Abstract
The greatest challenge in cancer therapy is to eradicate cancer cells with minimal damage to normal cells. Targeted therapy has been developed to meet that challenge, showing a substantially increased therapeutic index compared with conventional cancer therapies. Antibodies are important members of the family of targeted therapeutic agents because of their extraordinarily high specificity to the target antigens. Therapeutic antibodies use a range of mechanisms that directly or indirectly kill the cancer cells. Early antibodies were developed to directly antagonize targets on cancer cells. This was followed by advancements in linker technologies that allowed the production of antibody–drug conjugates (ADCs) that guide cytotoxic payloads to the cancer cells. Improvement in our understanding of the biology of T cells led to the production of immune checkpoint-inhibiting antibodies that indirectly kill the cancer cells through activation of the T cells. Even more recently, bispecific antibodies were synthetically designed to redirect the T cells of a patient to kill the cancer cells. In this Review, we summarize the different approaches used by therapeutic antibodies to target cancer cells. We discuss their mechanisms of action, the structural basis for target specificity, clinical applications and the ongoing research to improve efficacy and reduce toxicity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data used to make Figs. 1 and 6 are available from the American Cancer Society and the Antibody Society.
References
Sternberger, L. A. & Sternberger, N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl Acad. Sci. USA 80, 6126–6130 (1983).
Stark, S. E. & Caton, A. J. Antibodies that are specific for a single amino acid interchange in a protein epitope use structurally distinct variable regions. J. Exp. Med. 174, 613–624 (1991).
Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society www.antibodysociety.org/resources/approved-antibodies (2023). The Antibody Society is an association that supports research and development of antibody-based drugs and maintains an updated list of antibodies approved by the FDA and EMA.
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 1, 118–129 (2001).
Weiner, G. J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 15, 361–370 (2015).
Ho, M. Inaugural editorial: searching for magic bullets. Antib. Ther. 1, 1–5 (2018).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975). This seminal paper describes the hybridoma technology that enabled the production of monoclonal antibodies. Kohler and Milstein received the Nobel Prize for this work.
Hwang, W. Y. & Foote, J. Immunogenicity of engineered antibodies. Methods 36, 3–10 (2005).
Reff, M. E. et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83, 435–445 (1994). This paper describes the generation of rituximab, the first chimeric antibody that demonstrated significant tumour reduction and later became the standard of care for the treatment of patients with B cell lymphomas.
Goldstein, N. I., Prewett, M., Zuklys, K., Rockwell, P. & Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1, 1311–1318 (1995).
Looney, R. J. et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 50, 2580–2589 (2004).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986). This paper describes the process of CDR grafting that enabled the production of humanized antibodies. The majority of cancer antibodies utilize the humanized antibody format.
Carter, P. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Spieker-Polet, H., Sethupathi, P., Yam, P. C. & Knight, K. L. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl Acad. Sci. USA 92, 9348–9352 (1995).
Zhang, Y. F. & Ho, M. Humanization of rabbit monoclonal antibodies via grafting combined Kabat/IMGT/Paratome complementarity-determining regions: rationale and examples. MAbs 9, 419–429 (2017).
Parray, H. A. et al. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 85, 106639 (2020).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990). This paper describes the early phage display technologies that enabled the selection of antibodies against a range of antigens including cancer antigens. G. Smith and G. Winter received the Nobel Prize for their work on phage display-based antibody production.
Lonberg, N. et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 368, 856–859 (1994). This paper describes the development of transgenic mouse models that led to the production of fully human antibodies.
Lu, R. M. et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1 (2020).
Winter, G., Griffiths, A. D., Hawkins, R. E. & Hoogenboom, H. R. Making antibodies by phage display technology. Annu. Rev. Immunol. 12, 433–455 (1994).
Lu, S. et al. The rapid and highly parallel identification of antibodies with defined biological activities by SLISY. Nat. Commun. 14, 17 (2023).
Alfaleh, M. A. et al. Phage display derived monoclonal antibodies: from bench to bedside. Front. Immunol. 11, 1986 (2020).
Almagro, J. C., Daniels-Wells, T. R., Perez-Tapia, S. M. & Penichet, M. L. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front. Immunol. 8, 1751 (2017).
Booth, B. Human antibody discovery: of mice and phage. Forbes https://www.forbes.com/sites/brucebooth/2017/05/11/human-antibody-discovery-of-mice-and-phage/?sh=1f76520c7f26 (2017).
Jain, T. et al. Biophysical properties of the clinical-stage antibody landscape. Proc. Natl Acad. Sci. USA 114, 944–949 (2017).
Douglass, J. et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 6, eabd5515 (2021).
Hsiue, E. H. et al. Targeting a neoantigen derived from a common TP53 mutation. Science 371, eabc8697 (2021). This paper describes the generation of bispecific antibodies targeting the most common oncogenic mutation (R175H) in the tumour suppressor protein p53.
Boder, E. T., Midelfort, K. S. & Wittrup, K. D. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc. Natl Acad. Sci. USA 97, 10701–10705 (2000).
Georgiou, G. et al. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat. Biotechnol. 15, 29–34 (1997).
Ho, M., Nagata, S. & Pastan, I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl Acad. Sci. USA 103, 9637–9642 (2006).
Lipovsek, D. & Pluckthun, A. In-vitro protein evolution by ribosome display and mRNA display. J. Immunol. Methods 290, 51–67 (2004).
Wang, J. et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs 11, 1443–1451 (2019).
Marcus, R. et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 377, 1331–1344 (2017).
Vitolo, U. et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J. Clin. Oncol. 35, 3529–3537 (2017).
van Imhoff, G. W. et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J. Clin. Oncol. 35, 544–551 (2017).
Maloney, D. G. et al. A phase 3 randomized study (HOMER) of ofatumumab vs rituximab in iNHL relapsed after rituximab-containing therapy. Blood Adv. 4, 3886–3893 (2020).
