Abstract
Background:
Deficiencies in vitamin D directly impact children’s health and place minority and obese youth at risk for a range of health issues. The Institute of Medicine (IOM) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium has set both a recommended daily allowance (RDA) for vitamin D supplementation and a population-wide sufficiency target for the biomarker of vitamin D status, serum 25-hydroxyvitamin D (25(OH)D). However, new research suggests that the RDA is not sufficient to meet the target biomarker status for individuals who are heavy or who have dark skin. Our objective was to provide appropriate daily vitamin D supplementation levels for these individuals.
Methods:
Using data derived from the National Health and Nutrition Examination Survey (NHANES) and a recently published dosing formula, we calculated the required supplemental dose of vitamin D to meet the IOM target in children and adolescents.
Results:
To be sure that 95% of the target population meets the IOM’s population-wide biomarker target, some individuals require a daily dose of up to 2,000 international units (IUs) of supplemental vitamin D.
Conclusion:
Health professionals should work with their patients to encourage lifelong vitamin D supplement use at a dosage sufficient to obtain adequate 25(OH)D levels.
Similar content being viewed by others
Main
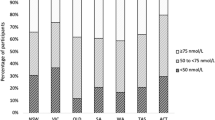
Current levels of sun exposure in the United States are low compared with levels during human evolution. Furthermore, levels of serum 25-hydroxyvitamin D (25(OH)D)—the biomarker currently used to determine vitamin D status—are typically substantially higher in sun-exposed subjects than that in the general US population (1,2,3). For example, levels in dark-skinned peoples near the equator average 46 ng/ml in traditionally living people in Tanzania (2° to 4°S) (4), 30 ng/ml in rural Ghana (6°N) (5), and 29 ng/ml in more developed Victoria, Seychelles (4°S) (5). Average levels in the United States are 15 ng/ml for non-Hispanic blacks, 20 ng/ml for Mexican-Americans, and 26 ng/ml for non-Hispanic whites (6). Skin color is one of the major determinants of vitamin D status because a given amount of sun exposure produces less vitamin D in darker skin than that in lighter skin (7,8). There is evidence that these differences in vitamin D levels are biologically related to US health disparities (6,9,10,11,12).
The second major determinant of vitamin D status is body weight. Until recently, it has been unclear whether low levels of vitamin D cause higher body weight or higher body weight causes low vitamin D status; however, recent studies have clarified that equivalent amounts of vitamin D are simply more diluted in those with higher body weights (13,14,15). Although vitamin D compounds are fat soluble, they are not sequestered in adipose cells but freely enter and leave (13). This means that to reach equivalent target serum levels of 25(OH)D, heavier individuals have to produce or ingest more vitamin D than lighter individuals.
There are three methods to increase vitamin D levels: through exposure to sunlight, diet, and supplementation. Because of the relationship between sun exposure and skin cancer in those with light skin, public health authorities have been recommending that the general population lower their sun exposure for over 30 y (16). It is possible to obtain vitamin D through diet; however, other than wild fish that eat a certain type of zooplankton, few food sources have ample amounts of vitamin D (17,18). In developed countries, some foods are fortified with low levels of vitamin D. While this fortification seems to have solved the problem of vitamin D deficiency for some individuals, others continue to be vitamin D deficient. Consequently, the only currently viable method of preventing vitamin D deficiency throughout the population is individual supplementation.
In early 2011, the Institute of Medicine (IOM) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (19) set the recommended daily allowance (RDA) for ages 1 y through 70 at 600 international unit (IUs)/d. The committee also suggested that a serum 25(OH)D level of 20 ng/ml or higher would ensure that practically all persons have levels sufficient to support the classic endocrine action of vitamin D, which impacts bone health. However, the committee said it could not determine the optimal level for the modern autocrine/paracrine action of vitamin D because of a lack of data. According to the committee, the modern understanding results in possible roles for higher vitamin D levels to reduce rates of carcinogenesis, cardiovascular disease, diabetes, falls, and preeclampsia while simultaneously improving immune response, neuropsychologic functioning, and physical performance (19).
