Abstract
To determine the impact of breast conservation on quality of life and identify treatment-related and other demographic factors associated with post-breast cancer treatment quality of life. A prospective study was conducted on 392 women who underwent breast cancer surgery at Hangzhou Cancer Hospital from January 1, 2013, to December 31, 2022. Operable breast cancer patients who had completed all treatments except endocrine therapy were included. Patients with tumor recurrence/metastasis, bilateral or male breast cancer, and other primary malignancies were excluded. After enrollment, patients were asked to complete the BREAST-Q scale, and their pathological and medical records were reviewed. Analysis of variance was used to compare the quality of life scores among the groups. Univariate and multivariate linear regression analyses were performed to identify independent factors associated with quality of life scores in different domains. Participants completed the BREAST-Q scale at a median of 4.6 years after surgery. Quality of life scores varied based on the therapeutic strategy. Breast conservation has significant advantages over mastectomy in terms of breast satisfaction, psychosocial, and sexual well-being. Compared to oncoplastic breast-conserving surgery, mastectomy was independently associated with decreased breast satisfaction, psychosocial, and sexual well-being, while conventional breast-conserving surgery showed comparable outcomes to oncoplastic breast-conserving surgery in terms of these factors. Breast conservation leads to an improvement in quality of life compared to mastectomy. Oncoplastic breast-conserving surgery does not lead to a decrease in quality of life compared to conventional breast-conserving surgery and offers better outcomes compared to mastectomy.
Similar content being viewed by others
Introduction
Breast cancer is a prevalent global malignancy1, and breast-conserving surgery (BCS) with adjuvant radiotherapy (RT) is a well-established treatment for early-stage breast cancer2,3. However, up to 30% of BCS recipients express dissatisfaction with their postoperative appearance, necessitating corrective interventions4. In the 1980s, European surgeons introduced "oncoplastic breast-conserving surgery" (OBCS), which incorporates plastic surgery techniques for post-BCS breast defect reconstruction5.
While OBCS offers satisfactory long-term oncological results and broadens treatment possibilities for patients who would typically undergo mastectomies6, it involves more extensive incisions, additional tissue manipulation, and potential flap reconstruction in comparison to conventional breast-conserving surgery (cBCS)7,8. The procedures involved in OBCS are more complex, time-consuming, and costly. Given these complexities, is it still worthwhile to pursue breast conservation by OBCS? Some researchers have proposed whether the use of OBCS should be reduced9.
Understanding the impact on the quality of life of breast cancer survivors is crucial given its significant influence on medical decision-making10,11. Despite the widespread utilization of OBCS to conserve the breast and enhance its aesthetics, research on its impact on quality of life is limited and complicated due to the variability of surgical approaches. Consequently, this study aimed to assess the effect of breast conservation by OBCS on the quality of life of patients with operable breast cancer treated at Hangzhou Cancer Hospital from January 1, 2013, to December 31, 2022, and to elucidate the treatment and demographic factors associated with postoperative quality of life.
Methods
Materials and methods
This prospective, cross-sectional, case–control study was conducted at a single center. The inclusion criteria were operable breast cancer patients treated at Hangzhou Cancer Hospital between January 1, 2013, and December 31, 2022, who had completed all treatments except endocrine therapy and provided participation consent. The exclusion criteria were patients with tumor recurrence/metastasis, bilateral or male breast cancer, or other primary malignancies. Participants were categorized into two groups: BCS group (cBCS with RT subgroup and OBCS with RT subgroup), and unilateral MAST group (MAST with RT subgroup and MAST without RT subgroup). This study utilized the BREAST-Q scale12, which includes separate modules for BCS and MAST without reconstruction. The BCS module was used for the OBCS with RT subgroup because OBCS in this study predominantly referred to oncoplastic lumpectomy/glandular remodeling. BREAST-Q assesses six distinct domains: satisfaction with breasts, psychosocial well-being, physical well-being, sexual well-being, satisfaction with overall outcome, and satisfaction with care. Due to the elapsed time between surgery and questionnaire completion in this study, the domains of satisfaction with the overall outcome and satisfaction with care were excluded. Each domain was scored on a scale from 0 to 100, with higher scores indicating an enhanced quality of life. Differences in BREAST-Q scores were categorized as small (2–3 points), moderate (4–7 points), and large (8–10 points)13. Patient characteristics, collected using the questionnaire, included employment status, educational level, marital status, and economic status. Patients’ medical and pathological records were reviewed to determine the disease tumor, node, and metastasis (TNM) staging14, erythroblastic oncogene B (ERBB2; formerly HER2/neu or HER2) status, hormone receptor status, and body mass index (BMI). Information on surgery, chemotherapy (yes/no), RT, and endocrine therapy (yes/no) was obtained using a questionnaire in conjunction with medical records. The lymphedema status (yes/no) was assessed using the questionnaire's question regarding arm swelling. This study was approved by the Ethics Committee of Hangzhou Cancer Hospital, and all participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and followed the guidelines of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guidelines.
