Abstract
A surface-spreading synaptonemal complex (SC) technique was used to analyse spermatocytes and oocytes of triploid turbot (Scophthalmus maximus) in order to visualise the process of chromosome synapsis. The most conspicuous characteristic of triploid oocytes is that, in the trivalents, the lateral elements of the SC were frequently associated in threes, either completely along the length of the trivalent, or partially, forming a variety of forked structures. In these nuclei, synapsis usually occurred among homologous chromosomes and the number of bivalents observed was significantly higher than that expected under the assumption of random chromosome association among all partners. However, the frequency of trivalents was very low in triploid spermatocytes, triple synapsis being also scarce. In these nuclei chromosomes that were excluded from homologous synapsis become engaged in random SC formation, and, therefore a considerable number of non-homologous associations are produced. The causes of the synaptic differences observed in triploid males and females of turbot and their possible relation to the sterility displayed by these animals are discussed.
Similar content being viewed by others
Introduction
All female, or sterile, populations have been obtained in many fish species by chromosome set manipulation through gynogenesis and triploidy, respectively (Thorgaard, 1983; Benfey, 1989). Recently, chromosome set manipulation has been applied to marine fishes important for European aquaculture including seabream, seabass and different flatfishes (Gorshkova et al, 1995; Howell et al, 1995; Garrido-Ramos et al, 1996;Felip et al, 1997).
The existence of three or more complete sets of chromosomes in autopolyploids implies that homologous chromosomes have to compete for synapsis during meiosis. Surface-spreading techniques for making whole mount preparations of synaptonemal complexes (SCs) allow a direct quantification of the different synaptic configurations. In most cases, only two chromosomes synapse at any one site but when changes of synaptic partner occur they lead to multivalent formation. If crossovers occur on both sites of a synapsis partner exchange (SPE), a pachytene multivalent will be manifested as a metaphase I multivalent (Sybenga, 1975). Triploid organisms segregate the chromosomes of trivalents randomly in meiosis, which leads to the production of aneuploid gametes. When fertilisation takes place, these gametes produce zygotes with multiple trisomy, which are frequently inviable. Most studies of reproductive physiology in triploid fishes have concluded that triploid females do not produce mature oocytes, although triploid males are able to produce mature, postmeiotic cells, albeit generally aneuploid and in small numbers (Benfey, 1999).
The culture of turbot (Scophthalmus maximus) is firmly established in Europe and triploid individuals have been obtained in order to avoid problems related to sexual maturation (Piferrer et al, 2000, 2001). Sexual maturation usually occurs in diploid males and often in females before they reach marketable size which leads to a reduction in the somatic growth and to an increase in mortality. A previous SC analysis in diploid turbot has revealed that the pachytene karyotype in both male and female nuclei consisted in 22 SCs, corresponding to two submetacentric chromosome pairs, 11 subtelocentric pairs and nine telocentric pairs. The mean length of SC complement in males was 205 ± 12 μm, individuals ranging from 170 to 226 μm, and in the female analysed it was 172 ± 29 μm. No bivalent exhibiting an atypical synaptic behaviour, that is often associated with heteromorphic sex chromosomes, was observed (Cuñado et al, 2001). Here we study, within a wider programme dedicated to analyse the performance of triploid turbot, the chromosome synaptic behaviour in spermatocytes and oocytes in order to ascertain the origin of the sterility in these fishes.
Materials and methods
Three young males and three young females of triploid turbot (S. maximus) were obtained from the Instituto Oceanográfico at Vigo (Pontevedra, Spain). Triploids constituted by two maternal (female pronucleus plus second polar body) and one paternal chromosome complements were obtained by cold shock after fertilisation following the method described by Piferrer et al (2000, 2001).
SC spreading was carried out according to the method used by Cuñado et al (2000). The electron microscope used was a Jeol 1200. The absolute lengths of synaptonemal complexes were measured from enlarged photographic prints using the Image Tool program.
Results
Synapsis in triploid females
Information was available from 33 oocytes among the 70 examined by electron microscopy. Zygotene nuclei were scarce, only four showing spreads suitable for analysis while 29 pachytene nuclei could be completely analysed. Pachytene data from the three triploid females were pooled because they showed similar patterns of SC formation. The observation that the earlier stretches of SC that appear at zygotene are made up of two chromosomes (Figure 1) suggests that triple synapsis does not occur by simultaneous alignment of the three chromosomes at zygotene but takes place slightly later.
