Featured
-
-
-
Article |
 Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling
Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling
Toll-like receptors (TLRs) are crucial to innate immunity. Activation of these proteins, and of receptors for the pro-inflammatory cytokines IL-1 and IL-18, leads to the recruitment of adaptor proteins such as MyD88. These in turn interact with further proteins such as IRAK2 and IRAK4. The crystal structure of the MyD88–IRAK2–IRAK4 death domain complex is now reported, explaining how these three proteins cooperate in TLR/IL-1R signalling.
- Su-Chang Lin
- , Yu-Chih Lo
- & Hao Wu
-
Letter |
 A conserved spider silk domain acts as a molecular switch that controls fibre assembly
A conserved spider silk domain acts as a molecular switch that controls fibre assembly
Spider silk proteins are remarkably soluble when stored at high concentration and yet can be converted to extremely sturdy fibres, through unknown molecular mechanisms. Here, the structure of the evolutionarily conserved carboxy-terminal domain of a silk protein is presented. The results provide evidence that the structural state of this domain is essential for controlled switching between the storage and assembly forms of silk proteins. Such molecular switches might see application in the design of versatile fibrous materials.
- Franz Hagn
- , Lukas Eisoldt
- & Horst Kessler
-
Letter |
 Self-assembly of spider silk proteins is controlled by a pH-sensitive relay
Self-assembly of spider silk proteins is controlled by a pH-sensitive relay
Spider silk proteins are remarkably soluble when stored at high concentration and yet can be converted to extremely sturdy fibres, through unknown molecular mechanisms. Here, the X-ray structure of the amino-terminal domain of a silk protein is presented, revealing how evolutionarily conserved polar surfaces might control self-assembly as the pH is lowered along the spider's silk extrusion duct. Such a mechanism might be applicable to the design of versatile fibrous materials.
- Glareh Askarieh
- , My Hedhammar
- & Stefan D. Knight
-
Article |
 Single-molecule dynamics of gating in a neurotransmitter transporter homologue
Single-molecule dynamics of gating in a neurotransmitter transporter homologue
Neurotransmitter:Na+ symporters (NSS) remove neurotransmitters from the synapse in a reuptake process that is driven by the Na+ gradient. Here, single-molecule fluorescence imaging assays have been combined with molecular dynamics simulations to probe the conformational changes that are associated with substrate binding and transport by a prokaryotic NSS homologue, LeuT. The findings are interpreted in the context of an allosteric mechanism that couples ion and substrate binding to transport.
- Yongfang Zhao
- , Daniel Terry
- & Jonathan A. Javitch
-
Column |
 Molecular modelling's $10-million comeback?
Molecular modelling's $10-million comeback?
Is Bill Gates's decision to invest in software company Schrödinger an early sign of a new computer-aided era for drug design, asks Derek Lowe. Or is it just another small step on what's been a rather lengthy journey?
- Derek Lowe
-
Letter |
 X-ray crystal structure of the light-independent protochlorophyllide reductase
X-ray crystal structure of the light-independent protochlorophyllide reductase
The ability of plants to 'green' in the dark is attributed to the activity of the dark-operative protochlorophyllide oxidoreductase (DPOR). This enzyme catalyses the stereospecific reduction of the C17≡C18 double bond of protochlorophyllide to form chlorophyllide a, the direct precursor of chlorophyll a. The X-ray crystal structure of the catalytic component of DPOR has now been solved. A chemical mechanism is proposed by which the reduction of the double bond may occur.
- Norifumi Muraki
- , Jiro Nomata
- & Yuichi Fujita
-
News & Views |
 Getting the metal right
Getting the metal right
Controversy has raged over the identity of the metal cofactor of membrane-bound methane monooxygenase, a methane-oxidizing enzyme. A study suggests that the answer is a cluster of two copper ions.
- J. Martin Bollinger Jr
-
Article |
 G domain dimerization controls dynamin's assembly-stimulated GTPase activity
G domain dimerization controls dynamin's assembly-stimulated GTPase activity
Dynamin is a protein that catalyses the fission of clathrin-coated endocytic vesicles from cellular membranes. To carry out fission, it must hydrolyse GTP. The mechanism by which it does so is unknown, although it does require dynamin's GTPase effector domain (GED). Here, the structure of a minimal GTPase–GED fusion protein constructed from human dynamin 1 is presented. The structure reveals the catalytic machinery and provides new insight into the mechanisms underlying dynamin-catalysed membrane fission.
