Abstract
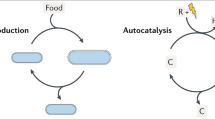
Darwinian evolution involves the inheritance and selection of variations in reproducing entities. Selection can be based on, among others, interactions with the environment. Conversely, the replicating entities can also affect their environment generating a reciprocal feedback on evolutionary dynamics. The onset of such eco-evolutionary dynamics marks a stepping stone in the transition from chemistry to biology. Yet the bottom-up creation of a molecular system that exhibits eco-evolutionary dynamics has remained elusive. Here we describe the onset of such dynamics in a minimal system containing two synthetic self-replicators. The replicators are capable of binding and activating a co-factor, enabling them to change the oxidation state of their environment through photoredox catalysis. The replicator distribution adapts to this change and, depending on light intensity, one or the other replicator becomes dominant. This study shows how behaviour analogous to eco-evolutionary dynamics—which until now has been restricted to biology—can be created using an artificial minimal replicator system.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The UPLC data generated and analysed in this article are included in its Supplementary Information in the form of integrated peak areas and exported traces of representative chromatograms. All other data generated or analysed during this study are included in this published article and its Supplementary Information. Waters UPLC software stores raw UPLC data in a database format from which they are not readily extractable. These data are available on request. Source data are provided with this paper.
References
Miikkulainen, R. & Forrest, S. A biological perspective on evolutionary computation. Nat. Mach. Intell. 3, 9–15 (2021).
De Jong, K. A. Evolutionary Computation: A Unified Approach (MIT Press, 2006).
Zeymer, C. & Hilvert, D. Directed evolution of protein catalysts. Annu. Rev. Biochem. 87, 31–157 (2018).
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. 57, 4143–4148 (2018).
Adamski, P. et al. From self-replication to replicator systems en route to de novo life. Nat. Rev. Chem. 4, 386–403 (2020).
Jakiela, S., Kaminski, T. S., Cybulski, O., Weibel, D. B. & Garstecki, P. Bacterial growth and adaptation in microdroplet chemostats. Angew. Chem. Int. Ed. 52, 8908–8911 (2013).
Behe, M. J. Experimental evolution, loss-of-function mutations, and ‘the first rule of adaptive evolution’. Q. Rev. Biol. 85, 419–445 (2010).
Mizuuchi, R., Ichihashi, N. & Yomo, T. Adaptation and diversification of an RNA replication system under initiation- or termination-impaired translational conditions. ChemBioChem 17, 1229–1232 (2016).
Govaert, L. et al. Eco-evolutionary feedbacks—theoretical models and perspectives. Funct. Ecol. 33, 13–30 (2019).
Schoener, T. W. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429 (2011).
Kosikova, T. & Philp, D. Exploring the emergence of complexity using synthetic replicators. Chem. Soc. Rev. 46, 7274–7305 (2017).
Clixby, G. & Twyman, L. Self-replicating systems. Org. Biomol. Chem. 14, 4170–4184 (2016).
Le Vay, K., Weise, L. I., Libicher, K., Mascarenhas, J. & Mutschler, H. Templated self-replication in biomimetic systems. Adv. Biosyst. 3, 1800313 (2019).
Hong, J. I., Feng, Q., Rotello, V. & Rebek, J. Competition, cooperation, and mutation—improving a synthetic replicator by light irradiation. Science 255, 848–850 (1992).
Yao, S., Ghosh, I., Zutshi, R. & Chmielewski, J. A pH-modulated, self-replicating peptide. J. Am. Chem. Soc. 119, 10559–10560 (1997).
Yao, S., Ghosh, I., Zutshi, R. & Chmielewski, J. A self-replicating peptide under ionic control. Angew. Chem. Int. Ed. 37, 478–481 (1998).
Leonetti, G. & Otto, S. Solvent composition dictates emergence in dynamic molecular networks containing competing replicators. J. Am. Chem. Soc. 137, 2067–2072 (2015).
Dadon, Z., Samiappan, M., Wagner, N. & Ashkenasy, G. Chemical and light triggering of peptide networks under partial thermodynamic control. Chem. Commun. 48, 1419–1421 (2012).