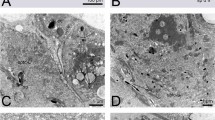
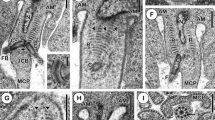
At zygotene, synapsis starts at or near chromosome ends in a pairwise fashion (Figure 1). Table 1 shows the frequencies of the different SC configurations observed in the fully traced pachytene nuclei of triploid females. Chromosomes of the different 22 trisomes (sets of three homologous chromosomes) formed usually either one bivalent plus one univalent or one trivalent (Figure 2a). Three unsynapsed homologous axes were not detected. Three types of bivalents were distinguished: standard (axes of equal length fully synapsed), those in which the third homologous axis showed a parallel alignment over long distances with respect to the synapsed chromosomes (Figure 2b) and heteromorphic (synapsis between unequal axes). Three types of trivalents were identified: (i) standard trivalents in which two chromosomes synapsed partially with different regions of the third chromosome (Figure 2g); (ii) fully synapsed trivalents showed triradial synapsis in which two of the branches presumably were homologously synapsed and the third one was non-homologously synapsed (Table 1); (iii) trivalents with triple synapsis, ie, the lateral elements of the SC were associated in threes, either completely along the length of the trivalent or partially, forming a variety of forked structures (Figures 2a, c–f, Table 1). The extent of triple synapsis ranged from 5% to 100% of the total length of the chromosome but only 20% of trivalents (25/123) showed more than 50%, usually involving distal and interstitial chromosome regions. Non-homologous synapsis was revealed by the presence of multivalents, triradial trivalents and partially self-synapsed univalents (Table 1).
Different aspects of synapsis in silver-stained spreads of triploid oocytes. (a) Electron micrograph of a pachytene nucleus. Arrowheads indicate some trivalents with triple synapsis. Arrows indicate some standard trivalents with a synapsis partner exchange (SPE). Asterisks mark some univalents. Bar represents 2.5 μm. Selected chromosome configurations (b–g): Bivalent with the third axis aligned (b). Trivalents with partial triple synapsis at interstitial (c–d) or distal regions (e). Almost fully triple synapsed trivalent (f). Standard trivalent with two SPEs (g).
The bivalent mean per triploid nucleus was 15.07 ± 0.57, a value far higher than expected, 7.33, under the random end-pairing model. That is, assuming random chromosome pairing, and that initiation of synapsis occurs at both ends without interstitial autonomous synapsis sites (ASSs). The expected bivalent mean value has been calculated as follows: if synapsis starts at each end, there will be a pairing between two of the three chromosomes. Thus, three pairings are possible, and the probability that the same two chromosomes get paired at both ends of a trivalent is one in three. One-third multiplied by 22 trisomes gives an expectation of 7.33 bivalents. The existence of both, standard trivalents with two SPEs and trivalents with triple synapsis at interstitial regions, invalidates this model since they indicate that synapsis can be initiated not only at the ends but also at intermediate chromosome regions. For instance, three ASSs with random pairing would produce 1/9 (11.2%) bivalents. This implies that the expected bivalent mean would be even less than 7.33 and, therefore the actual deviation between observed and expected bivalent frequencies are even higher.
Intercalary associations in trivalents may be caused either by synapsis partner exchange or through triple SC stretches. In the latter case the limited resolution of the fine structure, especially when triple synapsis is restricted to chromosome ends or regions close to them, precludes the assessment of the frequency of SPEs in these types of trivalents. Therefore, we have carried out an approach analysing only the number of SPEs in standard and triradial trivalents. Among 110 of these types of trivalents (Table 1), 97% (107/110) contained only one SPE and the remainder two SPEs. Most turbot chromosomes are telo- or subtelocentric, but centromeres were not consistently preserved in all chromosomes in SC spreads. To investigate the distribution of SPEs along the chromosomes, trivalent axes were divided into halves and the chromosome half containing the SPE was then divided into four intervals of equal length. Each SPE was included in one of the four intervals. Intervals were numbered from 1 to 4 in the direction from the end to the chromosome centre. The number of the interval where each SPE was located defined its position relative to the chromosome centre. The observed numbers of SPEs in each interval were plotted in a histogram shown in Figure 3. SPEs were located more often than expected on the basis of random distribution at or near the chromosome centre, intervals 3 and 4, rather than at the chromosome ends (χ2 = 32.16, d.f. = 3, P < 0.05).