Statistical analysis
The experimental data were statistically analyzed using SPSS (version 29.0) software, and categorical covariates were expressed as numbers (percentages). Analysis of variance (ANOVA) was used to compare quality of life scores among the different groups. Univariate and multivariate linear regression analyses were used to determine the independent factors associated with the quality of life scores in each domain. Variables with two-tailed P ≤ 0.15 in the univariate analysis were included in the multivariate analysis model using a stepwise method to establish the final multivariate model. Differences with P < 0.05 were considered statistically significant.
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of Hangzhou Cancer Hospital (approval number: [hzch-2023] HS no.007). Written informed consent was obtained from every patient.
Results
Patient enrollment
After screening, 623 eligible patients were invited, 456 provided written informed consent and completed the survey, but three were found to not meet the inclusion criteria after enrollment. After excluding 61 participants who only completed a brief questionnaire, a total of 392 patients’ data were included in the statistical analysis.
Patient, disease, and treatment characteristics
The interval between surgery and scale completion averaged 4.6 years (range: 0.33 to 9.83 years). Patient characteristics are detailed in Table 1. Majority were married, employed, had moderate economic status (income ¥30,000–200,000 per year), and high school or higher education. At surgery, 324 (82.7%) patients had a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) within the normal range (18.5 to 23.9 kg/m2), and 56 (14.3%) patients had a BMI of 24 kg/m2 or above. Among the patients, 39 (9.9%) had stage 0 breast cancer, 154 (39.3%) had stage I breast cancer, 158 (40.3%) had stage II breast cancer, and 41 (10.5%) had stage III breast cancer. The lesions on imaging before surgery of 253 (64.5%) patients measured two centimeters or less, 134 (34.2%) two to five centimeters, and 5 (1.3%) more than five centimeters. Chemotherapy was administered to 293 (74.7%) patients, with 121(30.9%) receiving neoadjuvant chemotherapy, and 273 (69.6%) patients received hormone therapy.
Treatment details including surgery, RT, and lymphedema are presented in Table 1. Among the patients, 88 (22.4%) underwent OBCS, 51 (13.0%) underwent cBCS, and 253 (64.5%) underwent unilateral MAST, among which 100 (25.5%) patients who underwent unilateral MAST received postoperative RT. All patients underwent axillary surgery, with 255 (65.1%) patients undergoing sentinel lymph node biopsy only and 137 (34.9%) patients undergoing axillary lymph node dissection. 61 (15.6%) patients reported having lymphedema.
