Featured
-
-
News & Views |
 Protection from the outside
Protection from the outside
Protein folding is a high-stakes process, with cell dysfunction and death being the unforgiving penalties for failure. Work in bacteria hints that organisms manage this process beyond the boundaries of the cytoplasm — and even the cell.
- Evan T. Powers
- & William E. Balch
-
Article |
 Structure and mechanism of the hexameric MecA–ClpC molecular machine
Structure and mechanism of the hexameric MecA–ClpC molecular machine
Regulated proteolysis by ATP-dependent proteases have a crucial role in protein quality control in cells. The Clp/Hsp100 proteins of the AAA+ superfamily of ATP-dependent chaperones unfold and translocate proteins into the proteolytic chamber of protease complexes. ClpC requires the adaptor protein MecA for activation and substrate targetting to the ClpCP protease complex. Here, a structural and biochemical analysis is presented of the MecA–ClpC complex revealing organizational principles and providing mechanistic insights into this complex molecular machine.
- Feng Wang
- , Ziqing Mei
- & Yigong Shi
-
News & Views |
 Unexpected interactions
Unexpected interactions
Unpaired electrons can exert effects that allow interatomic contacts in molecules to be detected more easily using nuclear magnetic resonance. One such effect reveals unusual interactions between certain atoms in a protein.
- Ivano Bertini
- & Claudio Luchinat
-
Letter |
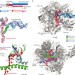 Structural basis of RNA polymerase II backtracking, arrest and reactivation
Structural basis of RNA polymerase II backtracking, arrest and reactivation
During gene transcription, RNA polymerase (Pol) II moves forward along DNA and synthesizes mRNA. However, Pol II can also move backwards and stall, which is important for regulatory purposes or when the polymerase hits an obstacle such as a nucleosome. This arrested state is reactivated by the transcription factor TFIIS. Here, a crystal structure is presented of a backtracked yeast Pol II complex in which the backtracked RNA can be observed, plus a structure of a backtracked complex that contains TFIIS. A model is presented for Pol II backtracking, arrest and reactivation during transcription elongation.
- Alan C. M. Cheung
- & Patrick Cramer
-
Research Highlights |
Glimpses of crystal growth
-
News & Views |
 A new look for the APC
A new look for the APC
Solving the structure of protein complexes is particularly challenging when they contain many subunits. In the case of the APC, a fruitful strategy has been to gain information by subtracting subunits. See Article p.227 and Letter p.274
- Ian Foe
- & David Toczyski
-
Article |
 Structural basis for the subunit assembly of the anaphase-promoting complex
Structural basis for the subunit assembly of the anaphase-promoting complex
The APC/C is a large multiprotein complex that functions as an E3 ubiquitin ligase to regulate the cell cycle. Here, the entire APC/C complex is reconstituted, and in combination with structural studies a pseudo-atomic model for 70% of the complex is provided. These results contribute towards a molecular understanding of the roles of individual subunits in APC/C assembly and their interactions with co-activators, substrates and regulatory proteins.
- Anne Schreiber
- , Florian Stengel
- & David Barford
-
Letter |
 Femtosecond X-ray protein nanocrystallography
Femtosecond X-ray protein nanocrystallography
The start-up of the new femtosecond hard X-ray laser facility in Stanford, the Linac Coherent Light Source, has brought high expectations for a new era for biological imaging. The intense, ultrashort X-ray pulses allow diffraction imaging of small structures before radiation damage occurs. This new capability is tested for the problem of structure determination from nanocrystals of macromolecules that cannot be grown in large crystals. Over three million diffraction patterns were collected from a stream of nanocrystals of the membrane protein complex photosystem I, which allowed the assembly of a three-dimensional data set for this protein, and proves the concept of this imaging technique.
- Henry N. Chapman
- , Petra Fromme
- & John C. H. Spence
-
Letter |
 Single mimivirus particles intercepted and imaged with an X-ray laser
Single mimivirus particles intercepted and imaged with an X-ray laser
The start-up of the new femtosecond hard X-ray laser facility in Stanford, the Linac Coherent Light Source, has brought high expectations for a new era for biological imaging. The intense, ultrashort X-ray pulses allow diffraction imaging of small structures before radiation damage occurs. This new capability is tested for the problem of imaging a non-crystalline biological sample. Images of mimivirus are obtained, the largest known virus with a total diameter of about 0.75 micrometres, by injecting a beam of cooled mimivirus particles into the X-ray beam. The measurements indicate no damage during imaging and prove the concept of this imaging technique.
