Abstract
A green technique that emerged as a promise in the degradation of numerous organic contaminants is photocatalysis. The aim of this study concerns photocatalytic degradation of organic using titanium dioxide nano particles (TiO2 NPs) which syntheses from ilmenite by different leaching methods using different ingredients such as HCl, HNO3 and Aqua Regia. The affecting factors such as rate of addition, reaction time, ilmenite grain size, acid to ilmenite ratio and reaction temperature were conducted. Comprehensive physicochemical characterization of Ilmenite and TiO2 NPs were conducted using different analytical techniques such as XRD, XRF, SEM, TEM and FTIR. Photocatalytic degradation of organics is confirmed by studies of affecting factors on the effectiveness of TiO2 NPs such as dose, agitation forces, light intensity, initial concentration, pH, time, and temperature. The removal percentages of TSS, COD, BOD and TN of organics were explored. From the results the maximum removal percentage of TSS were 97.3 and 96.9% before and after secondary treatment conducted using ferric chloride (FC). The maximum removal percentage of TKN, BOD, and COD before secondary treatment were conducted using mixture of TiO2 NPs, FC, and chitosan, which reached 44.2, 44 and 46.3%, respectively. The maximum removal percentage of TKN, BOD, and COD after secondary treatment were conducted using mixture of TiO2 NPs, FC, and chitosan, which reached 94.9, 99.7 and 99.6%, respectively. Overall, the results derived from this investigation suggest that the TiO2 NPs/UV holds significant advanced treatment of sewage water, making it a viable choice for water reuse applications.
Similar content being viewed by others
Introduction
The continued social and economic development and population growth are increasing pressure on the world's water resources, facing the challenges of water scarcity and deteriorating water quality1,2,3. Water scarcity is a serious issue that affects both humankind's way of life and the global economy4,5,6. One of the potential solutions to deal with water scarcity is utilizing non-conventional water resources7. Currently, emerging contaminants (ECs) have been detected in diverse effluents of surface water and treated municipal wastewater. ECs include pesticides, pharmaceuticals, and personal care and household items8 which, should be removed due to their toxicity, and other undesirable qualities9,10. Using the advanced oxidation processes (AOPs) is sufficient for these non-degradable pollutants in the wastewater drains that cannot be destroyed by conventional treatment9,11,12,13. AOPs, which depend on the non-selective reaction of hydroxyl radicals (⋅OH), are effective methods to degrade organic contaminant14,15. Heterogeneous AOPs include catalytic ozonation, photocatalytic ozonation, and heterogeneous photocatalysis16,17. The effective photocatalyst must be chemically and biologically inert, non-toxic, photoactive, economical, photostable, and able to use visible or near UV light. Catalysts include Si, WO3, ZnO, CdS, TiO2, ZnS, Fe2O3, SnO2, etc.18. Titanium dioxide (TiO2) is a commonly used catalyst due to its distinctive optical features, non-toxicity, low cost, and great photochemical stability19,20,21,22. There are various sources for titanium dioxide which is found in the form of ilmenite (40–80% TiO2) and mineral sand deposits such as anatase (> 95%TiO2), rutile (~ 95% TiO2) and leucoxene (> 65% TiO2)23. It’s important to create high-purity titanium dioxide from ilmenite by developing appropriate techniques with little environmental impact24. There are two established methods to produce titanium dioxide from ores: the chloride process and the sulfate process24,25. The chloride process is more efficient than sulphate method23,26. There is an important need to discover alternative techniques to extract high-grade TiO2 from titanium ores e.g., ilmenite which is available in large quantities in nature.
The goal of this paper is to extract titanium dioxide (TiO2) from ilmenite by different leaching agents such as: (1) HCl, HNO3, and Aqua Regia (mixture of nitric acid and hydrochloric acid) solutions. (2) Determine the optimal conditions for high-grade TiO2 extraction. (3) Characterization of raw ilmenite, intermediate materials and extracted titanium dioxide using different analytical techniques. (4) Conversion of titanium dioxide to titanium dioxide nano particle (TiO2NPs). (5) Chemical treatment of sewage wastewater using ferric chloride which is a byproduct of extracted titanium dioxide (6) utilization of TiO2 for removal of organics.
Materials and methods
Materials
Ilmenite samples were collected from the western side of Wadi Abu Ghalaga, South Eastern Desert of Egypt. Humic acid, hydrochloric acid (HCl) and nitric acid (HNO3) were obtained from the Fisher Scientific Company. Aqua regia solution was prepared in the laboratory (HNO3 and HCl, 1:3). Double distilled water was used. Dissolution experiments of ilmenite ore were conducted in rounded flasks attached to a condenser and stirrer.