Rugo, H. S. et al. Margetuximab versus trastuzumab in patients with previously treated HER2-positive advanced breast cancer (SOPHIA): final overall survival results from a randomized phase 3 trial. J. Clin. Oncol. 41, 198–205 (2023).
Price, T. J. et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 15, 569–579 (2014).
Furman, W. L. Monoclonal antibody therapies for high risk neuroblastoma. Biologics 15, 205–219 (2021).
Peter, H. H. et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J. Allergy Clin. Immunol. 146, 479–491.e5 (2020).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001). This is a groundbreaking clinical trial demonstrating the survival benefit of the HER2-targeting antibody trastuzumab in patients with breast cancer. Slamon received the Lasker–DeBakey award for his work related to HER2 targeting in breast cancer.
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Camidge, D. R. et al. Phase I study of 2- or 3-week dosing of telisotuzumab vedotin, an antibody-drug conjugate targeting c-Met, monotherapy in patients with advanced non-small cell lung carcinoma. Clin. Cancer Res. 27, 5781–5792 (2021).
Redman, J. M., Hill, E. M., AlDeghaither, D. & Weiner, L. M. Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 67, 28–45 (2015).
Mone, A. P. et al. Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia 20, 272–279 (2006).
Meyer, S. et al. New insights in type I and II CD20 antibody mechanisms-of-action with a panel of novel CD20 antibodies. Br. J. Haematol. 180, 808–820 (2018).
Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520 (2014).
Salfeld, J. G. Isotype selection in antibody engineering. Nat. Biotechnol. 25, 1369–1372 (2007).
Yu, J., Song, Y. & Tian, W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 13, 45 (2020).
Wang, X., Mathieu, M. & Brezski, R. J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 9, 63–73 (2018).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Golay, J. & Taylor, R. P. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies 9, 58 (2020).
Schneider-Merck, T. et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J. Immunol. 184, 512–520 (2010).
Rosner, T. et al. Immune effector functions of human IgG2 antibodies against EGFR. Mol. Cancer Ther. 18, 75–88 (2019).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003). This study of early structural biology has improved our understanding of trastuzumab binding to the HER2 extracellular subdomain 4 to inhibit HER2 function.
Hao, Y., Yu, X., Bai, Y., McBride, H. J. & Huang, X. Cryo-EM structure of HER2-trastuzumab-pertuzumab complex. PLoS ONE 14, e0216095 (2019).
Weisser, N. E. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat. Commun. 14, 1394 (2023).
Rouge, L. et al. Structure of CD20 in complex with the therapeutic monoclonal antibody rituximab. Science 367, 1224–1230 (2020).
Liu, R., Oldham, R. J., Teal, E., Beers, S. A. & Cragg, M. S. Fc-engineering for modulated effector functions-improving antibodies for cancer treatment. Antibodies 9, 64 (2020).
Nimmerjahn, F., Vidarsson, G. & Cragg, M. S. Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy. Nat. Immunol. 24, 1244–1255 (2023). This review describes the Fc domain modifications that alter IgG antibody effector functions.
Rugo, H. S. et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 7, 573–584 (2021).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017). This paper demonstrates the utility of the PD1 antibody, pembrolizumab, in a range of cancer types with mismatch repair deficiency. This led to the FDA approval of pembrolizumab in any cancer with mismatch repair deficiency which was the first tissue agnostic drug approval in oncology.
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Nghiem, P. T. et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 374, 2542–2552 (2016).
Sharma, P. et al. The next decade of immune checkpoint therapy. Cancer Discov. 11, 838–857 (2021).
Chamoto, K., Yaguchi, T., Tajima, M. & Honjo, T. Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1. Nat. Rev. Immunol. 23, 682–695 (2023). Together with Sharma et al. (2021), this review describes the mechanism of immune checkpoint-inhibiting antibodies in cancer. Allison and Honjo jointly received the Nobel Prize for their discoveries of a cancer therapy by inhibition of the immune checkpoint PD1.
Huard, B. et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl Acad. Sci. USA 94, 5744–5749 (1997).
Wang, J. et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 176, 334–347.e12 (2019).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Romano, E. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA 112, 6140–6145 (2015).
Kim, M. J. et al. Deletion of PD-1 destabilizes the lineage identity and metabolic fitness of tumor-infiltrating regulatory T cells. Nat. Immunol. 24, 148–161 (2023).
Sharma, A. et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 25, 1233–1238 (2019).
Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008 (2019).
Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346–1358 (2020).
Scapin, G. et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat. Struct. Mol. Biol. 22, 953–958 (2015).
You, W. et al. A network meta-analysis comparing the efficacy and safety of anti-PD-1 with anti-PD-L1 in non-small cell lung cancer. J. Cancer 9, 1200–1206 (2018).
Barlesi, F. et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 19, 1468–1479 (2018).
Lee, N. Y. et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 22, 450–462 (2021).
Monk, B. J. et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 22, 1275–1289 (2021).
Bang, Y. J. et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann. Oncol. 29, 2052–2060 (2018).
Lee, J. Y. et al. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat. Commun. 7, 13354 (2016).
Lee, H. T. et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 7, 5532 (2017).
Lee, H. T., Lee, S. H. & Heo, Y. S. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules 24, 1190 (2019).
Lin, D. Y. et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl Acad. Sci. USA 105, 3011–3016 (2008).
Liu, K. et al. Structural basis of anti-PD-L1 monoclonal antibody avelumab for tumor therapy. Cell Res. 27, 151–153 (2017).
Tang, S. & Kim, P. S. A high-affinity human PD-1/PD-L2 complex informs avenues for small-molecule immune checkpoint drug discovery. Proc. Natl Acad. Sci. USA 116, 24500–24506 (2019).