BREAST-Q results by breast surgery strategy
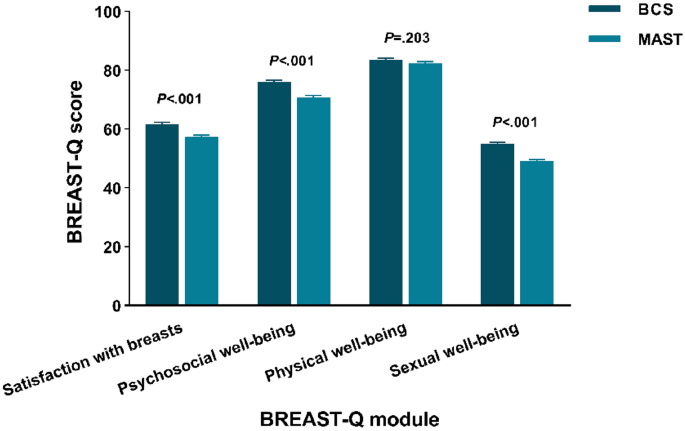
Figure 1 illustrates unadjusted mean BREAST-Q scores by breast surgery strategy. Satisfaction with breasts, psychosocial well-being and sexual well-being were significantly different among the groups (P < 0.001). BCS group showed higher scores in satisfaction with breasts (61.70), psychosocial well-being (76.01), physical well-being (83.52) and sexual well-being (55.06), while the scores for MAST group is lower (satisfaction with breasts: 57.30, psychosocial well-being: 70.83, physical well-being: 82.40 and sexual well-being: 49.21).
Satisfaction with breasts
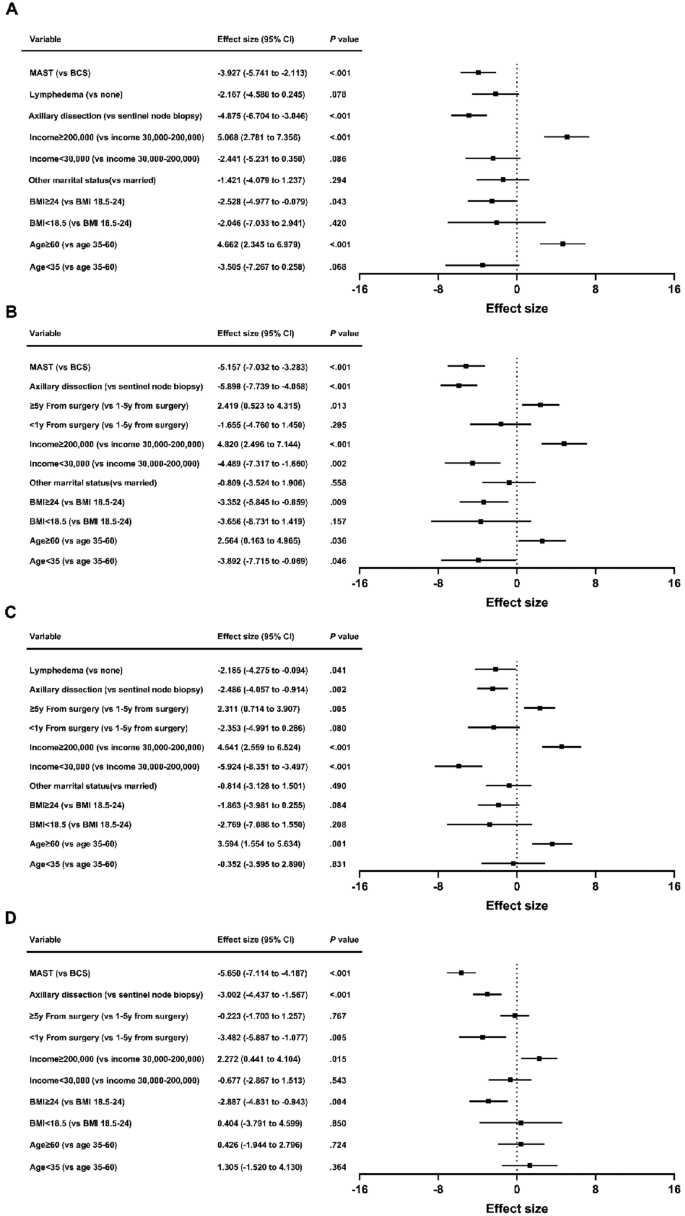
Higher scores in satisfaction with breasts correlated independently with age ≥ 60 (β = 4.662; 95% CI = 2.345 to 6.979; P < 0.001) and patient-reported income ≥ 200,000 (β = 5.068; 95% CI = 2.781 to 7.356; P < 0.001). Lower scores were associated with BMI ≥ 24 (β = − 2.528; 95% CI = − 4.977 to − 0.079; P = 0.043), axillary dissection (β = − 4.875; 95% CI = − 6.704 to − 3.046; P < 0.001) and MAST (β = − 3.927; 95% CI = − 5.741 to − 2.113; P < 0.001) (Fig. 2A). Patient-reported income < 30,000 and lymphedema showed significance only in univariate analysis. Other factors exhibited no significant association.