- M. Marvin Seibert
- , Tomas Ekeberg
- & Janos Hajdu
-
Letter |
 Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
A complex of RNA and protein known as the box C/D RNP catalyses the site-specific modification of RNAs with a 2′-O-methylation group. The structure of the full complex has now been solved, including the guide RNA and either of two substrate RNAs. This structure reveals how the guide and target RNAs are aligned, and how the methyltransferase subunit, fibrillarin, facilitates placement of the target ribose into the active site.
- Jinzhong Lin
- , Shaomei Lai
- & Keqiong Ye
-
Obituary |
 John Fenn (1917–2010)
John Fenn (1917–2010)
Chemist who enabled mass spectrometry to weigh up biology.
- Carol V. Robinson
-
News & Views |
 Peptide gets in shape for self-defence
Peptide gets in shape for self-defence
The transformation of tadpole to frog and of caterpillar to butterfly are two of the more obvious examples of metamorphosis. But molecular shape-shifting may occur in each of us as part of our innate antibacterial defence system. See Letter p.419
- Robert I. Lehrer
-
Letter |
 Atomic-level modelling of the HIV capsid
Atomic-level modelling of the HIV capsid
The HIV virion has a cone-shaped core composed of capsid proteins, which take either pentameric or hexameric form. The crystal structure of the capsid hexamer had been solved previously. Now the structure of the pentamer is provided, which allows the proposal of the first atomic-level model of the mature HIV capsid.
- Owen Pornillos
- , Barbie K. Ganser-Pornillos
- & Mark Yeager
-
News & Views |
 Finding the wet spots
Finding the wet spots
The functions of proteins are critically coupled to their interplay with water, but determining the dynamics of most water molecules at protein surfaces hasn't been possible. A new spectroscopic method promises to change that.
- Vincent J. Hilser
-
News & Views |
 Binding the receptor at both ends
Binding the receptor at both ends
G-protein-coupled receptors initiate a wide range of signalling pathways in cells. It seems that both a G protein and an agonist molecule must bind to the receptors to persistently activate them. See Article p.175 & Letters p.236 & p.241
- Stephen R. Sprang
-
News |
 Near-action shots of vital proteins
Near-action shots of vital proteins
Structures of G-protein-coupled receptors visualized in near-active states.
- Amy Maxmen
-
Letter |
 The structural basis for agonist and partial agonist action on a β1-adrenergic receptor
The structural basis for agonist and partial agonist action on a β1-adrenergic receptor
Here, the X-ray crystal structure of the β1 adrenergic receptor, a G-protein-coupled receptor, bound to four small molecules that either act as full agonists or partial agonists is solved. The structures show that agonist binding induces a contraction of the catecholamine-binding pocket relative to the antagonist-bound receptor. This work reveals the pharmacological differences between different ligand classes, which should facilitate the structure-based design of new drugs with predictable efficacies.
- Tony Warne
- , Rouslan Moukhametzianov
- & Christopher G. Tate
-
Article |
 Structure of a nanobody-stabilized active state of the β2 adrenoceptor
Structure of a nanobody-stabilized active state of the β2 adrenoceptor
The X-ray crystal structure of the human β2 adrenergic receptor, a G-protein-coupled receptor, in an agonist-bound 'active' state is solved. Comparison of this structure with a previously published structure of the same GPCR in an inactive state indicates that minor changes in the binding pocket of the protein lead to major changes elsewhere — there is a large outward movement of the cytoplasmic end of one of the transmembrane segments and rearrangements of two other transmembrane segments. This structure provides insights into the process of agonist binding and activation.
- Søren G. F. Rasmussen
- , Hee-Jung Choi
- & Brian K. Kobilka
-
Letter |
 Structure and function of an irreversible agonist-β2 adrenoceptor complex
Structure and function of an irreversible agonist-β2 adrenoceptor complex
The X-ray crystal structure of the human β2 adrenergic receptor, a G-protein-coupled receptor (GPCR), covalently bound to a small-molecule agonist is solved. Comparison of this structure with structures of this GPCR in an inactive state and in an antibody-stabilized active state reveals how binding events at both the extracellular and intracellular surfaces stabilize the active conformation of the receptor. Molecular dynamics simulations suggest that the agonist-bound active state spontaneously relaxes to an inactive-like state in the absence of a G protein.