Experimental procedure
As shown in Fig. 1 the systematic diagram of all experimental procedures is explored.
Leaching titanium dioxide and ferric chloride (FC) from ilmenite
Ilmenite samples were crushed and ground by using a grinding machine. The crushed samples were sieved through a 200-mesh sieve (75 µm). The ilmenite sample leached with acids under several conditions. After each leaching experiment, the product was filtered, and rinsed with distilled water, the filtrate called FC. Several affecting factors were studied including acids ratio, grain size, temperature, flow rate (amount of acid add (ml) per time(minute), and reaction time as follows:
Effect of acid ratio
The ratio of 1:1, 1:2.1:31:6 at a constant (flow rate (0.5 ml/min), time of reaction (3 h) ilmenite particle size 75 micron, and reaction temperature 120 °C.
Effect of reaction time
30, 60, 120, 180, 240 min were chosen at constant reaction time 3 h., flow rate (0.5 ml/min), ilmenite particle size 75-micron, ilmenite acid ratio (1:3) and reaction temperature 120 °C.
Effect of flow rate
0.5, 1, 3, and 9 ml/min were chosen as different follow rates with a constant (time of reaction is (3 h), ilmenite particle size 75-μm, ilmenite acid ratio (1:3) and reaction temperature 120 μC.
Effect of temperature
80, 100, 110, and 120 μC at a constant (flow rate (0.5 ml/min), time of reaction (3 h) ilmenite particle size 75-μm, and ilmenite acid ratio (1:3).
Effect of particle size of ilmenite
300, 200, 100, and 75 microns at a constant (flow rate (0.5 ml/min), time of reaction (3 h), reaction temperature 120 °C, and ilmenite acid ratio (1:3).
Conversion of the prepared titanium dioxide to titanium dioxide nano particles (TiO2 NPs)
5 g of the prepared titanium dioxide react with suitable acid with molar ratio (3:1), add 100 m distilled water, the product neutralize with ammonia (2 M) until pH 11, stirring, and heating at 90 °C for 30 min, filter the mixture, wash by distilled water, drying at 105 °C for 2 h., calcination at 550 °C, the product named titanium dioxide nano particles (TiO2 NPs).
Characterization of ilmenite and titanium dioxide nano particles (TiO2 NPs)
Characterization of raw ilmenite and titanium dioxide nano particles (TiO2 NPs) were carried out by using different analytical techniques such as transmission electron microscopy (TEM) using a Zeiss EM-90 operating at 80 kV tension. Scanning Electron Microscope (SEM) Model Jeol 6510 JSM, LA. Brunauer–Emmett–Teller (BET) by using N2 adsorption/desorption at 77 K using an automatic surface area device (BELSORP MINI X). X-ray diffraction (XRD) (Paralytical Philips APD-3720, Netherlands) with Cu–kα radiation (λ = 0.154 cm−1) and operated at 40 kV, 35 mA, 5 min scanning speed in the 2θ range of 5°–80°. Fourier transform infrared (FTIR) spectrum of TiO2 NPs was recorded in the range of 400–4000 cm−1 with a Bruker FT/IR-2000 spectrometer. X-ray fluorescence (XRF) technique using Axios MAX, PAN analytical, 40 kV, 50 Ma.
Application of (TiO2 NPs)/UV for removal of humic acid from synthetic solution
The humic acid of 50 mg/l was prepared, the effectiveness of TiO2 NPs/UV for photocatalytic degradation of humic acid was carried out, the affecting factors such as dose, light intensity, pH, initial concentration, and agitation forces were conducted.
Application of FC and TiO2 NPs/UV for real sample of sewage water treatment
A certain sample of sewage water before and after secondary treatment was treated using a binary system of ferric chloride and titanium dioxide nano particles (TiO2 NPs) for advanced treatment of sewage water. The main characteristics of sewage water such as COD, BOD, TSS, and TKN are analyzed according to standards methods27.
Utilization of the prepared FC in chemical treatment for sewage water
The sample of the prepared ferric chloride (FC) coagulant was used to remove some pollutants such as BOD, COD and TSS from sewage water where the treatment depends on precipitation, coagulation, and adsorption techniques by poly inorganic coagulants (PIC). Each sample was mixed with 8–13 ppm of PIC and agitated for 1 min rapid mixing (200 rpm), followed by slow mixing for five minutes and (40 rpm) and 30 min standing time. The concentrations of pollutants were measured in ore samples and in the filtrate according to standards methods27.