The genesis of our current report was a new vitamin D dosing formula developed by Zittermann et al. (15), who analyzed data from 144 cohorts in 94 randomized controlled trials involving vitamin D. Based on the vitamin D dose in each trial, the average increase in serum 25(OH)D, the average weight and age of the subjects, and other factors, the researchers developed a vitamin D dosing formula, pointing out that body weight was a much more important determinant of dose than had been previously realized. In addition, based on our own research (6), we knew that baseline vitamin D levels, which are included in the Zittermann’s formula, vary by skin color. Our goal, therefore, was to use body weight and skin color to provide more accurate recommended vitamin D3 supplemental dosages—dosages that would allow 95% of the US population aged from 1 to 21 y to reach the IOM’s 20 ng/ml serum 25(OH)D target. Our approach was to combine the Zittermann’s formula with nationally representative data from the continuous version of the National Health and Nutrition Examination Survey (NHANES) (20).
Results
The demographic characteristics of NHANES data we used are representative of the noninstitutionalized US population aged from 1 to 21 y (21).
Table 1 shows the required supplemental vitamin D dose, by weight and skin color, to meet the IOM’s goal of serum 25(OH)D levels of 20 ng/ml in 95% of the individuals in each table cell. Our calculation subtracts the mean dietary vitamin D intake from the dose calculated by the Zittermann’s formula. In the NHANES data, dietary intake includes vitamin D in foods both naturally and as a result of fortification, but not vitamin D from supplementation, which is provided as a separate measurement. No group has a dietary intake high enough to meet the required dose. As a practical matter, in the United States, the smallest vitamin D supplement contains 400 IUs. Because vitamin D supplements are typically only available over the counter in 400, 600, 1,000, 2,000, and 5,000 IU doses, we rounded the calculated dose up to the nearest available supplement size.
Although we used average data for specific racial and ethnic groups as a proxy for skin color, we want to emphasize that professionals using this table should consider the patient’s actual skin color, not race or ethnicity. Skin color is a continuous biological characteristic that ranges from very dark to very light. Skin color appears in categories in this table, but it does not occur that way in nature. An individual African-American, depending on the individual’s actual skin color, could be classified in any of the categories given here. Moreover, health professionals who concentrate on the patient’s actual skin color can use this table with patients of any race or ethnicity. We should also note that in developing its formula, the Zittermann’s team included ethnicity in its analysis but found that it was not relevant as an input to their formula, although it is relevant, as we have previously shown (6), to baseline 25(OH)D levels.
Table 2 shows the raw data used to calculate the required dosages. It should be noted that the column indicating serum 25(OH)D levels represents the level of individuals at the fifth percentile. This creates calculated doses sufficient for 95% of the population. In rows in which SEs are relatively small in relation to the means, the row has a larger sample size, while relatively large SEs indicate a smaller sample size (e.g., there are fewer individuals in the highest weight groups). The final two columns show the average level of vitamin D obtained from the diet and from current levels of supplementation. The dietary mean was used in the calculation to reduce the calculated dose by vitamin D obtained through the diet. The supplement mean was not used in the calculation but is provided here to show that current supplementation levels are far from adequate.
Discussion
This paper attempts to provide health professionals with practical advice based on the latest findings in vitamin D research relevant to the health of children and adolescents. Individuals with darker skin or higher body weights typically have vitamin D levels below what any health authorities consider adequate. We present the dosage required to meet the need of 95% of US children and adolescents at a level determined to be sufficient for practically all persons by the IOM’s Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. These dosages depend on skin color, because darker skin produces less vitamin D than lighter skin from equivalent amounts of sunlight, and on body weight, because vitamin D is more diluted in heavier individuals.