He, M. et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget 8, 67129–67139 (2017).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 36, 1714–1768 (2018).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Runcie, K., Budman, D. R., John, V. & Seetharamu, N. Bi-specific and tri-specific antibodies — the next big thing in solid tumor therapeutics. Mol. Med. 24, 50 (2018).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008). This paper describes the first-in-human clinical trial of the CD19-targeting bispecific antibody blinatumomab, which was the first bispecific antibody to achieve widespread adoption in the clinic and subsequently set the stage for the widespread adoption of the bispecific antibody format.
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019). This detailed review describes the different formats of bispecific antibodies.
Liddy, N. et al. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 18, 980–987 (2012).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
Ahn, M. J. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N. Engl. J. Med. 389, 2063–2075 (2023).
Park, K. et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J. Clin. Oncol. 39, 3391–3402 (2021).
Moores, S. L. et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 76, 3942–3953 (2016).
Dixit, S., Abraham, L., Weiser, N. & Gold, M. R. Super-resolution imaging studies of zanidatamab: providing insights into its bispecific mode of action. Cancer Res. 81, abstr. 1032 (2021).
Weiser, N. E. et al. The bispecific antibody zanidatamab’s (ZW25’s) unique mechanisms of action and durable anti-tumor activity in HER2-expressing cancers. Cancer Res. 81, abstr. 1005 (2021).
Meric-Bernstam, F. et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 23, 1558–1570 (2022).
Falchi, L., Vardhana, S. A. & Salles, G. A. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities. Blood 141, 467–480 (2023).
Tsuchikama, K., Anami, Y., Ha, S. Y. Y. & Yamazaki, C. M. Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 21, 203–223 (2024). This review focuses on the current state and future directions of ADCs in oncology.
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 7, 93 (2022).
Stapleton, N. M. et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2, 599 (2011).
Natsume, A., Niwa, R. & Satoh, M. Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC. Drug Des. Dev. Ther. 3, 7–16 (2009).
Flygare, J. A., Pillow, T. H. & Aristoff, P. Antibody-drug conjugates for the treatment of cancer. Chem. Biol. Drug Des. 81, 113–121 (2013).
Hingorani, D. V. et al. Monomethyl auristatin antibody and peptide drug conjugates for trimodal cancer chemo-radio-immunotherapy. Nat. Commun. 13, 3869 (2022).
Francisco, J. A. et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102, 1458–1465 (2003). This study describes the generation of a CD30-targeting ADC using a peptide cleavable linker. The ADC was later named brentuximab, and it is considered standard first-line therapy for patients with Hodgkin lymphoma and other CD30+ lymphomas. Multiple approved ADCs targeting other cancer antigens now use this drug–linker combination.
Ansell, S. M. et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 387, 310–320 (2022).
Horwitz, S. et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 33, 288–298 (2022).
Zhang, D. et al. Catalytic cleavage of disulfide bonds in small molecules and linkers of antibody-drug conjugates. Drug Metab. Dispos. 47, 1156–1163 (2019).
Moore, K. N. et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N. Engl. J. Med. 389, 2162–2174 (2023).
Kovtun, Y. V. et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66, 3214–3221 (2006).
Oflazoglu, E. et al. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin. Cancer Res. 14, 6171–6180 (2008).
Conilh, L., Sadilkova, L., Viricel, W. & Dumontet, C. Payload diversification: a key step in the development of antibody-drug conjugates. J. Hematol. Oncol. 16, 3 (2023).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016). This paper describes the generation of tetrapeptide linker that masks the hydrophobicity of the payload and allows attachment of a large number of hydrophobic payloads without affecting antibody pharmacokinetics. This linker–drug combination is now being used in multiple ADCs that are nearing regulatory approval.
Zammarchi, F. et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 131, 1094–1105 (2018).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Cortes, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022). This phase III clinical trial directly compares two HER2-targeting ADCs, showing the benefit of the ADC trastuzumab deruxtecan, which carries the payload DXd attached by a cleavable linker and possesses a high DAR.
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Kreitman, R. J. & Pastan, I. Immunotoxins: from design to clinical application. Biomolecules 11, 1696 (2021).
Li, M., Mei, S., Yang, Y., Shen, Y. & Chen, L. Strategies to mitigate the on- and off-target toxicities of recombinant immunotoxins: an antibody engineering perspective. Antib. Ther. 5, 164–176 (2022).
Kreitman, R. J. & Pastan, I. Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin. Cancer Res. 17, 6398–6405 (2011). This review of immunotoxins shows the development of the first cancer-targeting immunotoxin moxetumomab that received FDA approval for the treatment of hairy cell leukaemia.
Lin, P., Qi, J. & Liu, W. Expert’s views and perspectives: an interview with distinguished investigator Dr. Ira Pastan at the National Cancer Institute at NIH. Antib. Ther. 3, 163–166 (2020).
Kreitman, R. J. et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia 32, 1768–1777 (2018).
Kreitman, R. J. & Pastan, I. Contextualizing the use of moxetumomab pasudotox in the treatment of relapsed or refractory hairy cell leukemia. Oncologist 25, e170–e177 (2020).
Kim, Y. S. & Brechbiel, M. W. An overview of targeted alpha therapy. Tumour Biol. 33, 573–590 (2012).
Parakh, S., Lee, S. T., Gan, H. K. & Scott, A. M. Radiolabeled antibodies for cancer imaging and therapy. Cancers 14, 1454 (2022).
Witzig, T. E. et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 20, 2453–2463 (2002).
Kaminski, M. S. et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J. Clin. Oncol. 19, 3918–3928 (2001).
Prasad, V. The withdrawal of drugs for commercial reasons: the incomplete story of tositumomab. JAMA Intern. Med. 174, 1887–1888 (2014).
Alhaj Moustafa, M. et al. Real world long-term follow-up experience with yttrium-90 ibritumomab tiuxetan in previously untreated patients with low-grade follicular lymphoma and marginal zone lymphoma. Clin. Lymphoma Myeloma Leuk. 22, 618–625 (2022).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 19, 589–608 (2020).