Utilization of the prepared TiO2 NPs/UV in treatment of sewage water
The sample of the prepared TiO2 NPs was used to remove some pollutants such as BOD, COD, TKN, TOC and TSS from sewage water where the treatment depends on photocatalytic degradation. Each sample was mixed with 8–13 ppm of TiO2 NPs/UV and agitated for 30 min rapid mixing (200 rpm), followed by standing time for 30 min. The concentrations of pollutants were measured in ore samples and in the filtrate in Eq. (1) according to standards methods27.
where C0 and Ce are the initial and final concentrations (mg/l); respectively.
Results and discussion
Factors affecting the productivity of TiO2
Effect of reaction temperature
The endothermic nature of the reaction is clearly indicated Fig. 2a. The increase of temperature raises the production of TiO2. The result showed that as temperature increases the produced percentage of TiO2 increases. The optimum production condition is at 160 °C, the elevated temperature was required to overcome the binding between different metal oxides in ilmenites ore material.
Effect of reaction time
The effect of the contact time on the percentage of the produced TiO2 was shown in Fig. 2b. The results revealed that as the contact time increases, the TiO2 production increases where the upper productivity limit was at time 3 h of direct contact between the reagents. The long time for reaction was required also to overcome the binding between ingredients of ore material, which compatible with effect of temperature.
Effect of rate of addition
The flow rate has a significant effect on the production of TiO2 Fig. 2c. The result showed that as flow rates increase the produced TiO2 decreases. It became clear from the results that the best flow rate to obtain the highest production rate is 0.5 ml/min. The slow rate of addition gave the chance for formation product easily.
Effect of ilmenite grain size
The large, exposed surface area of the ilmenite enhances the reaction and then the production of TiO2 increases Fig. 2d. The result showed that as the grain size increases the production of TiO2 decreases where the best size for the optimum condition is 75 μm. The small size of ilmenite increases the rate of reaction due to the increase of surface area.
Effect of ilmenite:acid ratio
The relation between the ilmenite and acids ratio and the percentage of the produced TiO2 was illustrated in Fig. 2e. The result showed that ratio increases the productivity content of TiO2 increases. The optimum productivity limit was 1:3. which prof that stochiometric calculations are compatible with empirical formula.
From the results the optimum conditions of TiO2 as follows: time of reaction (3 h), temperature (160 °C), rate of reaction (1 ml/min), ilmenite acid ratio (1:3), and ilmenite grain size 75 μm.
The higher productivity was obtained using aqua regia due to double action of hydrochloric acid and nitric acid.
Characterization of materials
TEM and SEM characterizations
Scanning electron microscopy (SEM) was employed to examine the surface structure of TiO2 nanoparticles. Figure 3a,b shows the SEM image of synthesized TiO2 NPs, which clearly revealed their spherical shape with very little aggregation. Additionally, it was noted that all the particles were in the nanoscale range28,29,30,31,32,33,34.
TEM analysis provided information about the shape and size of TiO2 NPs. Figure 3c,d represents the TEM micrographs of synthesized TiO2 NPs. The result showed that the TiO2 NPs have an average particle size of 92 nm. The TiO2 nanoparticles have spherical and elliptical shapes with agglomerated morphology35.
Surface area characterization
The surface area, pore volume, and porosity of TiO2 nanoparticles were assessed using nitrogen adsorption/desorption measurements, specifically the Brunauer–Emmett–Teller (BET) method, as depicted in Fig. 4A and Table 1. The results indicated a Type II isotherm with a small H1 hysteresis loop, as per the IUPAC classification. In Figure A, the pore characteristics of TiO2 nanoparticles were observed to be in the meso/micro range, with a monolayer capacity (Vm) of 2.0 cm3/g and an average pore diameter of 28.29 nm. The surface area (SBET) for the nano TiO2 was measured at 88.15 m2/g, and the total pore volumes (Vp) were found to be 0.0616 cm3/g. To analyze the pore size distribution, the Barrett–Joyner–Halenda (BJH) method was applied, as presented in Fig. 4B and Table 1. The BJH curve revealed an average pore size of 40 nm, indicating that the majority of the nanoparticle pores are mesopores28,29,33,36.
XRD characterization
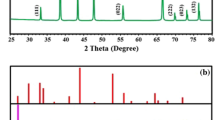
The XRD spectra of ilmenite ore and the synthesized TiO2 NPs is shown in Fig. 5. The XRD analysis as shown in Fig. 5A revealed the presence of two mineral phases: ilmenite and hematite. the ore is primarily composed of ferri-ilmenite with trace amounts of titano-hematite. The presence of the (104) peak at 2θ = 32.78 demonstrated the presence of ilmenite in the XRD pattern37,38,39.