Formula Assumptions
The dosages provided in this paper assume the supplement taken is vitamin D3, which is the version produced by animals, including humans. Vitamin D2 is produced by plants and may be preferred by vegetarians. However, according Zittermann’s findings, vitamin D2 is not as effective at raising serum 25(OH)D levels. While their formula included an adjustment for vitamin D2 supplements, it was not included in the adjusted formula used in this paper. In discussing the difference between the two versions with vegetarian patients, it may therefore be helpful for clinicians to know that vitamin D3 is manufactured by purifying lanolin—oils washed from wool—to obtain the same cholesterol-based vitamin D3 precursor found in human skin, then exposing that to ultraviolet light (22).
In addition to the adjustment for vitamin D2, Zittermann’s formula includes an adjustment for those who take a calcium supplement in addition to vitamin D. Although our raw calculated dosages would be low for those taking vitamin D2 or calcium, in general, the differences would be overwhelmed by the step of rounding the calculated dose up to the nearest available supplement size.
Supplement Adherence
Just as differences caused by rounding to available supplement dosages overwhelm the mathematical exactness of the Zittermann’s formula, differences in adherence to supplementation recommendations overwhelm the effectiveness of the recommendations themselves. Supplementation involves a lifelong commitment to a treatment that promises to prevent disease, rather than a temporary commitment to a treatment that promises to cure disease. Consequently, in addition to the issues around calculating a sufficient dose, there are important issues regarding adherence to the health professional’s recommendation regarding vitamin D supplements.
The literature on adherence to supplement regimens offers just one technique that has been shown to more than double adherence: providing a written prescription for the supplement, even though it is an over-the-counter product that does not require a prescription and will not be paid for by prescription plans (23). It can also be helpful to discuss the reasoning behind the prescription, such as the impact of skin color and body weight on vitamin D levels (24) and to involve parents in monitoring usage, especially if the parents themselves are taking supplements, and the children can be involved in making sure the entire family is adherent (25).
Finally, forgetfulness is the reason most often given for not taking supplements (25,26,27). Implementation intentions, which are plans for when, where, and how an action will be taken, have been shown to be helpful in thwarting forgetfulness in this context (27); however, health professionals must do more than simply tell the patient to make plans (28). Asking the patient questions such as, “Which drug store will you visit to buy these?”, “What time of day will you take them?”, and “How will you remember?” can be a simple but effective way to increase adherence, particularly if the patient makes a plan to take the supplement at the same time as a regular activity, such as waking up, going to bed, or at a specific meal (27). Because of the way vitamin D is metabolized and stored in the human body, it is even possible for a healthcare provider to ask a patient to take up to a 3-month dose in the provider’s presence to ensure adherence (29). For the same reason, patients can be advised to take skipped doses all at once.
Vitamin D Toxicity or Intoxication
Since the 1960s, health professionals have been taught that vitamin D supplementation can result in toxicity or intoxication (30). This belief stemmed from an increasing incidence of idiopathic infantile hypercalcemia soon after manufacturers began fortifying foods with vitamin D in the 1950s. However, recent genetic studies of other families of affected infants suggest that these cases of intoxication appeared only in individuals with specific mutations in the CYP24A1 gene, which creates the protein that breaks down vitamin D metabolites (31). More recently, reported cases of vitamin D intoxication have typically resulted from errors in manufacturing a fortified food or in compounding a supplement. Intoxicated individuals present with severe hypercalcemia, hypercalciuria, or nephrocalcinosis and their associated symptoms. Patients usually experience complete recovery after ending excessive supplementation. Individuals with diseases that lead to high calcium levels, such as the granulomatous diseases or lymphoma, as well as individuals with genetic mutations in the CYP24A1 gene, need to be treated with great care.