Dowden, H. & Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 18, 495–496 (2019).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Smietana, K., Siatkowski, M. & Moller, M. Trends in clinical success rates. Nat. Rev. Drug Discov. 15, 379–380 (2016).
Pham, E. et al. Preclinical assessment of a MUC12-targeted BiTE (bispecific T-cell engager) molecule. Mol. Cancer Ther. 20, 1977–1987 (2021).
Kebenko, M. et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE(R)) antibody construct, in patients with refractory solid tumors. Oncoimmunology 7, e1450710 (2018).
DeFrancesco, L. Cartography biosciences and nested therapeutics: diamonds in the rough. Nat. Biotechnol. https://doi.org/10.1038/d41587-023-00013-9 (2023).
Mullard, A. Claudin-18.2 attracts the cancer crowd. Nat. Rev. Drug Discov. 22, 683–686 (2023).
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat. Med. 29, 2133–2141 (2023).
Janne, P. A. et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 12, 74–89 (2022).
Wainberg, Z. A. et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 23, 1430–1440 (2022).
Paz-Ares, L. et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: an open-label, phase I study. J. Clin. Oncol. 41, 2893–2903 (2023).
Picozzi, V. et al. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open 5, e000668 (2020).
Kelly, W. K. et al. Xaluritamig, a STEAP1 x CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 14, 76–89 (2024).
Dannenfelser, R. et al. Discriminatory power of combinatorial antigen recognition in cancer T cell therapies. Cell Syst. 11, 215–228.e5 (2020).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 31, 71–75 (2013).
Banaszek, A. et al. On-target restoration of a split T cell-engaging antibody for precision immunotherapy. Nat. Commun. 10, 5387 (2019).
Oostindie, S. C. et al. Logic-gated antibody pairs that selectively act on cells co-expressing two antigens. Nat. Biotechnol. 40, 1509–1519 (2022).
Iozzo, R. V. & Schaefer, L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 (2015).
Ilieva, K. M. et al. Chondroitin sulfate proteoglycan 4 and its potential as an antibody immunotherapy target across different tumor types. Front Immunol. 8, 1911 (2018).
Filmus, J., Capurro, M. & Rast, J. Glypicans. Genome Biol. 9, 224 (2008).
Ho, M. & Kim, H. Glypican-3: a new target for cancer immunotherapy. Eur. J. Cancer 47, 333–338 (2011).
Li, N., Gao, W., Zhang, Y. F. & Ho, M. Glypicans as cancer therapeutic targets. Trends Cancer 4, 741–754 (2018).
Li, N. et al. The IgG4 hinge with CD28 transmembrane domain improves V(H)H-based CAR T cells targeting a membrane-distal epitope of GPC1 in pancreatic cancer. Nat. Commun. 14, 1986 (2023).
Kato, D. et al. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab. eLife 9, e49392 (2020).
Li, N., Fu, H., Hewitt, S. M., Dimitrov, D. S. & Ho, M. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc. Natl Acad. Sci. USA 114, E6623–E6631 (2017).
Bosse, K. R. et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell 32, 295–309.e12 (2017).
Li, N. et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Rep. Med. 2, 100297 (2021).
Phung, Y., Gao, W., Man, Y.-G., Nagata, S. & Ho, M. High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. mAbs 4, 592–599 (2012).
Li, D. et al. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology 158, 2250–2265.e20 (2020).
Nakano, K. et al. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 378, 279–284 (2009).
Shi, D. et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin. Cancer Res. 26, 3979–3989 (2020).
Loffler, A. et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95, 2098–2103 (2000). This paper describes the production of large quantities of a stable bispecific antibody targeting CD19 on B cell lymphomas. The CD19-targeting bispecific antibody was later named blinatumomab and was the first successful bispecific antibody that gained widespread adoption for cancer treatment.
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017). This phase III clinical trial establishes the survival benefit of blinatumomab therapy over chemotherapy in patients with ALL.
Maloney, D. G. et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84, 2457–2466 (1994).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
de Weers, M. et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 186, 1840–1848 (2011).
Carpenter, R. O. et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 19, 2048–2060 (2013).
Frerichs, K. A. et al. Preclinical activity of JNJ-7957, a novel BCMAxCD3 bispecific antibody for the treatment of multiple myeloma, is potentiated by daratumumab. Clin. Cancer Res. 26, 2203–2215 (2020).
Nichakawade, T. D. et al. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers. Nature https://doi.org/10.1038/s41586-024-07233-2 (2024).
Paul, S. et al. TCR β chain-directed bispecific antibodies for the treatment of T cell cancers. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abd3595 (2021).
Maciocia, P. M. et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 23, 1416–1423 (2017).
Pearlman, A. H. et al. Targeting public neoantigens for cancer immunotherapy. Nat. Cancer 2, 487–497 (2021). This review describes the prevalence of targetable public neoantigens in cancer.
Hwang, M. S. et al. Structural engineering of chimeric antigen receptors targeting HLA-restricted neoantigens. Nat. Commun. 12, 5271 (2021).
Hattori, T. et al. Creating MHC-restricted neoantigens with covalent inhibitors that can be targeted by immune therapy. Cancer Discov. 13, 132–145 (2023).
Wright, K. M. et al. Hydrophobic interactions dominate the recognition of a KRAS G12V neoantigen. Nat. Commun. 14, 5063 (2023).
Wu, D., Gallagher, D. T., Gowthaman, R., Pierce, B. G. & Mariuzza, R. A. Structural basis for oligoclonal T cell recognition of a shared p53 cancer neoantigen. Nat. Commun. 11, 2908 (2020).
Trenevska, I., Li, D. & Banham, A. H. Therapeutic antibodies against intracellular tumor antigens. Front. Immunol. 8, 1001 (2017).
Hamid, O. et al. 728O results from phase I dose escalation of IMC-F106C, the first PRAME × CD3 ImmTAC bispecific protein in solid tumors. Ann. Oncol. 33, S875 (2022).