Psychosocial well-being
Better psychosocial well-being correlated with age ≥ 60 (β = 2.564; 95% CI = 0.163 to 4.965; P = 0.036), patient-reported income ≥ 200,000 (β = 4.820; 95% CI = 2.496 to 7.144; P < 0.001), and ≥ 5y from surgery (β = 2.419; 95% CI = 0.523 to 4.315; P = 0.013). Poor psychosocial well-being was linked to age < 35 (β = − 3.892; 95% CI = − 7.715 to − 0.069; P = 0.046), BMI ≥ 24 (β = − 3.352; 95% CI = − 5.845 to − 0.859; P = 0.009), patient-reported income < 30,000 (β = − 4.489; 95% CI = − 7.317 to − 1.660; P = 0.002), axillary dissection (β = − 5.898; 95% CI = − 7.739 to − 4.058; P < 0.001) and MAST (β = − 5.157; 95% CI = − 7.032 to − 3.283; P < 0.001) (Fig. 2B). Chemotherapy was only significant in univariate analysis. Other variables showed no significant association.
Physical well-being
Factors associated with better physical well-being were age ≥ 60 (β = 3.594; 95% CI = 1.554 to 5.634; P = 0.001), patient-reported income ≥ 200,000 (β = 4.541; 95% CI = 2.559 to 6.524; P < 0.001), and ≥ 5y from surgery (β = 2.311; 95% CI = 0.714 to 3.907; P = 0.005). Conversely, patient-reported income < 30,000 (β = − 5.924; 95% CI = − 8.351 to − 3.497; P < 0.001), axillary dissection (β = − 2.486; 95% CI = − 4.057 to − 0.914; P = 0.002) and lymphedema (β = − 2.185; 95% CI = − 4.275 to − 0.094; P = 0.041) were associated with poorer physical well-being (Fig. 2C). < 1y from surgery was only significant in univariate analysis. Other factors lacked significant association.
Sexual well-being
Multivariate analysis indicated lower sexual well-being scores with BMI ≥ 24 (β = − 2.887; 95% CI = − 4.831 to − 0.943; P = 0.004), < 1y from surgery (β = − 3.482; 95% CI = − 5.887 to − 1.077; P = 0.005), axillary dissection (β = − 3.002; 95% CI = − 4.437 to − 1.567; P < 0.001), and MAST (β = − 5.650; 95% CI = − 7.114 to − 4.187; P < 0.001). Patient-reported income ≥ 200,000 (β = 2.272; 95% CI = 0.441 to 4.104; P = 0.015) correlated with elevated sexual well-being (Fig. 2D). Lymphedema was significant in univariate analysis. Other variables exhibited no significant correlation.
BREAST-Q results by local therapy strategy
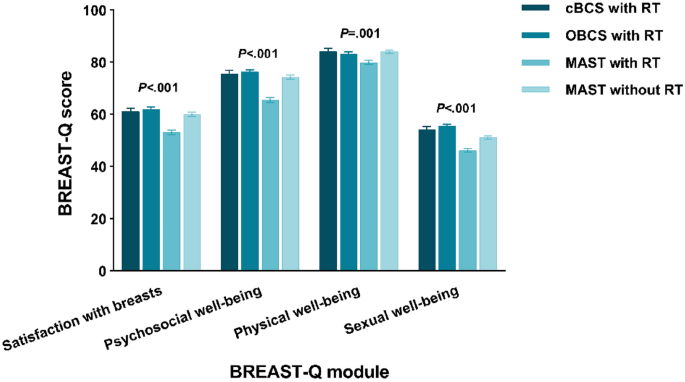
To assess if there were enhancements in quality of life among women who underwent OBCS, we performed similar analyses among the subgroups. Figure 3 illustrates unadjusted mean BREAST-Q scores by local therapy strategy. All four domains were significantly different (P < 0.05). OBCS with RT group showed highest scores in satisfaction with breasts (61.99), psychosocial well-being (76.27) and sexual well-being (55.53). cBCS with RT group yielded the highest physical well-being score (84.10). The lowest domain scores were in MAST with RT group (satisfaction with breasts: 53.11, psychosocial well-being: 65.49, physical well-being: 79.89 and sexual well-being: 46.24).