- Daniel M. Rosenbaum
- , Cheng Zhang
- & Brian K. Kobilka
-
Letter |
 Vernier templating and synthesis of a 12-porphyrin nano-ring
Vernier templating and synthesis of a 12-porphyrin nano-ring
Templates are widely used to arrange molecular components so they can be covalently linked into complex molecules that are not readily accessible by classical synthetic methods. But, as larger structures are targeted, the synthesis of the templates themselves becomes challenging. It is now shown that 'molecular Verniers' can solve this problem: using a template with six binding sites and molecular building blocks with four porphyrins acting as binding sites, a 12-porphyrin nano-ring with a diameter of 4.7 nm is created. The ease and efficiency of this synthesis establishes Vernier templating as a powerful new strategy for producing large monodisperse macromolecules.
- Melanie C. O’Sullivan
- , Johannes K. Sprafke
- & Harry L. Anderson
-
Letter |
 Sensing the anomeric effect in a solvent-free environment
Sensing the anomeric effect in a solvent-free environment
The anomeric effect is a chemical phenomenon that refers to an observed stabilization of six-membered carbohydrate rings when they contain an electronegative substituent at the C1 position of the ring. This stereoelectronic effect influences the three-dimensional shapes of many biological molecules, but the underlying physical origin is unclear. Here it is shown that complexes formed between a truncated peptide motif and an isolated sugar in the gas phase are nearly identical structurally; however, the strength of the polarization of their interactions with the peptide differs greatly. It will be important to re-evaluate the influence, and biological effects, of substituents at position C2 of the six-membered carbohydrate rings.
- Emilio J. Cocinero
- , Pierre Carcabal
- & Benjamin G. Davis
-
News & Views |
 Proteins in dynamic equilibrium
Proteins in dynamic equilibrium
Protein molecules in solution exist as an equilibrium of different conformations, but the sizes and shifts of these populations cannot be determined from static structures. A report now shows how they can be measured in solution.
- Pau Bernadó
- & Martin Blackledge
-
News & Views |
 Reader's block
Reader's block
Protein factors can regulate gene expression by binding to specifically modified DNA-associated proteins. Small molecules that selectively interfere with such interaction may be of therapeutic value. See Article p.1067 & Letter p.1119
- Sean D. Taverna
- & PhiliP A. Cole
-
Letter |
 Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
The initial crystal structure of LeuT, together with subsequent functional and structural studies, provided direct evidence for a single, high-affinity substrate-binding site. Recent binding, flux and molecular simulation studies, however, have been interpreted in terms of a model where there are two high-affinity binding sites: the second (S2) site is believed to be located within the extracellular vestibule. Here, direct measurement is performed of substrate binding to wild-type LeuT and to S2 site mutants using isothermal titration calorimetry, equilibrium dialysis and scintillation proximity assays. The conclusion is made that LeuT harbours a single, centrally located, high-affinity substrate-binding site.
- Chayne L. Piscitelli
- , Harini Krishnamurthy
- & Eric Gouaux
-
Letter |
 The assembly of a GTPase–kinase signalling complex by a bacterial catalytic scaffold
The assembly of a GTPase–kinase signalling complex by a bacterial catalytic scaffold
Pathogenic Escherichia coli translocate many proteins into the host cell to promote virulence. It is now shown that one of these proteins, EspG, which is present in enterohaemorrhagic E. coli, interferes with the host signalling network.
- Andrey S. Selyunin
- , Sarah E. Sutton
- & Neal M. Alto
-
Letter |
 Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis
Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis
The first X-ray crystal structure of a diterpene cyclase is reported — this enzyme, taxadiene synthase, catalyses the cyclization of an isoprenoid in the first committed step of the biosynthesis of the cancer chemotherapeutic drug Taxol. The C-terminal catalytic domain binds and activates the substrate in a manner seen in class I terpenoid cyclases, but the N-terminal domain and a third 'insertion' domain together adopt the fold of a class II terpenoid cyclase. It is proposed that this enzyme could be the ancestral progenitor of all terpenoid cyclases.