Figure 5B shows the sharp peaks appearance for TiO2 NPs at 2θ values 27.78°, 3642°, 39.54°, 41.62°, 44.38°, 54.66°, 56.98°, 63.14°, 64.34°, 69.26°, and 70.1° at corresponding Miller indices (110), (101) (004), (111), (210), (211), (220), (002), (310), (112) and (116) respectively, confirms the formation of highly crystalline TiO2 NPs28,29,31,36,40.
FTIR characterization
The FT-IR spectrum of Ilmenite is shown in Fig. 6a. The 467 cm−1 and 532 cm−1 bands in the ilmenite spectra were linked to the Fe–O bonding, which is the distinctive band of ilmenite. The bending mode of adsorbed water on the ilmenite surface was assigned to the band of 3415 cm−138,41,42.
The FT-IR spectrum of synthesized TiO2 nanoparticles is shown in Fig. 6b. Within this spectrum, the absorption peak at 3452.2 cm−1 corresponds to the –OH stretching, while the peak at 1635.5 cm−1 is associated with the –OH bending vibration, which indicates the presence of water as moisture. Moreover, the strong peak at 690.5 cm−1 is attributed to the Ti–O stretching band, which is a distinctive characteristic of TiO228,29,33,43,44,45.
XRF characterization
As shown in Table 2 the elemental analysis of ilmenite and Titanium dioxide nano particles (TiO2 NPs) explored by XRF, the main contents of metal oxides of ilmenite such as SiO2, Al2O3, Fe2O3, MgO, SO3, K2O, Na2O, Cr, MnO2 and TiO2 is 14.07, 3.75, 34.2, 4.88, 1.95, 0.08, 0.19, 1526, 1.63 and 32.72 respectively and loss of ignition is 6.3774 and any variation in chemical composition is due to different sources localities46,47. Whereas the major elemental oxide of TiO2 nanoparticles is 95.6 and loss of ignition is 4.435,47.
Affecting factors on the effectiveness of TiO2 NPs/UV for photocatalytic degradation of humic acid
Effect of dose
The relation between the dose and the percentage of the removal of TOC and COD at constant (agitation force 200 rpm, light intensity 500 lx, contact time 60 min, pH 7.5, temperature 298 K and Concentration 100 mg/l) was illustrated in Fig. 7a. The highest removal percentages of COD and TOC recorded at dose 600 mg/l. whereas the highest removal percentage which recorded by Mohammed et al. was 4 g/l48,49,50, Aljuboury et al. was 0.5 g/l51, Sirisha et al. was at a dosage of 1.5 g/l52. Also, Joy et al. was 0.51 g/l53 and Surendra et al. recorded that the highest removal percentage was at a dosage of 1 g/l54.
Effect of agitation forces
The relation between the RPM and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, light intensity 500 lx, contact time 60 min, pH 7.5, temperature 298 K and concentration 100 mg/l) was illustrated in Fig. 7b. The highest removal percentages of COD and TOC recorded at 200 rpm for 60 min of spinning. whereas the highest removal percentage which was recorded by Surendra et al. was at 600 rpm for 60 min of spinning54.
Effect of light intensity
The relation between the light intensity and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, agitation forces 100 rpm, contact time 60 min, pH 7.5, temperature 298 K and concentration 100 mg/l) was illustrated in Fig. 7c. The highest removal percentages of COD and TOC recorded at light intensity 800 lx. whereas the highest removal percentage which recorded at light intensity 3.0 mw/cm255,56,57.
Effect of initial concentration
The relation between concentration of TiO2 and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, agitation forces 100 rpm, contact time 60 min, pH 7.5, temperature 298 K and light intensity 500 lx) was illustrated in Fig. 7d. The highest removal percentages of COD and TOC recorded at concentration 10 mg/l. whereas the highest removal percentage which recorded by Sirisha et al. was 5 mg/l52. Also, Surendra et al. recorded that the highest removal percentage was at initial concentration of 20 mg/l54.
Effect of pH
The relation between pH and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, agitation forces 100 rpm, contact time 60 min, light intensity 500 lx, temperature 298 K and concentration 100 mg/l) was illustrated in Fig. 7e. The highest removal percentages of COD and TOC were recorded at pH 7.5. whereas the highest removal percentage which recorded by Mohammed et al. was at pH 7.548 and Sirisha et al. (92.41%) was at pH of 852. Also, Surendra et al. recorded that the highest removal percentage was at pH of 754.
Effect of time
The relation between time and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, agitation forces 100 rpm, pH 7.5, light intensity 500 lx, temperature 298 K and concentration 100 mg/l) was illustrated in Fig. 7f. The highest removal percentages of COD and TOC recorded at time 90 min. whereas the highest removal percentage which recorded by Mohammed et al. was 180 min48,49, Aljuboury et al. was 170 min51, and Surendra et al. recorded that the highest removal percentage was at 60 min54.