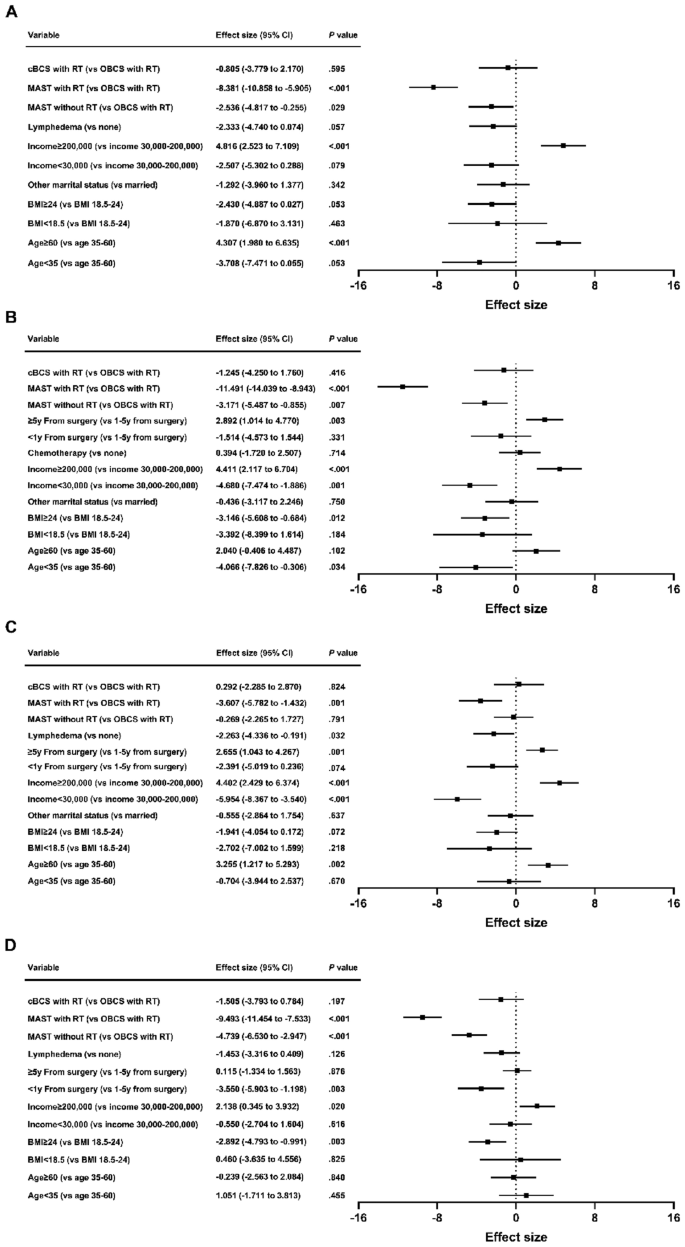
Multivariate analysis indicated that MAST with RT was associated with poor breast satisfaction (β = − 8.381; 95% CI = − 10.858 to − 5.905; P < 0.001), psychosocial well-being (β = − 11.491; 95% CI = − 14.039 to − 8.943; P < 0.001), physical well-being (β = − 3.607; 95% CI = − 5.782 to − 1.432; P = 0.001) and sexual well-being (β = − 9.493; 95% CI = − 11.454 to − 7.533; P < 0.001). MAST without RT was associated with decreased breast satisfaction (β = − 2.536; 95% CI = − 4.817 to − 0.255; P = 0.029), psychosocial well-being (β = − 3.171; 95% CI = − 5.487 to − 0.855; P = 0.007) and sexual well-being (β = − 4.739; 95% CI = − 6.530 to − 2.947; P < 0.001). cBCS with RT was not associated with BREAST-Q scores on univariate or multivariate analysis. The statistically significant factors correlated with BREAST-Q scores were mostly consistent with the outcomes of the breast surgery models (Fig. 4).