- Joshua S. Chappie
- , Sharmistha Acharya
- & Fred Dyda
-
Letter |
 Structural basis of oligomerization in the stalk region of dynamin-like MxA
Structural basis of oligomerization in the stalk region of dynamin-like MxA
Dynamin is a protein that catalyses the scission of clathrin-coated pits at the plasma membrane. The mechanisms of dynamin-catalysed scission remain poorly understood. Here, the structure of the stalk region of human MxA, a dynamin-like protein, is presented. A structural model of MxA oligomerization and stimulated GTP hydrolysis is put forward that has functional implications for all members of the dynamin family.
- Song Gao
- , Alexander von der Malsburg
- & Oliver Daumke
-
Letter |
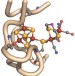 Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG
Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG
Hydrogen metabolism is facilitated by the activity of three hydrogenase enzymes. The catalytic core of the [FeFe]-hydrogenase (HydA), called the H-cluster, exists as a [4Fe4S] subcluster linked to a modified 2Fe subcluster. Here, Chlamydomonas reinhardtii HydA was expressed in a genetic background that did not contain the other hydrogenase biosynthetic genes. The structure of this HydA was then solved, revealing the stepwise manner by which the H-cluster is synthesized, and offering insight into how HydA might have evolved.
- David W. Mulder
- , Eric S. Boyd
- & John W. Peters
-
News |
 Japan rolls out elite science funds
Japan rolls out elite science funds
FIRST scheme targets large grants to world-leading researchers.
- David Cyranoski
-
News Feature |
 Protein folding: The dark side of proteins
Protein folding: The dark side of proteins
Almost every human protein has segments that can form amyloids, the sticky aggregates known for their role in disease. Yet cells have evolved some elaborate defences, finds Jim Schnabel.
- Jim Schnabel
-
Letter |
 Crystal structure of the FTO protein reveals basis for its substrate specificity
Crystal structure of the FTO protein reveals basis for its substrate specificity
The fat mass and obesity-associated (FTO) gene has been associated with increased body weight. The FTO protein has DNA/RNA demethylase activity. Here, the crystal structure of human FTO in complex with the mononucleotide 3-methylthymidine is presented. The structure provides a basis for understanding the substrate specificity of FTO, and should serve as a foundation for the design of FTO inhibitors.
- Zhifu Han
- , Tianhui Niu
- & Jijie Chai
-
Letter |
 Super-resolution biomolecular crystallography with low-resolution data
Super-resolution biomolecular crystallography with low-resolution data
X-ray crystallography has become the most common way for structural biologists to obtain the three-dimensional structures of proteins and protein complexes. However, crystals of large macromolecular complexes often diffract only weakly (yielding a resolution worse than 4 Å), so new methods that work at such low resolution are needed. Here a new method is described by which to obtain higher-quality electron density maps and more accurate molecular models of weakly diffracting crystals.
- Gunnar F. Schröder
- , Michael Levitt
- & Axel T. Brunger
-
Letter |
 Recognition of a signal peptide by the signal recognition particle
Recognition of a signal peptide by the signal recognition particle
Nascent secretory or membrane proteins contain an amino-terminal signal peptide that mediates their targeting to the plasma membrane (in prokaryotes) or endoplasmic reticulum (in eukaryotes). This peptide is recognized by the signal recognition particle (SRP). A universally conserved component of the SRP is known as SRP54 (Ffh in bacteria). Here, the crystal structure of Sulfolobus solfataricus SRP54 fused to a signal peptide is presented, revealing how the signal peptide is recognized by SRP54.
- Claudia Y. Janda
- , Jade Li
- & Kiyoshi Nagai
-
Letter |
 Tracking G-protein-coupled receptor activation using genetically encoded infrared probes
Tracking G-protein-coupled receptor activation using genetically encoded infrared probes
Rhodospsin is a G-protein-coupled receptor that is responsible for vision in dim light. Light isomerizes the protein's retinal chromophore and triggers concerted movements of several transmembrane helices. Here, an approach involving mutant rhodopsins and infrared spectroscopy enabled changes in the electrostatic environment to be seen as rhodopsin proceeded along its activation pathway. Early conformational changes were observed that precede the well-known larger movements of the transmembrane helices.
- Shixin Ye
- , Ekaterina Zaitseva
- & Reiner Vogel
-
Letter |
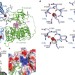 Structural basis for receptor recognition of vitamin-B12–intrinsic factor complexes
Structural basis for receptor recognition of vitamin-B12–intrinsic factor complexes
Vitamin B12 (cobalamin) is an essential coenzyme in mammals, and is taken up from the diet. The proteins required for its uptake are the gastric intrinsic factor (IF) and the ileal endocytic cubam receptor, which is in turn formed from the proteins cubilin and amnionless. Here, the crystal structure is presented of the complex between IF–cobalamin and the IF–cobalamin-binding region (CUB) of cubilin. The structure illustrates how numerous CUB domains function together as modular ligand-binding regions.