Effect of temperature
The relation between degree of temperature and the percentage of the removal of TOC and COD at constant (dose 400 mg/l, agitation forces 100 rpm, pH 7.5, light intensity 500 lx, contact time 60 min and concentration 100 mg/l) was illustrated in Fig. 7g. The highest removal percentages of COD and TOC recorded at temperature 298 (K). whereas the highest removal percentage which recorded by Sirisha et al. was 333 K52. Also, Surendra et al. recorded that the highest removal percentage was at temperature of 70 °C54.
Application of binary system on real sample of sewage water treatment
From Table 3, the removal percentages of TKN, BOD, COD and TSS before secondary treatment using FC are 32, 29, 34 and 97.3, respectively. The removal percentages of TKN, BOD, COD and TSS before secondary treatment using TiO2 NPs/UV are 93.5, 99.5, 99.6 and 84.7, respectively. The removal percentages of TKN, BOD, COD and TSS before secondary treatment using TiO2 NPs/UV and FC are 94.7, 99.7, 99.7 and 85.5, respectively. The removal percentages of TKN, BOD, COD and TSS before secondary treatment using TiO2 NPs/UV, Fc and chitosan are 94.9, 99.7, 99.6 and 87.2, respectively.
From Table 4, the removal percentages of TKN. BOD, COD and TSS after secondary treatment using FC are14.8, 9.9, 13.2 and 96.9, respectively. The removal percentages of TKN. BOD, COD and TSS before secondary treatment using TiO2 NPs/UV are15.4, 14.2, 22.3 and 69.8, respectively. The removal percentages of TKN. BOD, COD and TSS before secondary treatment using TiO2 NPs/UV and FC are 33.8, 31.8, 33.3 and 45.5, respectively. The removal percentages of TKN. BOD, COD and TSS before secondary treatment using TiO2 NPs/UV, FC and chitosan are 44.2, 44, 46.3 and 54.5, respectively. The maximum percentages removal of COD, BOD and TSS in sewage wastewater reached 90, 92, and 93%, respectively58, the maximum removals of TSS, COD and BOD are 94.2, 89.2, and 76.9% respectively using PAlFeClSi59. the ultimate removal percentages of TSS, COD and BOD were 92.0, 89.0, and 91.0%, respectively by PAlFeCl + Si60.
Conclusion
This paper investigated the leaching of ilmenite to extract titanium dioxide via utilizing leaching procedures with various ingredients such as hydrochloric acid, nitric acid, and Aqua Regia then application of titanium dioxide plus ultraviolet radiation in advanced wastewater treatment. The affecting factors on titanium dioxide extraction such as ilmenite to acid ratio, reaction time, ilmenite grain size, rate of addition and reaction temperature were conducted. The best leaching conditions obtained were Ilmenite to acid ratio: 1:3 respectively, time: 3 h, grain size: 75 μm, temperature: 160 °C, rate of addition: 0.5 ml/min. Titanium dioxide nano particles (TiO2 NPs) were prepared. Comprehensive physico-chemical characterization of Ilmenite and TiO2 NPs were characterized using different analytical techniques. All the analytical techniques proved the formation of titanium dioxide and titanium dioxide nanoparticles. Affecting factors on the effectiveness of TiO2 NPs for photocatalytic degradation such as dose, agitation forces, light intensity, initial concentration, pH, time, and temperature are conducted. The optimum conditions for TiO2 NPs/UV photocatalytic degradation obtained were dose 600 mg/l, agitation force 200 rpm for 60 min of spinning, light intensity 800 lx, initial concentration 10 mg/l, pH 7.5, time 90 min and temperature 298 (K). The removal percentages of Total suspended solids (TSS), chemical oxygen demands (COD), biological oxygen demand (BOD)and total nitrogen (TN) were explored. According to results, the maximum removal percentage of TSS were 97.3 and 96.9% before and after secondary treatment which were conducted using FC. The maximum removal percentage of TKN, BOD, and COD before secondary treatment were conducted using mixture of TiO2 NPs, FC, and chitosan, which reached 44.2, 44 and 46.3%, respectively. The maximum removal percentage of TKN, BOD, and COD after secondary treatment were conducted using mixture of TiO2 NPs, FC, and chitosan, which reached 94.9, 99.7 and 99.6%, respectively. All in all, the results derived from this investigation suggest that the TiO2 NPs/UV holds significant promise for effective advanced treatment of sewage water, making it a viable and appropriate choice for water reuse applications.
Data availability
All relevant data are included in the paper.