Patient and treatment factors associated with breast satisfaction (A), psychosocial well-being (B), physical well-being (C) and sexual well-being (D) scores by local therapy strategy. cBCS: conventional breast-conserving surgery; OBCS: oncoplastic breast-conserving surgery; MAST: mastectomy; RT: radiotherapy; BMI: body mass index; CI: confidence interval.
Discussion
The rates of BCS and breast reconstruction after mastectomy are significantly lower in China than in Western countries15. One contributing factor is that Chinese women typically have smaller breast sizes than women in Western countries, while presenting with larger breast tumor volumes at the time of initial diagnosis, making BCS challenging. Additionally, some Chinese patients adhere to outdated beliefs and have concerns about potential impacts on treatment outcomes or cancer recurrence associated with BCS. OBCS provides acceptable long-term oncological outcomes and has extended treatment options for patients who would traditionally be candidates for mastectomies6. In recent years, there has been a clear change in the emphasis of surgical oncology in China, with a growing emphasis on utilizing modern oncoplastic surgical techniques to perform more breast conserving surgeries. Given the increasing prevalence of OBCS, it is essential to examine its impact on quality of life.
In this single-center prospective study, discernible disparities in quality of life surfaced among patients with breast cancer undergoing various local treatment strategies within ten years of surgery. Patients opting for more extensive surgery, particularly when combined with RT, experienced diminished quality of life; satisfaction with breasts; and psychosocial, physical, and sexual well-being. This aligns with findings from prior studies. Engel et al.’s study16 has shown that patients undergoing BCS reports a higher quality of life compared to those opting for mastectomy. This improvement is often linked to the conservation of the breast and the associated psychological advantages. BCS enables breast conservation, leading to enhanced body image and self-esteem. Patients undergoing BCS may experience less psychological distress and enjoy better psychosocial well-being due to breast conservation. Additionally, BCS has a lesser impact on sexual well-being in comparison to mastectomy, as it retains natural breast tissue.
This study’s findings concur with those of Otsuka et al.’s study17 in that oncoplastic surgery improved satisfaction with breasts. However, in Otsuka et al.’s study, the quality of life score was not elevated by OBCS (major breast surgery: 154.5 ± 24.6; minor breast surgery: 159.0 ± 20.8; OBCS: 158.7 ± 14.0). Although differences exist between major breast surgery and OBCS, the difference is not pronounced. In the present study, psychosocial and sexual well-being scores were elevated compared to MAST. Additionally, patients who underwent OBCS had better physical well-being scores than those who underwent MAST with RT and equal physical well-being scores than those who underwent MAST without RT. This may be attributable to the omission of RT, reduced chemotherapy and lymphedema in the MAST without RT group. Previous studies18,19 have highlighted RT, chemotherapy, and lymphedema as adverse determinants of quality of life.
Rose et al.20 suggested that patients who underwent OBCS showed significant improvement in the “psychosocial well-being” module compared to cBCS, while no significant differences were observed between the two groups in the “physical health,” “breast satisfaction,” and “sexual health” modules. Furthermore, a meta-analysis21 indicated improved quality of life with OBCS compared with cBCS in patients with early-stage breast cancer, with better physical and psychological well-being, higher self-esteem, and a more stable body image, leading to improved social and emotional functioning. However, the clinical studies included in the meta-analysis were predominantly small- sample studies from single centers, and the surgical approaches varied. This study identified no significant differences in any of the quality of life modules between the patients who underwent OBCS and those who underwent cBCS, which is consistent with the findings of de Oliveira-Junior et al22. This may be because the present study’s follow-up time was longer, and several aspects of OBCS will decline over time23. In our study, the tumor lesion on imaging before surgery averaged 2.11 ± 0.67 cm in OBCS subgroup, and 1.62 ± 0.52 cm in cBCS subgroup. Smaller lesions are more likely to undergo cBCS, resulting in comparable cosmetic outcomes between the two surgical groups. Moreover, the limited number of BCS patients in our study is a significant factor that limits the ability to detect differences in quality of life between OBCS and cBCS subgroups.