Dadon, Z., Samiappan, M., Safranchik, E. Y. & Ashkenasy, G. Light-induced peptide replication controls logic operations in small networks. Chem. Eur. J. 16, 12096–12099 (2010).
Riess, B., Grotsch, R. K. & Boekhoven, J. The design of dissipative molecular assemblies driven by chemical reaction cycles. Chem 6, 552–578 (2020).
Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotech. 13, 882–889 (2018).
Merindol, R. & Walther, A. Materials learning from life: concepts for active, adaptive and autonomous molecular systems. Chem. Soc. Rev. 46, 5588–5619 (2017).
Sorrenti, A., Leira-Iglesias, J., Markvoort, A. J., de Greef, T. F. A. & Hermans, T. M. Non-equilibrium supramolecular polymerization. Chem. Soc. Rev. 46, 5476–5490 (2017).
Engwerda, A. H. et al. Coupled metabolic cycles allow out-of-equilibrium autopoietic vesicle replication. Angew. Chem. Int. Ed. 59, 20361–20366 (2020).
Morrow, S. M., Colomer, I. & Fletcher, S. P. A chemically fuelled self-replicator. Nat. Commun. 10, 1011 (2019).
Pross, A. Seeking to uncover biology’s chemical roots. Emerg. Top. Life Sci. 3, 435–443 (2019).
Pross, A. What Is Life?: How Chemistry Becomes Biology (Oxford Univ. Press, 2016).
Yang, S. et al. Chemical fueling enables molecular complexification of self-replicators. Angew. Chem. Int. Ed. 60, 11344–11349 (2021).
Bandela, A. K. et al. Primitive selection of the fittest emerging through functional synergy in nucleopeptide networks. Proc. Natl Acad. Sci. USA 118, e2015285118 (2021).
Wagner, N., Hochberg, D., Peacock-Lopez, E., Maity, I. & Ashkenasy, G. Open prebiotic environments drive emergent phenomena and complex behavior. Life 9, 45 (2019).
Kamioka, S., Ajami, D. & Rebek, J. Autocatalysis and organocatalysis with synthetic structures. Proc. Natl Acad. Sci. USA 107, 541–544 (2010).
Arsene, S., Ameta, S., Lehman, N., Griffiths, A. D. & Nghe, P. Coupled catabolism and anabolism in autocatalytic RNA sets. Nucleic Acids Res. 46, 9660–9666 (2018).
Santiago, G. M., Liu, K., Browne, W. R. & Otto, S. Emergence of light-driven protometabolism on recruitment of a photocatalytic cofactor by a self-replicator. Nat. Chem. 12, 603–607 (2020).
Ottele, J., Hussain, A. S., Mayer, C. & Otto, S. Chance emergence of catalytic activity and promiscuity in a self-replicator. Nat. Catal. 3, 547–553 (2020).
Black, S. P., Sanders, J. K. M. & Stefankiewicz, A. R. Disulfide exchange: exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 43, 1861–1872 (2014).
Malakoutikhah, M. et al. Uncovering the selection criteria for the emergence of multi-building-block replicators from dynamic combinatorial libraries. J. Am. Chem. Soc. 135, 18406–18417 (2013).
Colomb-Delsuc, M., Mattia, E., Sadownik, J. W. & Otto, S. Exponential self-replication enabled through a fibre elongation/breakage mechanism. Nat. Commun. 6, 7427 (2015).
Rekondo, A. et al. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horizons 1, 237–240 (2014).
Nevejans, S., Ballard, N., Miranda, J. I., Reck, B. & Asua, J. M. The underlying mechanisms for self-healing of poly (disulfide)s. Phys. Chem. Chem. Phys. 18, 27577–27583 (2016).
Sreerama, N. & Woody, R. W. Computation and analysis of protein circular dichroism spectra. Methods Enzymol. 383, 318–351 (2004).
LeVine, H. III Thioflavine-T interaction with synthetic Alzheimers-disease β-amyloid peptides—detection of amyloid aggregation in solution. Protein Sci. 2, 404–410 (1993).