- Christian Brix Folsted Andersen
- , Mette Madsen
- & Gregers R. Andersen
-
News & Views |
 When four become one
When four become one
Every machine is made of parts. But, as the new structure of the HIV integrase enzyme in complex with viral DNA shows, one could not have predicted from the individual parts just how this machine works.
- Robert Craigie
-
Article
| Open Access Signatures of mutation and selection in the cancer genome
Signatures of mutation and selection in the cancer genome
Homozygous gene deletions in cancer cells occur over recessive cancer genes (where they can confer selective growth advantage) or over genes at fragile sites of the genome (where they are thought to reflect increased DNA breakage). Here, a large number of homozygous deletions in a collection of cancer cell lines are identified and analysed to derive structural signatures for the two different types of deletion. More deletions are found in inherently fragile regions, and fewer overlying recessive genes.
- Graham R. Bignell
- , Chris D. Greenman
- & Michael R. Stratton
-
Article |
 Active site remodelling accompanies thioester bond formation in the SUMO E1
Active site remodelling accompanies thioester bond formation in the SUMO E1
The post-translational modification of cellular proteins by ubiquitin (Ub) and ubiquitin-like (Ubl) proteins — such as SUMO — regulates a broad array of cellular processes. E1 enzymes activate Ub and Ubl in two steps, by carboxy-terminal adenylation and thioester bond formation to a catalytic cysteine, but the structural basis for the intermediates remains unknown. Crystal structures for SUMO E1 in complex with SUMO adenylate and tetrahedral intermediate analogues are now reported and analysed.
- Shaun K. Olsen
- , Allan D. Capili
- & Christopher D. Lima
-
Letter |
 TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity
TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity
T lymphocytes, which are an integral part of most adaptive immune responses, recognize foreign antigens through the binding of antigenic peptide–major histocompatibility complex (pMHC) molecules on other cells to specific T-cell antigen receptors (TCRs). Using single-molecule microscopy and fluorescence resonance energy transfer, the kinetics of TCR–pMHC binding are now measured in situ, revealing accelerated kinetics and increased affinity when compared with solution measurements.
- Johannes B. Huppa
- , Markus Axmann
- & Mark M. Davis
-
News & Views |
 Transformative encounters
Transformative encounters
Researchers have met the challenge of capturing transient states of the SUMO E1 activating enzyme. Their pictures show radically different crystal structures for two of the steps in this enzyme's activity.
- Brenda A. Schulman
- & Arthur L. Haas
-
Letter |
 Directional water collection on wetted spider silk
Directional water collection on wetted spider silk
Many plants and animals make use of biological surfaces with structural features at the micro- and nanometre-scale that control the interaction with water. The appearance of dew drops on spider webs is an illustration of how they are one such material capable of efficiently collecting water from air. The water-collecting ability of the capture silk of the Uloborus walckenaerius spider is now shown to be the result of a unique fibre structure that forms after wetting.
- Yongmei Zheng
- , Hao Bai
- & Lei Jiang
-
Letter |
 Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers
Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers
The antiviral drugs amantadine and rimantadine target the M2 protein of influenza A virus, making an understanding of its structure important for the study of drug resistance. The results of a recent crystal structure of M2 differ from those of a solution NMR structure with regards to binding of these drugs, indicating a different mechanism of inhibition in each case. Here, using solid-state NMR spectroscopy, two different amantadine-binding sites are shown to exist in the phospholipid bilayers of M2.
- Sarah D. Cady
- , Klaus Schmidt-Rohr
- & Mei Hong
-
Letter |
 Experimental evidence for a frustrated energy landscape in a three-helix-bundle protein family
Experimental evidence for a frustrated energy landscape in a three-helix-bundle protein family
The primary sequence of a protein defines its free-energy landscape and thus determines the rate constants of folding and unfolding, with theory suggesting that roughness in the energy landscape leads to slower folding. However, obtaining experimental descriptions of this landscape is challenging. Landscape roughness is now shown to be responsible for the slower folding and unfolding times observed in the R16 and R17 domains of α-spectrin relative to the similar R15 domain.
- Beth G. Wensley
- , Sarah Batey
- & Jane Clarke
-
Letter |
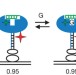 Multiple native states reveal persistent ruggedness of an RNA folding landscape
Multiple native states reveal persistent ruggedness of an RNA folding landscape
The 'thermodynamic hypothesis' proposes that the sequence of a biological macromolecule defines its folded, active structure as a global energy minimum in the folding landscape; however, it is not clear whether there is only one global minimum or several local minima corresponding to active conformations. Here, using single-molecule experiments, an RNA enzyme is shown to fold into multiple distinct native states that interconvert.