In addition to the type of surgery, other clinical factors such as BMI (≥ 24), income (< 30,000), < 1y from surgery, axillary dissection, and lymphedema were negatively correlated with quality of life. Identifying these risk factors can facilitate early postoperative intervention and ultimately improve the postoperative quality of life of patients with breast cancer. Age (≥ 60) and ≥ 5y from surgery were associated with enhanced quality of life. Breast cancer patients can experience significant effects from the disease itself and the ongoing adjuvant therapies, both after diagnosis and during the treatment process24. These are all factors that lead to decreased quality of life within 5 years, especially within 1 year, rather than ≥ 5y after surgery. Moreover, good economic status was associated with better satisfaction with breasts, and psychosocial, physical, and sexual well-being. Patients with improved financial circumstances can access higher-quality healthcare services, opt for more expensive treatment options that may improve aesthetic outcomes. The financial advantage also affords patients more opportunities for supportive care, counseling, and resources to manage the challenges of breast cancer treatment and recovery, resulting in a decrease in stress, anxiety, and depression. These enhancements can have a positive impact on patients’ self-perception, confidence, and overall satisfaction with their breast appearance, all of which are closely connected to sexual health and intimacy. Notably, other studies25,26 found an association between economic status and quality of life.
This study has some limitations. It was a cross-sectional, single-time, survey-based prospective study; therefore, the baseline quality of life of patients before surgery was not recorded, which may have influenced their choice of surgical approach and postoperative quality of life. Additionally, this study did not identify patients who chose MAST due to refusal of BCS; patients who selected MAST based on personal preferences may have different quality-of-life scores. Furthermore, this study did not include patients with postmastectomy breast reconstructions, which may improve quality of life of postmastectomy patients. Finally, given that this was a single-center small-sample study, studies with larger sample sizes are required to further confirm the findings of this study. Nevertheless, patient-reported questionnaires can provide basic information on quality of life and assist in identifying potential areas requiring intervention during the patient’s survival period.
Conclusion
OBCS is an acceptable option for patients with larger tumors who are not suitable for cBCS because it allows them to conserve their breasts6. This study demonstrated that patients who had their breast conserved reported a higher quality of life compared to mastectomy patients. Despite extensive incisions, additional tissue manipulation, and potential flap reconstruction, patients who underwent OBCS did not report a lower quality of life than those who underwent cBCS. Furthermore, they experienced significantly enhanced quality of life compared with patients who underwent MAST, particularly in the domains of satisfaction with breasts, psychosocial well-being, and sexual well-being. Quality of life data should be incorporated into decision support tools to assist patients with breast cancer in selecting the surgical approach, and discussions with patients should include information regarding quality of life to ensure that they understand the long-term impacts of different surgical approaches. This is particularly crucial because most patients with breast cancer have an extended postoperative survival period. Our data can support further improvements in Chinese breast surgical care for better survival and quality of life.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to Chinese law but are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Fisher, B. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 347(16), 1233–1241 (2002).
Veronesi, U. et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 347(16), 1227–1232 (2002).
Clough, K. B., Cuminet, J., Fitoussi, A., Nos, C. & Mosseri, V. Cosmetic sequelae after conservative treatment for breast cancer: Classification and results of surgical correction. Ann. Plast. Surg. 41(5), 471–481 (1998).
Audretsch, W. et al. Oncoplatic surgery in breast conserving therapy and flap supported operability. In Proceedings of the Annual Symposium on Breast Surgery and Body Contouring. Santa Fe, New Mexico (1993).
Calabrese, C. et al. Oncoplastic conservative surgery for breast cancer: Long-term outcomes of our first ten years experience. Eur. Rev. Med. Pharmacol. Sci. 22(21), 7333–7342 (2018).