Synapsis in triploid males
Information was available from 24 pachytene nuclei among the 65 examined by electron microscopy. Data from the three triploid males were pooled because they showed similar synaptic patterns. In most nuclei, well-defined multivalents and bivalents were located in the periphery of the spread with some evidence of a parallel alignment between the unsynapsed or partially synapsed homologous axes. On the contrary, the axes corresponding to univalents were in the centre of the spread forming a complex network in which SC stretches were visualised. An exceptional nucleus with complete synapsis is shown in Figure 4. The impossibility of unequivocal identification of homologous synaptic situations has led us to consider only two parameters related to the synaptic process in spermatocytes: the number of fully synapsed bivalents per nucleus and the number of SPEs per trivalent.
Table 1 summarises the SC configurations that could be unambiguously established. The extremely high bivalent mean per triploid nucleus, 23.58 ± 0.48, contrasts with the low trivalent mean, 1.04 ± 0.2 and indicates that in most nuclei the number of bivalents exceed 22, that is the maximum value assuming homologous synapsis. This finding and the existence of complex multivalents and heteromorphic bivalents indicate that, in contrast to the situation observed in females, non-homologous synapsis is a widespread phenomenon in triploid males. In addition, the frequency of bivalents observed was even higher than that of females and cannot be explained under the assumptions of the random end pairing model. Triple synapsis was rarely observed and never exceeded 20% of the chromosome length. The distribution of SPEs was analysed in standard and fully synapsed trivalents (Figure 3) and showed that among 20 trivalents, only one contained two SPEs. As in females SPEs were located at, or near, the chromosome centre rather than at chromosome ends (χ2 = 8.9, d.f. = 3, P < 0.05).
Discussion
In Crepis and Allium triploids alignment of homologous chromosomes over considerable distances is maintained into pachytene (Loidl and Jones, 1986; Vincent and Jones, 1993), however only occasional alignment in distal regions has been reported in triploid and primary trisomics of rye (Santos et al, 1995; Díez et al, 2001). An intermediate situation could be observed in triploid females of turbot where often terminal and interstitial associations of non-synapsed chromosomes seem to contribute to their alignment (Figure 2b). On the contrary, examples of alignment were less frequent in triploid males.
Triple synapsis occurs in triploid turbot with higher frequency in females than in males. To date, the existence of triple synapsis had been only described in human foetuses with trisomy 21 (Wallace and Hultén, 1983; Speed, 1984) and triploids of a few organisms. For instance, the basidiomycete Coprinus cinereus (Rasmussen et al, 1981), solanaceous plants (Sherma et al, 1989), domestic fowl (Solar et al, 1991), Lolium multiflorum (Thomas and Thomas, 1994), Saccharomyces cerevisiae (Loidl, 1995) and Nile Tilapia (Carrasco, 1998), although only Coprinus, fowl, yeast and turbot showed triple synapsis extended to the whole chromosome complement. The effect of sex on parameters such as SC length (Wallace and Hultén, 1985), chiasma frequency (Jones and Croft, 1989; Cano and Santos, 1990) and the triggering of the metaphase I checkpoint (Roeder and Bailis, 2000) is well known. Therefore, the sex differences observed in triploid turbot regarding the synaptic behaviour, ie, frequency of trivalents, amount of triple synapsis and behaviour of univalents, may not be surprising. They could be conditional upon structural and mechanical differences present during meiotic prophase I regarding, for instance, chromatin organisation, activation of synaptic initiation sites or time of meiotic progression.
In mammals, the presence of asynapsed chromosomes at pachytene triggers a checkpoint that removes cells via a p53-independent apoptotic pathway (Odorisio et al, 1998). If a similar mechanism is active in turbot, the spermatocytes, which showed an overall higher degree of saturation of synapsis than oocytes, would have more possibilities to overcome the pachytene checkpoint and, consequently, some production of spermatozoa could be expected if they also overcome the metaphase I checkpoint. In fact, we have observed spermatozoa in triploid males, although with a low frequency. On the contrary, triploid females probably would not produce mature oocytes or would do very occasionally. Further studies on the reproductive physiology of triploid turbot are necessary to test this hypothesis.