Abbreviations
- ECs:
-
Emerging contaminants
- AOPs:
-
Advanced oxidation processes
- APHA 2022:
-
American Public Health Association 2022
- BET:
-
Brunauer–Emmett–Teller
- BJH:
-
Barrett–Joyner–Halenda
- BOD:
-
Biological oxygen demand
- COD:
-
Chemical oxygen demand
- FC:
-
Ferric chloride
- FTIR:
-
Fourier transform infra-red
- LOI:
-
Loss of ignition
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmittance electron microscopy
- TiO2 NPs:
-
Titanium dioxide nanoparticles
- TKN:
-
Total Kjeldahl nitrogen
- TSS:
-
Total suspended solids
- XRF:
-
X-ray fluorescence
References
Elkorashey, R. M. Utilizing chemometric techniques to evaluate water quality spatial and temporal variation A case study: Bahr El-Baqar drain—Egypt. Environ. Technol. Innov. 26, 102332 (2022).
ElFetyany, M., Farag, H. & Abd El Ghany, S. H. Assessment of national water footprint versus water availability—case study for Egypt. Alex. Eng. J. 60, 3577–3585 (2021).
Suhan, M. B. K. et al. Sustainable pollutant removal and wastewater remediation using TiO2-based nanocomposites: A critical review. Nano-Struct. Nano-Obj. 36, 101050 (2023).
Ingrao, C., Strippoli, R., Lagioia, G. & Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 9, e18507 (2023).
Koop, S. H. A., Grison, C., Eisenreich, S. J., Hofman, J. & van Leeuwen, K. J. Integrated water resources management in cities in the world: Global solutions. Sustain. Cities Soc. 86, 104137 (2022).
Ding, G. K. C. Managing Water Scarcity—The Role of Sustainable Water Management in Reference Module in Earth Systems and Environmental Sciences (Elsevier, 2023). https://doi.org/10.1016/B978-0-323-90386-8.00063-2.
Khairy, S., Shaban, M., Negm, A. M., Eldeen, O. W. & Ramadan, E. M. Drainage water reuse strategies: Case of El-Bats drain, Fayoum Governorate. Egypt. Ain Shams Eng. J. 13, 101681 (2022).
van Hamelsveld, S. et al. Effects of selected emerging contaminants found in wastewater on antimicrobial resistance and horizontal gene transfer. Emerg. Contam. 9, 100257 (2023).
Mena, E., Rey, A. & Beltrán, F. J. TiO2 photocatalytic oxidation of a mixture of emerging contaminants: A kinetic study independent of radiation absorption based on the direct-indirect model. Chem. Eng. J. 339, 369–380 (2018).
Liu, M. et al. The role of metal-organic frameworks in removing emerging contaminants in wastewater. J. Clean. Prod. 429, 139526 (2023).
Roškarič, M., Žerjav, G., Zavašnik, J. & Pintar, A. The influence of synthesis conditions on the visible-light triggered photocatalytic activity of g-C3N4/TiO2 composites used in AOPs. J. Environ. Chem. Eng. 10, 107656 (2022).
Thuan, D. V. et al. Adsorption and photodegradation of micropollutant in wastewater by photocatalyst TiO2/rice husk biochar. Environ. Res. 236, 116789 (2023).
Hassan, S. S. M. et al. Enhanced photocatalytic degradation of chlorinated pesticides and polychlorinated biphenyls using Mo–TiO2/GO/MS nanocomposite. Opt. Mater. (Amst.) 142, 114084 (2023).
Wang, J. et al. The advanced treatment of textile printing and dyeing wastewater by hydrodynamic cavitation and ozone: Degradation, mechanism, and transformation of dissolved organic matter. Environ. Res. 215, 114300 (2022).
Hasanzadeh, M. et al. Persulfate-assisted heterogeneous photocatalytic degradation of furfural from aqueous solutions using TiO2–ZnO/biochar composite. Heliyon 9, e21421 (2023).
AlMayyahi, A. & Al-asadi, H. A. A. Advanced oxidation processes (AOPs ) for wastewater treatment and reuse: A brief review. Asian J. Appl. Sci. Technol 2, 18–30 (2018).
Pandis, P. K. et al. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 6, 8 (2022).
Mohammed, N., Palaniandy, P., Shaik, F., Deepanraj, B. & Mewada, H. Statistical analysis by using soft computing methods for seawater biodegradability using ZnO photocatalyst. Environ. Res. 227, 115696 (2023).
Armaković, S. J., Savanović, M. M. & Armaković, S. Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts 13, 26 (2022).
Li, X. et al. Visible light-driven multi-motion modes CNC/TiO2 nanomotors for highly efficient degradation of emerging contaminants. Carbon N. Y. 155, 195–203 (2019).