Hofheinz, R. D. et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26, 44–48 (2003).
Scott, A. M. et al. A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 9, 1639–1647 (2003).
Benson, A. B. III et al. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 22, 241-e15 (2017).
Hecht, J. R. et al. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the second-line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 22, 243-e23 (2017).
Bejarano, L., Jordao, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Gomez-Roca, C. et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naive or experienced for immune checkpoint blockade. J. Immunother. Cancer 10, e004076 (2022).
Ira Seth, W. et al. A phase 1a dose-escalation study of PY159, a monoclonal antibody targeting TREM1 (triggering receptor expressed on myeloid cells 1). J. Clin. Oncol. 41, 2523 (2023).
Amita P. et al. A phase 1a dose-escalation study of PY314, a TREM2 (triggering receptor expressed on macrophages 2) targeting monoclonal antibody. J. Clin. Oncol. https://doi.org/10.1200/JCO.2022.40.16_suppl.2648 (2022).
Byrne, K. T. & Vonderheide, R. H. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 15, 2719–2732 (2016).
Byrne, K. T. et al. Neoadjuvant selicrelumab, an agonist CD40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin. Cancer Res. 27, 4574–4586 (2021).
O’Hara, M. H. et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. 22, 118–131 (2021).
Curigliano, G. et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 27, 3620–3629 (2021).
Kim, H. R. et al. Cobolimab with dostarlimab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC): COSTAR lung. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2022.07.183 (2022).
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27, 1419–1431 (2021).
Bluhm, J. et al. CAR T cells with enhanced sensitivity to B cell maturation antigen for the targeting of B cell non-Hodgkin’s lymphoma and multiple myeloma. Mol. Ther. 26, 1906–1920 (2018).
Friedman, K. M. et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum. Gene Ther. 29, 585–601 (2018).
Golay, J. et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 98, 3383–3389 (2001).
Teeling, J. L. et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 177, 362–371 (2006).
Perez, H. L. et al. Antibody-drug conjugates: current status and future directions. Drug Discov. Today 19, 869–881 (2014).
Sharma, P. & Kranz, D. M. Recent advances in T-cell engineering for use in immunotherapy. F1000Res https://doi.org/10.12688/f1000research.9073.1 (2016).
Cancer Research Institute. Approval timeline of active immunotherapies. Cancer Research Institute https://www.cancerresearch.org/regulatory-approval-timeline-of-active-immunotherapies (2024).
Johnson, P. C., Gainor, J. F., Sullivan, R. J., Longo, D. L. & Chabner, B. Immune checkpoint inhibitors — the need for innovation. N. Engl. J. Med. 388, 1529–1532 (2023).
Revisiting checkpoint blockade. Nat. Biotechnol. 40, 981 (2022).
Postel-Vinay, S. et al. First-in-human phase I study of the OX40 agonist GSK3174998 with or without pembrolizumab in patients with selected advanced solid tumors (ENGAGE-1). J. Immunother. Cancer 11, e005301 (2023).
Davis, E. J. et al. First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors. J. Immunother. Cancer 10, e004235 (2022).
Andre, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743.e13 (2018).
Qin, S. et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer 18, 155 (2019).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Gilead. Gilead statement on the discontinuation of magrolimab study in AML with TP53 mutations. Gilead https://www.gilead.com/news-and-press/company-statements/gilead-statement-on-the-discontinuation-of-magrolimab-study-in-aml-with-tp53-mutations (2023).
Gilead. Gilead to discontinue phase 3 ENHANCE study of magrolimab plus azacitidine in higher-risk MDS. Gilead https://www.gilead.com/news-and-press/press-room/press-releases/2023/7/gilead-to-discontinue-phase-3-enhance-study-of-magrolimab-plus-azacitidine-in-higher-risk-mds (2023).
Zhao, B., Zhao, H. & Zhao, J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther. Adv. Med. Oncol. 12, 1758835920937612 (2020).
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Ying Cheng et al. Phase I/II combination study of tifcemalimab with toripalimab in patients with refractory extensive stage small cell lung cancer (ES-SCLC). J. Clin. Oncol. 41, 8579 (2023).
Shenderov, E. et al. Neoadjuvant enoblituzumab in localized prostate cancer: a single-arm, phase 2 trial. Nat. Med. 29, 888–897 (2023).
Ansell, S. M. et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 4, 1917–1926 (2020).
Claus, C., Ferrara-Koller, C. & Klein, C. The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. MAbs 15, 2167189 (2023).
Elizabeth Budde, M. et al. Preliminary results of a phase 1 dose escalation study of the first-in-class IgM based bispecific antibody Igm-2323 (anti-CD20 x anti-CD3) in patients with advanced B-cell malignancies. Blood 136, 45–46 (2020).
Wang, B. T. et al. Multimeric anti-DR5 IgM agonist antibody IGM-8444 is a potent inducer of cancer cell apoptosis and synergizes with chemotherapy and BCL-2 inhibitor ABT-199. Mol. Cancer Ther. 20, 2483–2494 (2021).
Li, K., Yun, R., Chai, M., Yakkundi, P. & Rosete, R. Novel CD38xCD3 bispecific IgM T cell engager, IGM-2644, potently kills multiple myeloma cells though complement and T cell dependent mechanisms. Cancer Res. 83, 2959 (2023).
Wang, B. et al. Anti-DR5 agonist IgM antibody IGM-8444 combined with SMAC mimetic birinapant induces strong synergistic tumor cytotoxicity. Cancer Res. 82, 1068 (2022).
Klein, C., Brinkmann, U., Reichert, J. M. & Kontermann, R. E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-024-00896-6 (2024). This review focuses on the current state and future directions of bispecific antibodies in oncology.
Dickinson, M. J. et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 387, 2220–2231 (2022).
Bacac, M. et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin. Cancer Res. 24, 4785–4797 (2018).