The presence of only one SPE in most turbot trivalents is in agreement with previous observations on the initiation of synapsis in diploid turbot (Cuñado et al, 2001) and is consistent with the random end-pairing model. If synapsis involving pairs of homologues proceeded more or less simultaneously from chromosome ends, completion of synapsis should result in trivalents with a single SPE more or less centrally located 67% of the time (see Sybenga, 1975). This value corresponds to a bivalent mean of 7.33. However, there are interstitial ASSs, since interstitial triple synapsis or two SPEs are observed in some trivalents (Table 1). On these grounds, we would expect a frequency of trivalents that would be higher than 67% depending on the number of ASSs, and consequently a very low frequency of pachytene bivalents would be attained (Vincent and Jones, 1993). Surprisingly, triploid turbot showed an extremely high bivalent mean number per nucleus: 15.07 (females) and 23.58 (males). Two alternative and possibly complementary explanations could help to understand these findings: (i) If the triploids are obtained by the fusion of a male haploid gamete and a female diploid gamete resulting from the fusion of a female pronucleus and the second polar body as reported by Piferrer et al (2000), the two sets of chromatids of each female gamete should show a great genetic similarity. This fact could lead to synaptic preferences between the chromosomes depending on their origin. (ii) It is also possible that synapsis started between any two chromosomes of a homologous set from one end only and proceeded very quickly. Because of the small size of turbot chromosomes it would extend all along the bivalent before another initiation event took place. The differences in the bivalent frequencies between male and female turbot could be ascribed again to the specific characteristics of the synaptic process in each sex.
References
Benfey, TJ (1989). A bibliography of triploid fish, 1943 to 1988. Can Tech Rep Fish Aqua Sci, 1682: 33.
Benfey, TJ (1999). The physiology and behaviour of triploid fishes. Rev Fish Sci, 7: 39–67.
Cano, MI, Santos, JL (1990). Chiasma frequencies and distributions in gomphocerine grasshoppers: a comparative study between sexes. Heredity, 64: 17–23.
Carrasco, LAP (1998). The effects of induced trploidy on the reproduction of the rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oerochromis niloticus). PhD Thesis, Institute of Aquaculture, University of Stirling, UK.
Cuñado, N, Garrido-Ramos, MA, de La Herrán, R, Ruíz-Rejón, C, Ruíz-Rejón, M, Santos, JL (2000). Organization of repetitive DNA sequences at pachytene chromosomes of gilthead seabream Sparus aurata (Pisces, Perciformes). Chromosome Res, 8: 67–72.
Cuñado, N, Terrones, J, Sánchez, L, Martínez, P, Santos, JL (2001). Synaptonemal complex analysis in spermatocytes and oocytes of turbot, Scophthalmus maximus (Pisces, Scophtalmidae). Genome, 44: 1143–1147.
Díez, M, Santos, JL, Cuñado, N, Naranjo, T (2001). Meiosis in primary trisomics of rye: considerations for models of chromosome pairing. Chromosome Res, 9: 13–23.
Felip, A, Zanuy, S, Carrillo, M, Martínez, G, Ramos, J, Piferrer, F (1997). Optimal conditions for the induction of triploidy in the sea bass (Dicentrarchus labrax L.). Aquaculture, 152: 287–298.
Garrido-Ramos, M, de La Herrán, R, Lozano, R, Cárdenas, S, Ruíz-Rejón, C, Ruíz-Rejón, M (1996). Induction of triploidy in offspring of gilthead seabream (Sparus aurata) by means of heat shock. J Appl Ichthyol, 12: 53–55.
Gorshkova, G, Gorshkov, S, Haldani, A, Gordin, H, Knibb, W (1995). Chromosome set manipulation in marine fish. Aquaculture, 158: 157–158.
Howell, BR, Baynes, SM, Thompson, D (1995). Progress towards the identification of the sex-determining mechanisms of the sole, Solea solea (L.) by the induction of diploid gynogenesis. Aquacult Res, 26: 135–140.
Jones, GH, Croft, JA (1989). Chromosome pairing and chiasma formation in spermatocytes and oocytes of Dendrocoelum lacteum (Turbellaria, Tricladida); a cytogenetical and ultrastructural study. Heredity, 63: 97–106.
Loidl, J (1995). Meiotic chromosome pairing in triploid and tetraploid Saccharomyces cerevisiae. Genetics, 139: 1511–1520.