Limitations
The data set on which the Zittermann’s formula is based does not include any children younger than 10 y. Consequently, the Zittermann’s paper cautions against using its formula with young children. Moreover, the 2001–2002 and 2003–2004 cycles of NHANES did not collect serum 25(OH)D data on children younger than 6 y, and the other cycle did not collect serum 25(OH)D data on children younger than 1 y, resulting in excessive missing data for young children in the NHANES serum 25(OH)D data set. Although we did use the formula with children younger than 10 y, the results we obtained with the calculation were lower than the IOM’s recommended amounts and were replaced by the recommended amounts in developing Table 1 . It should be noted that this recommendation is particularly important for breastfed babies unless the mother is taking ample vitamin D. On average, breastfed babies have serum 25(OH)D levels about 60% of the mother’s level (32). Consequently, the mother would have to have a serum 25(OH)D level above 33 ng/ml to achieve a 20 ng/ml level in the baby.
The RCTs used by Zittermann et al. also did not include individuals who received vitamin D supplements at a rate higher than 60 IUs/kg/d. The dosages recommended in Table 1 do not go above this level. The IOM does not recommend using serum 25(OH)D tests to screen for vitamin D deficiency. In general, health professionals can quickly determine the probability of vitamin D deficiency by considering a patient’s weight, skin color, and, for markedly tanned individuals, level of sun exposure. On the other hand, there is no option other than a serum 25(OH)D test to determine whether a prescribed dose is adequate or excessive.
We should also note that in response to the IOM’s recommendations, the Endocrine Society released Clinical Practice Guidelines (33), which recommended higher daily requirements and a minimum serum 25(OH)D level of 30 ng/ml as a more appropriate goal given what is known about vitamin D. However, using the Zittermann’s formula to determine supplement requirements at that level results in values most health professionals would consider extraordinarily high (in some cases >6,000 IU/d). Practically speaking, most health professionals would not currently consider recommending such high levels without supervision; thus, these levels are unrealistic to suggest for long-term unsupervised implementation among young patients without further research. Consequently, these results do not incorporate the Endocrine Society’s supplementation recommendations.
Finally, the Zittermann’s formula accounted for 54% of the variance in serum 25(OH)D levels, so there are other, mostly unknown, factors that impact 25(OH)D levels that were not included in the model used to develop the dosages presented here. For example, one of these factors is total calcium intake (34) (not simply whether a calcium supplement is taken, as in the Zittermann’s formula) and another is the methylation levels of the genes that produce the enzymes that convert vitamin D into 25(OH)D and that metabolize vitamin D compounds (35).
This paper presents a novel table intended for the use of health professionals that gives the dose of vitamin D supplementation required to meet the minimum serum 25(OH)D target in 95% of US children and adolescents by body weight and skin color. Health professionals can determine the probability of vitamin D deficiency in a patient by considering these variables in addition to the patient’s potential level of sun exposure, which is typically quite low in the United States. Vitamin D deficiency is a serious concern in patients of color and in patients with high body weights and is suspected of being at least partially responsible for the health disparities between these groups and the general population. Accurate vitamin D supplement use among youth may contribute to reducing these disparities.
Methods
We began by making several mathematical conversions to the Zittermann’s formula. Our version calculates supplemental doses rather than increases in 25(OH)D; it measures doses in IU of vitamin D rather than micrograms (1 μg = 40 IUs); it measures 25(OH)D in the US standard of nanograms per milliliter (ng/ml) rather than in the metric standard of nanomoles per liter (1 ng/ml = 2.496 nmol/l); and it subtracts average dietary intake from the formula’s calculated dose. The converted formula, in spreadsheet format, where Weight is body weight in kilograms, Age is in years, Baseline is the current 25(OH)D level in ng/ml, Goal is the target 25(O(H)D level in ng/ml, Diet is average dietary intake in IUs, and Dose is the supplemental dose in IUs is:
Dose = (Weight * 40 * EXP((−49.4 − (0.22*Age) + (0.325*Baseline) + (2.496*(Goal − Baseline)))/16.03)) − Diet
We used this formula to calculate the dosages shown in Table 1 before rounding. In order to do the calculation, we needed to know the mean age, weight, baseline 25(OH)D level, and dietary vitamin D intake of the individuals in each body weight by skin color group. We obtained these data through analysis of the continuous version of the NHANES (20). NHANES data collection protocols were approved by the National Center for Health Statistics Research Ethics Review Board, and our work with NHANES was approved by the Institutional Review Board of Teachers College, Columbia University.