Nolan-Stevaux, O. et al. AMG 509 (xaluritamig), an anti-STEAP1 XmAb 2+1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer. Cancer Discov. 14, 90–103 (2024).
Li, G. et al. Novel CD123xCD3 bispecific IgM antibody, IGM-2537, potently induces T-cell mediated cytotoxicity of acute myeloid leukemia cells with minimal cytokine release. Cancer Res. 83, 2933 (2023).
Genevive, H. H. et al. Pharmacodynamics and biomarker correlates of imvotamab (IGM-2323), the first-in-class CD20xCD3 bispecific IgM antibody with dual mechanisms of action, in patients with advanced B cell malignancies. Blood 140, 6436–6438 (2022).
Luke, J. J. et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat. Med. 29, 2814–2824 (2023).
Dovedi, S. J. et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 11, 1100–1117 (2021).
Gao, X. et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 24, 1134–1146 (2023).
Chen, B. et al. A phase Ib/II study of cadonilimab (PD-1/CTLA-4 bispecific antibody) plus anlotinib as first-line treatment in patients with advanced non-small cell lung cancer. Br. J. Cancer 130, 450–456 (2024).
Li, Q. et al. The anti-PD-L1/CTLA-4 bispecific antibody KN046 in combination with nab-paclitaxel in first-line treatment of metastatic triple-negative breast cancer: a multicenter phase II trial. Nat. Commun. 15, 1015 (2024).
Kvarnhammar, A. M. et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J. Immunother. Cancer 7, 103 (2019).
Karin Lee et al. Preclinical studies support clinical development of AZD2936, a monovalent bispecific humanized antibody targeting PD-1 and TIGIT. J. Immunother. Cancer 10, A489 (2023).
Shapir Itai, Y. et al. Bispecific dendritic-T cell engager potentiates anti-tumor immunity. Cell 187, 375–389.e18 (2024).
Zhao, L. et al. A novel CD19/CD22/CD3 trispecific antibody enhances therapeutic efficacy and overcomes immune escape against B-ALL. Blood 140, 1790–1802 (2022).
Wu, L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020).
Pardon, E. et al. A general protocol for the generation of nanobodies for structural biology. Nat. Protoc. 9, 674–693 (2014).
Li, D. et al. Camel nanobody-based B7-H3 CAR-T cells show high efficacy against large solid tumours. Nat. Commun. 14, 5920 (2023).
Xu, J. et al. Nanobodies from camelid mice and llamas neutralize SARS-CoV-2 variants. Nature 595, 278–282 (2021). This paper describes the generation of a transgenic mouse (nanomouse), which expresses camelid variable heavy domain of heavy chain (VHH) genes. Such transgenic mouse platforms enable the production of nanobodies from mice.
Hu, Y. et al. RenNano® mice: a heavy-chain-only antibody platform for the generation of nanobody therapeutics. Cancer Res. 83, LB210 (2023).
De Genst, E. et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl Acad. Sci. USA 103, 4586–4591 (2006).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Jovcevska, I. & Muyldermans, S. The therapeutic potential of nanobodies. BioDrugs 34, 11–26 (2020).
Lucchi, R., Bentanachs, J. & Oller-Salvia, B. The masking game: design of activatable antibodies and mimetics for selective therapeutics and cell control. ACS Cent. Sci. 7, 724–738 (2021). This review describes the recent advancements in activatable antibodies in cancer and other diseases.
Sulea, T. et al. Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. MAbs 12, 1682866 (2020).
Kang, J. C. et al. Engineering a HER2-specific antibody-drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 37, 523–526 (2019).
Zhang, Y. et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 29, 609–627 (2019).
Desnoyers, L. R. et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 5, 207ra144 (2013).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Boni, V. et al. Praluzatamab ravtansine, a CD166-targeting antibody-drug conjugate, in patients with advanced solid tumors: an open-label phase I/II Trial. Clin. Cancer Res. 28, 2020–2029 (2022).
Boustany, L. M. et al. A probody T cell-engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity. Cancer Res. 82, 4288–4298 (2022).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Xu, S. Internalization, trafficking, intracellular processing and actions of antibody-drug conjugates. Pharm. Res. 32, 3577–3583 (2015).
Saunders, K. O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 10, 1296 (2019).
Brandl, F., Busslinger, S., Zangemeister-Wittke, U. & Pluckthun, A. Optimizing the anti-tumor efficacy of protein-drug conjugates by engineering the molecular size and half-life. J. Control. Release 327, 186–197 (2020).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Senior, M. Cancer-targeting antibody-drug conjugates drive dealmaking frenzy. Nat. Biotechnol. 42, 362–366 (2024).
Weng, W. et al. Antibody-exatecan conjugates with a novel self-immolative moiety overcome resistance in colon and lung cancer. Cancer Discov. 13, 950–973 (2023).
Li, B. T. et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 386, 241–251 (2022).
Siena, S. et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 22, 779–789 (2021).
Esfandiari, A., Cassidy, S. & Webster, R. M. Bispecific antibodies in oncology. Nat. Rev. Drug Discov. 21, 411–412 (2022).
do Pazo, C., Nawaz, K. & Webster, R. M. The oncology market for antibody-drug conjugates. Nat. Rev. Drug Discov. 20, 583–584 (2021).
Criscitiello, C., Morganti, S. & Curigliano, G. Antibody-drug conjugates in solid tumors: a look into novel targets. J. Hematol. Oncol. 14, 20 (2021).
Michael, L. W. et al. Zilovertamab vedotin targeting of ROR1 as therapy for lymphoid cancers. NEJM Evid. https://doi.org/10.1056/EVIDoa2100001 (2021).
Saura Manich, C. et al. LBA15 — primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 32, S1283–S1346 (2021).
Powles, T. B. et al. LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann. Oncol. 34, S1340 (2023).
Antignani, A. et al. Targeting receptors on cancer cells with protein toxins. Biomolecules https://doi.org/10.3390/biom10091331 (2020).