- Sergey V. Solomatin
- , Max Greenfeld
- & Daniel Herschlag
-
News Feature |
 Chemistry: Breaking the billion-hertz barrier
Chemistry: Breaking the billion-hertz barrier
Researchers in France have switched on the world's most powerful nuclear magnetic resonance instrument. Ananyo Bhattacharya asks whether it will attract new life to NMR spectroscopy.
- Ananyo Bhattacharya
-
Article |
 Structure of a bacterial homologue of vitamin K epoxide reductase
Structure of a bacterial homologue of vitamin K epoxide reductase
The γ-carboxylation of many blood coagulation factors relies on the generation of vitamin K hydroquinone by the enzyme vitamin K epoxide reductase (VKOR), of which the anticoagulant warfarin is an inhibitor. Here, the X-ray crystal structure of a bacterial homologue of VKOR is presented; the results have implications for the mechanism of action of mammalian VKOR and explain how mutations can cause warfarin resistance.
- Weikai Li
- , Sol Schulman
- & Tom A. Rapoport
-
Letter |
 Mechanism of folding chamber closure in a group II chaperonin
Mechanism of folding chamber closure in a group II chaperonin
Group II chaperonins are present in eukaryotes and archaea and are essential mediators of cellular protein folding. This process is critically dependent on the closure of a built-in lid, which is triggered by ATP hydrolysis, but the structural rearrangements and molecular events leading to lid closure are unknown. Using cryo-electron microscopy, the structures of an archaeal group II chaperonin in the open and closed states are now reported, providing details of this mechanism.
- Junjie Zhang
- , Matthew L. Baker
- & Wah Chiu
-
Letter |
 Mechanism of substrate recognition and transport by an amino acid antiporter
Mechanism of substrate recognition and transport by an amino acid antiporter
The amino acid antiporter AdiC is important for the survival of enteric bacteria such as Escherichia coli in extremely acid environments. Although the structure of substrate-free AdiC is known, how the substrate (arginine or agmatine) is recognized and transported by AdiC remains unclear. The crystal structure of an E. coli AdiC variant bound to arginine is now reported and analysed.
- Xiang Gao
- , Lijun Zhou
- & Yigong Shi
-
Article |
 Coupled chaperone action in folding and assembly of hexadecameric Rubisco
Coupled chaperone action in folding and assembly of hexadecameric Rubisco
Form I Rubisco, one of the most abundant proteins in nature, catalyses the fixation of atmospheric CO2 in photosynthesis. The limited catalytic efficiency of Rubisco has sparked extensive efforts to re-engineer the enzyme to enhance agricultural productivity. To bring this goal closer, the formation of cyanobacterial form I Rubisco is now analysed by in vitro reconstitution and cryo-electron microscopy.
- Cuimin Liu
- , Anna L. Young
- & Manajit Hayer-Hartl
-
Letter |
 Structural basis for the photoconversion of a phytochrome to the activated Pfr form
Structural basis for the photoconversion of a phytochrome to the activated Pfr form
Phytochromes regulate numerous photoresponses in plants and microorganisms through their ability to photointerconvert between a red-light-absorbing, ground state (Pf) and a far-red-light-absorbing, photoactivated state (Pfr). The structures of several phytochromes as Pf have been determined previously; here, the three-dimensional solution structure of the bilin-binding domain as Pfr is described. The results shed light on the structural basis for photoconversion to the activated Pfr form.
- Andrew T. Ulijasz
- , Gabriel Cornilescu
- & Richard D. Vierstra
-
Article |
 Targeting Bcr–Abl by combining allosteric with ATP-binding-site inhibitors
Targeting Bcr–Abl by combining allosteric with ATP-binding-site inhibitors
GNF-2 is a recently discovered, selective allosteric Bcr–Abl inhibitor. Solution NMR, X-ray crystallography, mutagenesis and hydrogen exchange mass spectrometry are now used to show that GNF-2 binds to the myristate-binding site of Abl, leading to changes in the structural dynamics of the ATP-binding site. The results show that the combination of allosteric and ATP-competitive inhibitors can overcome resistance to either agent alone.
- Jianming Zhang
- , Francisco J. Adrián
- & Nathanael S. Gray
-
Letter |
 Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor
Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor
G-protein-coupled receptors (GPCRs) mediate the majority of cellular responses to hormones and neurotransmitters and are the largest group of therapeutic targets for a range of diseases. The extracellular surface (ECS) of GPCRs is diverse and therefore an ideal target for the discovery of subtype-selective drugs. Here, NMR spectroscopy is used to investigate ligand-specific conformational changes around a central structural feature in the ECS of a GPCR.
- Michael P. Bokoch
- , Yaozhong Zou
- & Brian K. Kobilka

 The folding cooperativity of a protein is controlled by its chain topology
The folding cooperativity of a protein is controlled by its chain topology