Continuous NHANES data are collected on a 2-y cycle at randomly selected US sites. Dietary intake data for vitamin D are currently available for all individuals in two cycles (2007–2010), while serum 25(OH)D data are currently available for individuals aged 6 y and older in two cycles (2001–2004) and for individuals aged 1 y and older in one cycle (2005–2006). Although it is not used in the formula, we also calculated and report mean vitamin D supplementation levels for each group to determine whether current supplementation levels are adequate. Supplement usage data are available for all individuals in all six cycles (1999–2010). Because there is no overlap in the NHANES cycles for dietary vitamin D and serum 25(OH)D levels, we chose to use all available data for each analysis. NHANES researchers collect data at an in-home interview followed by a physical examination, questionnaires, and interviews at a mobile examination center (36). All of the data used in this study were collected at the mobile examination center or in subsequent second-day dietary interviews. Subjects self-report dietary and supplement data in detailed interviews, and researchers collect a blood sample for the serum 25(OH)D measurement, which is completed using a Diasorin radioimmunoassay (36). NHANES released adjusted 25(OH)D data in November 2010 to correct drifts in assay performance; we used the adjusted data. Skin color is rarely measured outside anthropology; therefore, we used race and ethnicity as a proxy for skin color. NHANES includes nationally representative samples for three race/ethnicity groups: non-Hispanic blacks, Mexican-Americans, and non-Hispanic whites. We assumed that, on average, those who self-identify as non-Hispanic black have darker skin than those who self-identify as Mexican-American, who themselves have, on average, darker skin than those who self-identify as non-Hispanic white.
Statistical Analysis
NHANES relies on a complex survey design using both clustering and stratification, which is presented in detail elsewhere (36). We analyzed the data with the statistical program R, version 3.0.2 (37), using the Survey package, version 3.28-2 (38) to provide the necessary methods for analyzing complex survey data (39). Sample weights provided with the NHANES data adjust for unequal probabilities of selection (some subpopulations were oversampled), nonresponse adjustments, and other adjustments (21). We used dietary weights when calculating dietary data and mobile examination center weights when calculating serum 25(OH)D status and vitamin D supplement intake, appropriately adjusted for the number of cycles of available data. For each analysis, we deleted cases with missing information on any of the variables in that analysis as well as cases for individuals older than 21 y, resulting in 7,764 unweighted cases for the dietary vitamin D analysis, 10,961 unweighted cases for the serum 25(OH)D analysis, and 30,128 unweighted cases for the supplemental vitamin D analysis.
Statement of Financial Support
This project was completed with no financial support.
Disclosure
There are no financial ties to this study, nor any potential or perceived conflicts of interest.
References
Haddad JG, Chyu KJ . Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab 1971;33:992–5.
Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007;92:2130–5.
Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD . Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab 2012;37:798–802.
Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA . Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr 2012;108:1557–61.
Durazo-Arvizu RA, Camacho P, Bovet P, et al. 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr 2014;100:908–14.
Weishaar T, Vergili JM . Vitamin D status is a biological determinant of health disparities. J Acad Nutr Diet 2013;113:643–51.
Clemens TL, Adams JS, Henderson SL, Holick MF . Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74–6.
Murray FG . Pigmentation, sunlight, and nutritional disease. Am Anthropol 1934;36:438–45.
Bibuld D . Health disparities and vitamin D. Clin Rev Bone Miner Metab 2009;7:63–76.
Grant WB . Differences in vitamin-D status may explain black-white differences in breast cancer survival rates. J Natl Med Assoc 2008;100:1040.
Grant WB, Peiris AN . Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc 2010;11:617–28.
Peiris AN, Bailey BA, Peiris P, Copeland RJ, Manning T . Race and vitamin D status and monitoring in male veterans. J Natl Med Assoc 2011;103:492–7.