Pemmaraju, N. et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N. Engl. J. Med. 380, 1628–1637 (2019).
Saleh, M. N. et al. Antitumor activity of DAB389IL-2 fusion toxin in mycosis fungoides. J. Am. Acad. Dermatol. 39, 63–73 (1998).
Sartor, O. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Strosberg, J. et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Michael, S. H. et al. First-in-human safety, imaging and dosimetry of [68Ga]Ga-DPI-4452, a novel CA IX-targeting peptide, in patients with clear cell renal cell carcinoma. J. Clin. Oncol. https://doi.org/10.1200/JCO.2024.42.4_suppl.37 (2024).
Dolgin, E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 36, 1125–1127 (2018).
Sathekge, M. M. et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 25, 175–183 (2024).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Rubin, I. & Yarden, Y. The basic biology of HER2. Ann. Oncol. 12, S3-8 (2001).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Saunders, L. R. et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 7, 302ra136 (2015).
Vos, J. L. et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 12, 7348 (2021).
Acknowledgements
S.P. M.F.K., C.B., N.P. and S.Z. were supported by The Virginia and D.K. Ludwig Fund for Cancer Research, the Lustgarten Foundation for Pancreatic Cancer Research, The Commonwealth Fund, the Bloomberg~Kimmel Institute for Cancer Immunotherapy, and the National Institutes of Health (NIH) Cancer Center Support grant P30 CA006973. S.P. was supported by NCI grant K08CA270403, the Leukaemia Lymphoma Society Translation Research Program award, the American Society of Hematology Scholar award, and the Swim Across America Translational Cancer Research Award. M.F.K. was supported by NIH/NIAID grant 1R21AI176764-01, the Jerome Greene Foundation, the Cupid Foundation, the Lupus Research Alliance Lupus Innovation Award, the Rheumatology Research Foundation Investigator Award, the Harrington Discovery Institute Harrington Scholar-Innovator Award, the Sol Goldman MS Research Program, and the Bisciotti Foundation Translational Fund. C.B. was supported by NCI grant R37 CA230400. M.H. was supported by the Intramural Research Program of NIH, NCI and Center for Cancer Research (Z01 BC010891, ZIA BC010891 and ZIC BC 011891).
Author information
Authors and Affiliations
Contributions
S.P., K.M.W., S.B.G. and M.H. researched data for the article. S.P., S.B.G., M.H. and A.vE. contributed substantially to discussion of the content. S.P., K.M.W., S.B.G., M.H., A.vE. and S.Z. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The Johns Hopkins University has filed patent applications related to technologies described in this paper on which S.P., M.F.K., N.P., D.M.P. and S.Z. are listed as inventors. M.H. is an inventor on NIH patents in antibody and cell therapies and may receive blinded royalties from the NIH. N.P. is a founder of Thrive Earlier Detection, an Exact Sciences company. N.P. is a consultant to Thrive Earlier Detection. N.P. and S.Z. hold equity in Exact Sciences. N.P. and S.Z. are founders of and/or consultants to and own equity in ManaT Bio., Neophore and Personal Genome Diagnostics. N.P. holds equity in Haystack Oncology and CAGE Pharma. N.P. is a consultant to Vidium. S.P. owns equity in Gilead, is a consultant to Merck and received payment from IQVIA. M.F.K. is a consultant to Argenx, Atara Biotherapeutics, Revel Pharmaceuticals, Sana Biotechnology and Sanofi. S.Z. has a research agreement with BioMed Valley Discoveries, Inc. C.B. is a consultant for Depuy-Synthes, Bionaut Labs, Galectin Therapeutics, Haystack Oncology and Privo Technologies. C.B. is a co-founder of OrisDx and Belay Diagnostics. D.M.P. reports grant and patent royalties through institution from BMS, a grant from Compugen, stock from Trieza Therapeutics and Dracen Pharmaceuticals, and founder equity from Potenza; is a founder of and consultant to and owns equity in ManaT Bio; is a consultant for Aduro Biotech, Amgen, Astra Zeneca (Medimmune/Amplimmune), Bayer, DNAtrix, Dynavax Technologies Corporation, Ervaxx, FLX Bio, Rock Springs Capital, Janssen, Merck, Tizona and Immunomic-Therapeutics; is on the scientific advisory board of Five Prime Therapeutics, Camden Nexus II, WindMil; and is on the board of directors for Dracen Pharmaceuticals. K.M.W. and S.B.G. are employees of Merck & Co., Inc. at the time of submission and may have stock ownership in Merck & Co., Inc., Rahway, NJ, USA.
Peer review
Peer review information
Nature Reviews Cancer thanks Falk Nimmerjahn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
American Cancer Society: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
Antibody Society: https://www.antibodysociety.org/resources/approved-antibodies/
Supplementary information
Glossary
- Anaphylaxis
-
A severe and potentially life-threatening reaction owing to exposure to an allergen such as an antibody or other medication. Common symptoms of anaphylaxis include swelling of the face and throat, difficulty in breathing, an increase in heart rate, a drop in blood pressure and loss of consciousness.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). A mechanism through which antibodies bind to target cells followed by recruitment of immune cells such as NK cells and macrophages to kill the target cells. The immune cells secrete cytotoxic granules (perforins and granzymes), and induce FAS signalling leading to target cell death.
- Antibody-dependent cellular phagocytosis
-
(ADCP). A mechanism through which antibodies bind to target cells, which in turn stimulates immune cells such as macrophages to engulf and degrade the target cells.
- B cell cloning
-
Isolation and expansion of single B cells that produce the desired monoclonal antibodies, to obtain the antibody-coding sequence.
- Bystander effects
-
With antibody–drug conjugates (ADCs), refers to a phenomenon wherein neighbouring cells near the target cancer cell are killed by the released cytotoxic payload. This effect can enhance the overall potency of the ADC by causing a broader destruction of cancer cells beyond the primary target cell.