Loidl, J, Jones, GH (1986). Synaptonemal complex spreading in Allium. I. Triploid A. sphaerocephalon. Chromosoma, 93: 420–428.
Odorisio, T, Rodríguez, TA, Evans, EP, Clarke, AR, Burgoyne, PS (1998). The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat Genet, 18: 257–261.
Piferrer, F, Cal, RM, Álvarez-Blazquez, B, Sánchez, L, Martínez, P (2000). Induction of triploidy in the turbot (Scophthalmus maximus). I. Ploidy determination and the effects of cold shocks. Aquaculture, 188: 79–90.
Piferrer, F, Cal, RM, Gómez, C, Bouza, C, Martínez, P (2002). Induction of triploidy in turbot (Scophthalmus maximus). II. Effects of cold shock timing and induction of triploidy in a large volume of eggs. Aquaculture. (in press).
Rasmussen, SW, Holm, PB, Lu, BC, Zickler, D, Sage, J (1981). Synaptonemal complex formation and distribution of recombination nodules in pachytene trivalents of triploid Coprinus cinereus. Carlsberg Res Commun, 46: 347–360.
Roeder, GS, Bailis, JM (2000). The pachytene checkpoint. TIG, 16: 395–403.
Santos, JL, Cuadrado, MC, Díez, M, Romero, C, Cuñado, N, Naranjo, T, Martínez, M (1995). Further insights on chromosomal pairing of autopolyploids: a triploid and tetraploids of rye. Chromosoma, 104: 298–307.
Sherman, JD, Stack, SM, Anderson, LK (1989). Two-dimensional spreads of synaptonemal complexes from solanaceous plants. IV. Synaptic irregularities. Genome, 32: 743–753.
Solari, AJ, Thorne, MH, Sheldon, L, Gillies, CB (1991). Synaptonemal complexes of triploid (ZZW) chickens: Z-Z pairing predominates over Z-W pairing. Genome, 32: 743–753.
Speed, RM (1984). Meiotic configurations in female trisomy 21 foetuses. Hum Genet, 66: 176–180.
Sybenga, J (1975). Meiotic Configurations. Springer-Verlag: Berlin.
Thomas, HM, Thomas, B (1994). Meiosis in triploid Lolium. I. Synaptonemal complex formation and chromosomes configurations at metaphase I in aneuploid autotriploid L. multiflorum. Genome, 37: 181–189.
Thorgaard, GH (1983). Chromosome set manipulation and sex control in fish. In: Hoar WS, Randall DJ, Donalson EM (eds) Fish Physiology, vol. 9B, Academic Press: New York, pp. 405–434.
Vincent, JE, Jones, GH (1993). Meiosis in autopolyploid Crepis capillaris.I. Triploids and trisomics; implications for models of chromosome pairing. Chromosoma, 102: 195–206.
Wallace, BMN, Hultén, MA (1983). Meiotic configurations in female trisomy 21 foetuses. Hum Genet, 66: 176–180.
Wallace, BMN, Hultén, MA (1985). Meiotic chromosome pairing in normal human female. Ann Hum Genet, 49: 215–226.
Acknowledgements
We are indebted to J Barrios and S Vidal for their valuable technical assistance. We also thank the contribution of F Piferrer (Instituto Ciencias del Mar, Barcelona) and to RM Cal (Instituto Español de Oceanografía, Vigo) for obtaining and supplying the triploid individuals. This work was supported by grant PB98–0107 awarded by the Dirección General de Enseñanza Superior (DGES, Spain) and by the Spanish Government FEDER grant 1FD1997–2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuñado, N., Terrones, J., Sánchez, L. et al. Sex-dependent synaptic behaviour in triploid turbot, Scophthalmus maximus (Pisces, Scophthalmidae). Heredity 89, 460–464 (2002). https://doi.org/10.1038/sj.hdy.6800165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800165
Keywords
This article is cited by
-
Gene expression analysis at the onset of sex differentiation in turbot (Scophthalmus maximus)
BMC Genomics (2015)
-
Consolidation of the genetic and cytogenetic maps of turbot (Scophthalmus maximus) using FISH with BAC clones
Chromosoma (2014)
-
Triploid planarian reproduces truly bisexually with euploid gametes produced through a different meiotic system between sex
Chromosoma (2014)