specimen Preparation for Transmission Electron Microscopy (Princeton Univ. Press, 1998).
Acknowledgements
Funding was provided by the National Institutes of Health (NIH) (R01 AI155405 to J.S.M.), a Catalyst Award from the Falk Medical Research Trust (to J.S.M.), the German Research Foundation (HA 6963/2-1 to S.H.) and the Hessen State Ministry of Higher Education, Research, and the Arts (HMWK) (DRUID-C6 to S.H.). D.J.S. acknowledges support from the NIH (T32 HL134643) and the MCW Cardiovascular Center’s A.O. Smith Fellowship Scholars Program. Screening used equipment procured through an NIH equipment grant (1S10OD032248-01 to T.P.S. and L.S.). We acknowledge the Research Computing Center at the Medical College of Wisconsin, and P. Kerber and F. Peterson for maintaining the MCW NMR facilities, the Indiana University Mass Spectrometry Center for HRMS analysis, the MCW Electron Microscopy Core and the Imaging Unit of the Biomedical Research Center Seltersberg.
Author information
Authors and Affiliations
Contributions
D.J.S. and J.S.M. conceived the study. D.J.S., S.K.P., S.G., L.B., C.M.R., E.G.C. and E.S. performed experiments, analyzed data and generated figures. L.S., T.P.S., S.H. and J.S.M. supervised the work. D.J.S. and J.S.M. wrote the manuscript with input from all authors. All authors participated in revisions and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
J.S.M., D.J.S., L.S. and T.P.S. have pending patent applications for the compounds described in this study. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dose-response curves of Different Northern Hemisphere Analogs.
Responses of Sm.TRPMPZQ to the molecules are shown in blue circles, Fh.TRPMPZQ in red circles, and control (untransfected cells) red diamonds. All data are presented as mean ± SEM. N = 3 biological replicates comprised of technical duplicates except for the following: untransfected cells, technical duplicates; compounds 4 and 7, Sm.TRPMPZQ and Fh.TRPMPZQ, n = 9 biological replicates comprised of technical duplicates and compound 9, Sm.TRPMPZQ and Fh.TRPMPZQ, n = 4 biological replicates comprised of technical duplicates.
Extended Data Fig. 2 Dose-response curves of Different Southern Hemisphere Analogs.
Responses of Sm.TRPMPZQ to the molecules are shown in blue circles, Fh.TRPMPZQ in red circles, and control (untransfected cells) red diamonds. All data are presented as mean ± SEM. N = 3 biological replicates comprised of technical duplicates except for the following: untransfected cells, technical duplicates; compound 27, Fh.TRPMPZQ, n = 4 biological replicates comprised of technical duplicates; compound 30, Sm.TRPMPZQ, n = 9 biological replicates comprised of technical duplicates.
Extended Data Fig. 3 Dose-response curves of Different Aromatic Core Analogs.
Responses of Sm.TRPMPZQ to the drugs are shown in blue circles, Fh.TRPMPZQ in red circles, and control (untransfected cells) red diamonds. All data are presented as mean ± SEM of n = 3 biological replicates comprised of technical duplicates except for untransfected cells which are presented as technical duplicates.
Extended Data Fig. 4 Effects of BZQ on F. hepatica after prolonged culture.
Tegumental damage in F. hepatica after extended treatment with BZQ. Immature liver flukes were treated for 72 h with BZQ (12.5 µM) or DMSO (0.5%) as control and ventral surfaces were imaged by scanning electron microscopy. Large collapsed blisters (arrows) were found after exposure (two identical replicates are shown). Scale bars = 500 µm (top row) and 10 µm (bottom row).
Extended Data Fig. 5 Effects of various benzamidoquinazolinones on F. hepatica.
(A–C) Motility of (A) adult, (B) triclabendazole (TCBZ)-sensitive immature, and (C) TCBZ-resistant immature F. hepatica after treatment with compound 1 (gold triangles) and compound 6 (purple triangles) compared with application of TCBZ (open circles) or DMSO (1.25%, open grey squares). Panel A: DMSO and both TCBZ, n = 3 flukes each; compounds 1 and 6, n = 4 flukes each. Panel B: DMSO and TCBZ, n = 4 flukes each; compound 1, n = 10 flukes; compound 6, n = 9 flukes. Panel C: DMSO, n = 6 flukes; TCBZ, n = 4 flukes; compound 1, n = 10 flukes; compound 6, n = 9 flukes; all data presented as mean ± SEM. (D–I) Tegumental damage in F. hepatica. Immature liver flukes were treated for 24 h with DMSO (control, D & E), Cmpd 1 (6.25 µM, F & G) or compound 6 (6.25 µM, H & I). 1 and 6 caused bleb formation (arrows) after exposure. Images are representative of three replicates. Scale bars = 500 µm (top row) and 10 µm (bottom row).
Supplementary information
Supplementary Information
Synthetic Chemistry Procedures and Characterization Data (Supplementary Figs. 1–143).
Supplementary Video 1
Vehicle-treated (DMSO, 1.25%) adult F. hepatica. Treatment with vehicle has no effect on fluke motility, and the typical undulating movement is still observed.
Supplementary Video 2
(±)-PZQ-treated (50 µM) adult F. hepatica. Treatment with (±)-PZQ has no effect on fluke motility compared with vehicle treatment (Supplementary Video 1), and the typical undulating movement is observed.
Supplementary Video 3
BZQ-treated (6.25 µM) adult F. hepatica. Treatment of the liver fluke with BZQ causes rapid and protracted contraction and paralysis when compared with vehicle treatment (Supplementary Video 1). The video shows a fully contracted and paralyzed adult liver fluke.
Supplementary Video 4
Supplementary Video 4. TCBZ-treated (50 µM) adult F. hepatica. Treatment of the liver fluke with TCBZ has detrimental effects, observed as decreased motion with little undulation compared with vehicle-treated worms (Supplementary Video 1). This is different than the contractile, spastic paralysis observed upon BZQ treatment (Supplementary Video 3).
Supplementary Code
Exported XYZ coordinates (from Maestro) for the model of BZQ in Sm.TRPMPZQ as depicted in Fig. 4.
Source data
Source Data Fig. 1
Values for plotted data in Fig. 1a–f,i,j.
Source Data Fig. 2
Values for plotted data in Fig. 2b,d,e.
Source Data Fig. 3
Values for plotted data in Fig. 3i–m.
Source Data Fig. 4
Values for plotted data in Fig. 4e,f.
Source Data Extended Data Fig. 1
Values for concentration–response curves plotted in Extended Fig. 1.
Source Data Extended Data Fig. 2
Values for concentration–response curves plotted in Extended Fig. 2.
Source Data Extended Data Fig. 3
Values for concentration–response curves plotted in Extended Fig. 3.
Source Data Extended Data Fig. 5
Values for data plotted in panels A-C.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sprague, D.J., Park, SK., Gramberg, S. et al. Target-based discovery of a broad-spectrum flukicide. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01298-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01298-3