- Capillary leak syndrome
-
Condition characterized by the leakage of fluid from small blood vessels (capillaries) into surrounding tissues. This leakage leads to a decrease in blood volume and can result in low blood pressure along with oedema (swelling) in various parts of the body, including the lungs, and organ failure.
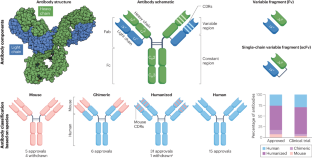
- Complementarity-determining regions
-
(CDRs). Specific regions within the antibody heavy and light chain variable domains that bind to the target antigen.
- Complement-dependent cytotoxicity
-
(CDC). A mechanism through which antibodies bind to the target cell followed by activation of the complement system, leading to lysis of the target cells.
- Cytokine release syndrome
-
(CRS). Systemic inflammation characterized by a constellation of symptoms such as fever, hypotension and hypoxia and mediated by the release of multiple cytokines from the immune cells of patients. CRS is a typical adverse effect observed with the use of T cell engager (TCE) bispecific antibodies.
- Cytopenias
-
A reduction in the number of circulating blood cells, such as red blood cells (erythrocytes), white blood cells (leukocytes) and/or platelets (thrombocytes). Cytopenias can be caused by several factors including exposure to drugs or antibodies that hinder the growth of new cells.
- Drug–antibody ratio
-
(DAR). The number of drugs attached to each antibody in an ADC.
- Fragment antigen binding
-
(Fab). An antibody which consists of two identical Fab fragments and one Fc fragment. Each Fab fragment is responsible for binding to a specific antigen. The Fab fragment is obtained by cleaving the antibody at specific sites using enzymes, such as papain or pepsin.
- Fragment crystallizable
-
(Fc). Fc fragment interacts with various immune cells through Fc receptors and with complement proteins that contribute to the immune response generated by the antibody. Each antibody class and subclass has unique Fc regions. Understanding the Fc fragment is crucial in the design of therapeutic antibodies because modifications to this region can impact the pharmacokinetics, effector functions and therapeutic efficacy of an antibody.
- Haemolytic uremic syndrome
-
A rare but serious condition that is characterized by the combination of haemolytic anaemia (destruction of red blood cells), thrombocytopenia (low platelet count) and acute kidney injury. It can be mediated by bacterial infections (such as Escherischia coli) or exposure to drugs and antibodies.
- Hydrophobicity
-
Refers to the property of being repelled by water. Hydrophobic substances are insoluble or poorly soluble in water. The hydrophobicity of the payload can affect the overall stability of the ADC. Highly hydrophobic payloads may lead to aggregation or destabilization of the ADC structure, potentially impacting its efficacy and safety.
- Microsatellite instability-high
-
(MSI-H). Cells with mismatch-repair deficiency resulting in high mutation burden and altered microsatellite (tract of repetitive DNA) sequences. MSI-H cancers are associated with a higher response to immune checkpoint-inhibiting antibodies.
- Monoclonal antibodies
-
Identical antibodies that bind to a specific part of the target antigen (epitope) and are derived from single clones of immune cells (such as B cells, plasma cells or hybridoma cells).
- Myelodysplastic syndromes
-
A group of disorders characterized by abnormal production and maturation of blood cells in the bone marrow. In myelodysplastic syndromes, the bone marrow fails to produce enough healthy blood cells, leading to low levels of red blood cells (anaemia), white blood cells (leukopenia) and platelets (thrombocytopenia).
- Neonatal Fc receptor
-
(FcRn). A receptor expressed by vascular endothelial cells and immune cells, which binds to the Fc portion of IgG antibodies. IgG antibody binding to FcRn leads to receptor-mediated internalization and recycling of the IgG, which is responsible for the long IgG half-life (about 21 days) in circulation.
- Peripheral neuropathy
-
A potential side effect that can occur owing to the cytotoxic payload component of the ADC affecting the peripheral nerves. Peripheral neuropathy caused by ADCs can manifest as numbness, tingling, burning sensations or pain in the hands, feet or other extremities.
- Public neoantigens
-
A public neoantigen is derived from a mutated protein and is found in multiple individuals with the same type of cancer. This shared characteristic makes public neoantigens particularly important in cancer immunotherapy because therapies targeting these common neoantigens can benefit a broad patient population. Common public neoantigens include BRAFV600E, KRASG12D, KRASG12C and TP53R175H. By contrast, private neoantigens are unique to an individual patient with cancer. Targeting private neoantigens requires the development of personalized therapies such as custom cancer vaccines and T cell-based therapies.
- Single-chain variable fragment
-
(scFv). An engineered antibody fragment composed of variable regions of the heavy and light chains combined into a single peptide chain by a linker. The scFv retains the ability to bind specifically to a target antigen, similar to a full-size antibody. The advantages of scFv include its smaller size (~25 kDa), which facilitates easier production and manipulation.
- Single-domain antibodies
-
Also known as nanobodies, are antibodies derived from camelids that consist of only a variable heavy domain and as a result have a relatively low molecular weight (~15 kDa), hence the name nanobody. By contrast, human antibodies consist of variable heavy and light domains and have higher molecular weights (a full-length IgG antibody is ~150 kDa and an scFv is ~ 25 kDa).
- Topoisomerase
-
Enzymes that maintain proper function and stability of DNA by cleaving DNA to relieve torsional strain and supercoiling occurring owing to processes such as DNA replication. Topoisomerase inhibitors disrupt this ability to maintain DNA and cause cell death.
- Tumour antigens
-
Proteins and other antigenic molecules expressed on the surface of tumour cells that can be targeted by therapeutic antibodies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paul, S., Konig, M.F., Pardoll, D.M. et al. Cancer therapy with antibodies. Nat Rev Cancer (2024). https://doi.org/10.1038/s41568-024-00690-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41568-024-00690-x