Mohamedahmed, A. Y. Y. et al. Comparison of surgical and oncological outcomes between oncoplastic breast-conserving surgery versus conventional breast-conserving surgery for treatment of breast cancer: A systematic review and meta-analysis of 31 studies. Surg. Oncol. 42, 101779 (2022).
Knowles, S. et al. An alternative to standard lumpectomy: A 5-year case series review of oncoplastic breast surgery outcomes in a Canadian setting. Can. J. Surg. 63(1), E46–E51 (2020).
Bonci, E. A., Anacleto, J. C. & Cardoso, M. J. Sometimes it is better to just make it simple. De-escalation of oncoplastic and reconstructive procedures. Breast. 69, 265–273 (2023).
Ohsumi, S. et al. Factors associated with health-related quality-of-life in breast cancer survivors: Influence of the type of surgery. Jpn. J. Clin. Oncol. 39(8), 491–496 (2009).
Luini, A. et al. The evolution of the conservative approach to breast cancer. Breast. 16(2), 120–129 (2007).
Pusic, A. L. et al. Development of a new patient-reported outcome measure for breast surgery: The BREAST-Q. Plast. Reconstr. Surg. 124(2), 345–353 (2009).
Voineskos, S. H., Klassen, A. F., Cano, S. J., Pusic, A. L. & Gibbons, C. J. Giving meaning to differences in BREAST-Q scores: Minimal important difference for breast reconstruction patients. Plast. Reconstr. Surg. 145(1), 11e–20e (2020).
American Joint Committee on Cancer. AJCC Cancer Staging Manual 8th edn, 589–628 (Springer, 2017).
Chen, Y. et al. Current trends of breast reconstruction after mastectomy for breast cancer patients in China: A survey report. Zhonghua Zhong Liu Za Zhi. 36(11), 851–857 (2014).
Engel, J., Kerr, J., Schlesinger-Raab, A., Sauer, H. & Hölzel, D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 10(3), 223–231 (2004).
Otsuka, S., Watanabe, N., Sasaki, Y. & Shimojima, R. Postoperative courses of breast reconstruction using inferior adipofascial tissue repair. Breast Cancer. 22(6), 570–577 (2015).
Chu, C. N., Hu, K. C., Wu, R. S. & Bau, D. T. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy. BMC Cancer. 21(1), 330 (2021).
Mokhtari-Hessari, P. & Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes. 18(1), 338 (2020).
Rose, M. et al. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res. Treat. 180(1), 247–256 (2020).
Aristokleous, I. & Saddiq, M. Quality of life after oncoplastic breast-conserving surgery: A systematic review. ANZ J. Surg. 89(6), 639–646 (2019).
de Oliveira-Junior, I. et al. Oncoplastic surgery in breast-conserving treatment: Patient profile and impact on quality of life. Breast Care 16(3), 243–253 (2021).
Maguire, P. D., Adams, A. & Nichols, M. A. Oncoplastic surgery and radiation therapy for breast conservation: Early outcomes. Am. J. Clin. Oncol. 38(4), 353–357 (2015).
Ohsumi, S., Shimozuma, K., Kuroi, K., Ono, M. & Imai, H. Quality of life of breast cancer patients and types of surgery for breast cancer—current status and unresolved issues. Breast Cancer. 14(1), 66–73 (2007).
Bowen, D. J. et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res. Treat. 106(1), 85–95 (2007).
Lathan, C. S. et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J. Clin. Oncol. 34(15), 1732–1740 (2016).
Disclaimers
The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Hangzhou Cancer Hospital is intended nor should be inferred.
Funding
This research was financed by the Medical and Health Research Project of Zhejiang Province, China (No. 2023KY964).
Author information
Authors and Affiliations
Contributions
YW and SX contributed to the conception, design, wrote the manuscript and analyzed the data; YH was responsible for the execution and for data collection; and SW supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., He, Y., Wu, S. et al. Disparities in quality of life among patients with breast cancer based on surgical methods: a cross-sectional prospective study. Sci Rep 14, 11364 (2024). https://doi.org/10.1038/s41598-024-62105-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62105-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.