Drincic AT, Armas LA, Van Diest EE, Heaney RP . Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–8.
Vimaleswaran KS, Berry DJ, Lu C, et al.; Genetic Investigation of Anthropometric Traits-GIANT Consortium. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383.
Zittermann A, Ernst JB, Gummert JF, Börgermann J . Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr 2014;53:367–74.
U.S. Department of Health Education and Welfare. Healthy People: The Surgeon General’s Report on Health Promotion and Disease Prevention. Washington, DC: Superintendent of Documents, 1979.
Schmid A, Walther B . Natural vitamin D content in animal products. Adv Nutr 2013;4:453–62.
U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 22. 2009.
Ross AC, Taylor C, Yaktine A, Del Valle H . Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press, 2011.
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. 1999–2010.
Johnson CL, Paulose-Ram R, Ogden CL . NHANES Analytic Guidelines 1999–2010. Vital and Health Statistics 2(161). Hyattsville, MD: National Center for Health Statistics (NCHS), 2013.
Holick MF . Vitamin D deficiency. N Engl J Med 2007;357:266–81.
Hill DA, Cacciatore M, Lamvu GM . Electronic prescribing influence on calcium supplementation: a randomized controlled trial. Am J Obstet Gynecol 2010;202:236.e1–5.
Greenley RN, Stephens KA, Nguyen EU, et al. Vitamin and mineral supplement adherence in pediatric inflammatory bowel disease. J Pediatr Psychol 2013;38:883–92.
Au LE, Harris SS, Jacques PF, Dwyer JT, Sacheck JM . Adherence to a vitamin D supplement intervention in urban schoolchildren. J Acad Nutr Diet 2014;114:86–90.
Brunner R, Dunbar-Jacob J, LeBoff M, et al. Predictors of adherence in the women’s health initiative calcium and vitamin D trial. Behav Med 2008;34:144–55.
Lourenço LB, Rodrigues RC, Spana TM, Gallani MC, Cornélio ME . Action and coping plans related to the behavior of adherence to drug therapy among coronary heart disease outpatients. Rev Lat Am Enfermagem 2012;20:821–9.
de Nooijer J, Jansen R, van Assema P . The use of implementation intentions to promote vitamin D supplementation in young children. Nutrients 2012;4:1454–63.
Mallet E, Philippe F, Castanet M, Basuyau JP . [Administration of a single Winter oral dose of 200,000 IU of vitamin D3 in adolescents in Normandy: evaluation of the safety and vitamin D status obtained]. Arch Pediatr 2010;17:1042–6.
Fraser D . The relation between infantile hypercalcemia and vitamin D–public health implications in North America. Pediatrics 1967;40:1050–61.
Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011;365:410–21.
Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW . High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med 2006;1:59–70.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.
Lips P . Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl 2012;243:60–4.
Zhou Y, Zhao LJ, Xu X, et al. DNA methylation levels of CYP2R1 and CYP24A1 predict vitamin D response variation. J Steroid Biochem Mol Biol 2013.
Zipf G, Chiappa M, Porter KS, et al. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. Washington, DC: National Center for Health Statistics (NCHS), 2013.
R Core Team. R: A Language and Environment for Statistical Computing. 3.0.2 edn. Vienna, Austria: R Foundation for Statistical Computing, 2013.
Lumley T . Survey: Analysis of Complex Survey Samples. 3.28-2 edn. Seattle, WA: 2012.
Lumley T . Analysis of complex suvey samples. J Stat Softw 2004;9:1–19.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weishaar, T., Rajan, S. Importance of body weight and skin color in determining appropriate vitamin D3 supplement doses for children and adolescents. Pediatr Res 77, 370–375 (2015). https://doi.org/10.1038/pr.2014.190
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2014.190
This article is cited by
-
The role of single nucleotide variant rs3819817 of the Histidine Ammonia-Lyase gene and 25-Hydroxyvitamin D on bone mineral density, adiposity markers, and skin pigmentation, in Mexican population
Journal of Endocrinological Investigation (2023)