Maity, S. et al. Caught in the act: mechanistic insight into supramolecular polymerization-driven self-replication from real-time visualization. J. Am. Chem. Soc. 142, 13709–13717 (2020).
Pal, A. et al. Controlling the structure and length of self-synthesizing supramolecular polymers through nucleated growth and disassembly. Angew. Chem. Int. Ed. 54, 7852–7856 (2015).
Otto, S., Furlan, R. L. & Sanders, J. K. Dynamic combinatorial libraries of macrocyclic disulfides in water. J. Am. Chem. Soc. 122, 12063–12064 (2000).
Henrıquez, C., Bueno, C., Lissi, E. & Encinas, M. Thiols as chain transfer agents in free radical polymerization in aqueous solution. Polymer 44, 5559–5561 (2003).
RÖder, B., Hanke, T., Oelckers, S., Hackbarth, S. & Symietz, C. Photophysical properties of pheophorbide a in solution and in model membrane systems. J. Porphyrins Phthalocyanines 4, 37–44 (2000).
Ogilby, P. R. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 39, 3181–3209 (2010).
Semisotnov, G. et al. Study of the ‘molten globule’ intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31, 119–128 (1991).
Entradas, T., Waldron, S. & Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 204, 111787 (2020).
Volterra, V. Variations and fluctuations of the number of individuals in animal species living together. ICES J. Mar. Sci. 3, 3–51 (1928).
Lewontin, R. C. The units of selection. Annu. Rev. Ecol. Syst. 1, 1–18 (1970).
Markovitch, O., Ottelé, J., Veldman, O. & Otto, S. Automated device for continuous stirring while sampling in liquid chromatography systems. Commun. Chem. 3, 1–4 (2020).
Valbuena, A., Maity, S., Mateu, M. G. & Roos, W. H. Visualization of single molecules building a viral capsid protein lattice through stochastic pathways. ACS Nano 14, 8724–8734 (2020).
Acknowledgements
The authors thank W. R. Browne (University of Groningen) for the phosphorescence and EPR measurements and O. Markovitch (University of Groningen) for providing guidance on the usage of UPLC stirring device. This work was supported by the Simons Foundation (553330; K.L.), Marie Curie Individual Fellowships (PSR 786350; K.L.), the oLife Cofund programme (847675; A.B.), the ERC (AdG 741774), the China Scholarship Council (J.W.) and the Dutch Ministry of Education, Culture and Science (Gravitation programme 024.001.035; A.K. and S.O.).
Author information
Authors and Affiliations
Contributions
K.L. and S.O. conceived the concept. K.L. designed, performed experiments and analysed the data. A.B. assisted in the data analysis and contributed to the scientific discussion. C.E. performed the experiments related to HS-AFM. C.E. and W.H.R. analysed the data related to HS-AFM. A.K. performed the experiments related to TEM. J.W. performed the experiments related to UPLC–MS. K.L. A.B. and S.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks David Lynn, Subhabrata Maiti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–58 and Tables 1 and 2.
Supplementary Video 1
HS-AFM showing the growth of 3-mer fibres from the ends liberated by fibre breakage.
Supplementary Video 2
HS-AFM showing the growth of a single 3-mer fibre.
Source data
Source Data Fig. 2
Relative peak area of components in the sample obtained from UPLC chromatograms.
Source Data Fig. 3
Relative/absolute peak areas of components in the sample obtained from UPLC chromatograms; calculation of the relative change of the peak areas, the concentration of bound precursor, the adsorption efficiency, and replication rate.
Source Data Fig. 4
Relative peak areas of components in the sample obtained from UPLC chromatograms.
Source Data Fig. 5
Raw spectral data; absolute peak areas of the monomer in UPLC chromatograms, and calculation of oxidation rates from them; Relative peak areas of components in the sample obtained from UPLC chromatograms.
Source Data Fig. 6
Relative peak areas of components in the sample in UPLC chromatograms.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, K., Blokhuis, A., van Ewijk, C. et al. Light-driven eco-evolutionary dynamics in a synthetic replicator system. Nat. Chem. 16, 79–88 (2024). https://doi.org/10.1038/s41557-023-01301-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01301-2