Nair, N. G., Gandhi, V. G., Modi, K. & Shukla, A. Photocatalytic degradation of levofloxacin by GO-TiO2 under visible light. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2023.12.049 (2024).
Ataabadi, M. R. & Jamshidi, M. Improved photocatalytic degradation of methylene blue under visible light using acrylic nanocomposite contained silane grafted nano TiO2. J. Photochem. Photobiol. A Chem. 443, 114832 (2023).
Thambiliyagodage, C., Wijesekera, R. & Bakker, M. G. Leaching of ilmenite to produce titanium based materials: A review. Discov. Materi. https://doi.org/10.1007/s43939-021-00020-0 (2021).
Nguyen, T. H. & Lee, M. S. A review on the recovery of titanium dioxide from ilmenite ores by direct leaching technologies. Miner. Process. Extr. Metall. Rev. 40, 231–247 (2019).
Kordzadeh-Kermani, V., Schaffie, M., Hashemipour Rafsanjani, H. & Ranjbar, M. A modified process for leaching of ilmenite and production of TiO2 nanoparticles. Hydrometallurgy 198, 105507 (2020).
Jabit, N. & Senanayake, G. Characterization and leaching kinetics of ilmenite in hydrochloric acid solution for titanium dioxide production. J. Phys. Conf. Ser. 1082, 012089 (2018).
APHA. Standard Methods for the Examination of Water and Wastewater: 20th edition (2022).
Ur Rehman, K. et al. A Coronopus didymus based eco-benign synthesis of Titanium dioxide nanoparticles (TiO2 NPs) with enhanced photocatalytic and biomedical applications. Inorg. Chem. Commun. 137, 109179 (2022).
Maurya, I. C., Singh, S., Senapati, S., Srivastava, P. & Bahadur, L. Green synthesis of TiO2 nanoparticles using Bixa orellana seed extract and its application for solar cells. Sol. Energy 194, 952–958 (2019).
Singh, S., Maurya, I. C., Tiwari, A., Srivastava, P. & Bahadur, L. Green synthesis of TiO2 nanoparticles using citrus limon juice extract as a bio-capping agent for enhanced performance of dye-sensitized solar cells. Surf. Interfaces 28, 101652 (2022).
Ramya, S. et al. TiO2 nanoparticles derived from egg shell waste: Eco synthesis, characterization, biological and photocatalytic applications. Environ. Res. 214, 113829 (2022).
Goulart-Gonçalves, S. et al. Direct synthesis of TiO2 nanoparticles without heat treatment: Effect of order of addition and precursor/reducing ratio. J. Phys. Chem. Solids 180, 111368 (2023).
Saini, R. & Kumar, P. Green synthesis of TiO2 nanoparticles using Tinospora cordifolia plant extract and its potential application for photocatalysis and antibacterial activity. Inorg. Chem. Commun. 156, 111221 (2023).
Gohari, G. et al. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 10, 912 (2020).
Arunmetha, S. et al. Effect of processing methods on physicochemical properties of titania nanoparticles produced from natural rutile sand. Adv. Powder Technol. 24, 972–979 (2013).
Van, N. T. K. et al. Straightforward sonoelectrochemical synthesis of TiO2 nanoparticles for photocatalytic removal of Cr(VI) in water. Mater. Lett. 349, 134800 (2023).
Lee, R.-B., Juan, J.-C., Lai, C.-W. & Lee, K.-M. Ilmenite: Properties and photodegradation kinetic on Reactive Black 5 dye. Chinese Chem. Lett. 28, 1613–1618 (2017).
Pamplona de Sousa, G. C., Gomes da Silva, K. C. & Alves Júnior, C. Absorber film deposition by hollow cathode discharge for solar thermal collectors application. Int. J. Mater. Res. 113, 635–643 (2022).
Yu, Q. et al. Application of waste engine oil for improving ilmenite flotation combined with sodium oleate collector. Minerals 11, 1242 (2021).
Prakash, M. & Ghosh, A. K. An investigation on optimization of instantaneous synthesis of TiO2 nanoparticles and it’s thermal stability analysis in PP-TiO2 nanocomposite. Solid State Sci. 120, 106707 (2021).
Yuan, Z., Zhao, X., Meng, Q., Xu, Y. & Li, L. Effect of selective coating of magnetite on improving magnetic separation of ilmenite from titanaugite. Miner. Eng. 149, 106267 (2020).
Tran, C. V. et al. Facile fabrication and characterizations of nanostructured Fe2O3-TiO2 composite from Ilmenite ore. Int. J. Adv. Eng. Manage. Sci. 4, 574–578 (2018).
Gupta, S. & Jain, P. Eco-friendly synthesis of TiO2 nanoparticles by Moringa oleifera leaves, their characterization and impact on spinach seeds (Spinacia oleracea). Mater. Today Proc. 89, 91–95 (2023).
Lu, X., Lv, X., Sun, Z. & Zheng, Y. Nanocomposites of poly(l-lactide) and surface-grafted TiO2 nanoparticles: Synthesis and characterization. Eur. Polym. J. 44, 2476–2481 (2008).
Rab, N., Chong, F. K., Mohamed, H. I. & Lim, W. H. Preparation of TiO 2 nanoparticles by hydrolysis of TiCl 4 using water and glycerol solvent system. J. Phys. Conf. Ser. 1123, 012065 (2018).
Yaraghi, A. et al. Aeration leaching of iron from nitrided malaysian ilmenite reduced by polystyrene-coal reductant. Proced. Chem. 19, 715–720 (2016).
Mahmoud, M. H. H., Afifi, A. A. I. & Ibrahim, I. A. Reductive leaching of ilmenite ore in hydrochloric acid for preparation of synthetic rutile. Hydrometallurgy 73, 99–109 (2004).
Mohammed, N., Palaniandy, P. & Shaik, F. Optimization of solar photocatalytic biodegradability of seawater using statistical modelling. J. Indian Chem. Soc. 98, 100240 (2021).
Nayeemuddin, M., Palaniandya, P., Shaik, F. & Mewada, H. Experimental and computational analysis for optimization of seawater biodegradability using photo catalysis. IIUM Eng. J. 24, 11–33 (2023).
Mohammed, N., Palaniandy, P., Shaik, F. & Mewada, H. Statistical modelling of solar photocatalytic biodegradability of seawater using combined photocatalysts. J. Inst. Eng. Ser. E 104, 251–267 (2023).
Aljuboury, D. A. & Shaik, F. Optimization of the petroleum wastewater treatment process using TiO2/Zn photocatalyst. South Afr. J. Chem. Eng. 38, 61–69 (2021).
Sirisha, U. et al. Synthesized TiO2 nanoparticles for the application of photocatalytic degradation of synthetic toxic dye acridine orange. Mater. Today Proc. 62, 3444–3449 (2022).
Joy, V. M., Dutta, S., Feroz, S. & Devi, G. Nanophotocatalytic treatment of seawater using TiO2 immobilized and suspension system under solar irradiation. J. Water Process Eng. 43, 102263 (2021).
Surendra, B., Guduru, N., Sohan Joshua, M., Ram Kiran, B. & Vangalapati, M. Influence of chemically synthesized TiO2 nanoparticles for photocatalytic degradation of herbicide atrazine. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2023.09.058 (2023).
Cheng, H.-Y., Chang, K.-C., Lin, K.-L. & Ma, C.-M. Study on isopropanol degradation by UV/TiO2 nanotube. in 020006 (2018). https://doi.org/10.1063/1.5030310.
Chen, D. et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 268, 121725 (2020).
Puri, N. & Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ. Res. 227, 115786 (2023).
Utilization of poly inorganic coagulants impregnated with activated silica derived from rice husk ash in treatment of grey water. Water Energy Food Environ. 1, 13–23 (2020).
Abo-El-Enein, S. A., Eissa, M. A., Diafullah, A. A., Rizk, M. A. & Mohamed, F. M. Utilization of a low cost agro-residue for production of coagulant aids and their applications. J. Hazard. Mater. 186, 1200–1205 (2011).
Mohamed, F. M. & Alfalous, K. A. The effectiveness of activated silica derived from rice husk in coagulation process compared with inorganic coagulants for wastewater treatment. Egypt. J. Aquat. Res. 46, 131–136 (2020).
Acknowledgements
This publication is funded through the United States Agency for International Development (USAID). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. The authors also are thanks to Science and Technology Development Fund (STDF) for funding this project and the Science and Technology Center of Excellence (STCE), Faculty of Earth Sciences, Beni-Suef University, Egypt.
Funding
This work was funded by the Science and Technology Development Fund (STDF) under Grant Number (46896).
Author information
Authors and Affiliations
Contributions
M.G.K., experiment, writing-original draft preparation; F.M.M., experiment, data curation, writing-original draft preparation; A.E.H., methodology, investigation, data curation; F.S.H., data collection, editing. Experimental operation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kholief, M.G., Hesham, A.EL., Hashem, F.S. et al. Synthesis and utilization of titanium dioxide nano particle (TiO2NPs) for photocatalytic degradation of organics. Sci Rep 14, 11327 (2024). https://doi.org/10.1038/s41598-024-53617-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53617-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.