- Mustafa Köksal
- , Yinghua Jin
- & David W. Christianson
-
News & Views |
 One protein, many functions
One protein, many functions
The Lassa virus nucleoprotein coats the viral genome to make a template for RNA synthesis. A study shows that it also binds the 'cap' structure of cellular messenger RNAs and directs immune evasion using a novel mechanism. See Article p.779
- Félix A. Rey
-
Letter |
 The mechanism of sodium and substrate release from the binding pocket of vSGLT
The mechanism of sodium and substrate release from the binding pocket of vSGLT
Here, a comprehensive study of the sodium/galactose transporter (vSGLT) is presented, consisting of molecular dynamics simulations, biochemical characterization and a new crystal structure of the 'inward-open' conformation. These experiments show that sodium exit causes a reorientation of transmembrane helix 1, opening an inner gate required for substrate exit, while also triggering minor rigid-body movements in two sets of transmembrane helical bundles. This cascade of conformational changes is responsible for the proper timing of ion and substrate release.
- Akira Watanabe
- , Seungho Choe
- & Jeff Abramson
-
News & Views |
 An alphavirus puzzle solved
An alphavirus puzzle solved
Alphaviruses infect their host by binding to cellular receptors and fusing with cell membranes. New studies define the receptor-binding protein of these viruses and its regulation of the membrane-fusion reaction. See Letters p.705 & p.709
- Margaret Kielian
-
News & Views |
 Weighing up protein folding
Weighing up protein folding
Labelling molecules by fast oxidation allows mass spectrometry to study protein folding at submillisecond time resolution. The method also brings a wealth of structural information about protein folding within reach.
- Martin Gruebele
-
Letter |
 Structural changes of envelope proteins during alphavirus fusion
Structural changes of envelope proteins during alphavirus fusion
The E1 and E2 glycoproteins of alphaviruses form heterodimers and assemble into spikes on the virus surface, which mediate receptor binding and endocytosis. When the virion encounters acidic pH in the endosome E1 and E2 dissociate and E1 triggers fusion with the endosomal membrane. Two papers now provide the first crystal structures for glycoprotein complexes incorporating E2 at acidic and neutral pH, respectively. Together they provide insight into how fusion activation is controlled in alphaviruses.
- Long Li
- , Joyce Jose
- & Michael G. Rossmann
-
Letter |
 Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein
Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein
A crystal structure of bacterial RNA polymerase (RNAP) bound to the transcription inhibitor Gfh1 reveals the mechanism of inhibition by Gfh1 and an alternative ratcheted state of RNAP.
- Shunsuke Tagami
- , Shun-ichi Sekine
- & Shigeyuki Yokoyama
-
Letter |
 Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
During translation, tRNAs enter the ribosome and then move sequentially through three sites, known as A, P and E, as they transfer their attached amino acids onto the growing peptide chain. How the ribosome facilitates tRNA translocation between the sites remains largely unknown. Now a study uses multiparticle cryoelectron microscopy of a ribosome bound to the translation elongation factor, EF-G, to get information about tRNA movement. It identifies two new substates and sees that translocation is linked to unratcheting of the 30S ribosomal subunit.
- Andreas H. Ratje
- , Justus Loerke
- & Christian M. T. Spahn
-
Letter |
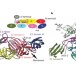 Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
The E1 and E2 glycoproteins of alphaviruses form heterodimers and assemble into spikes on the virus surface, which mediate receptor binding and endocytosis. When the virion encounters acidic pH in the endosome E1 and E2 dissociate and E1 triggers fusion with the endosomal membrane. Two papers now provide the first crystal structures for glycoprotein complexes incorporating E2 at acidic and neutral pH, respectively. Together they provide insight into how fusion activation is controlled in alphaviruses.
- James E. Voss
- , Marie-Christine Vaney
- & Félix A. Rey
-
News |
 Elusive protein factory mapped at last
Elusive protein factory mapped at last
Victory claimed in race to determine structure of eukaryotic ribosome.
- Daniel Cressey
-
Research Highlights |
Structural biology: Dopamine receptor revealed
-
Article |
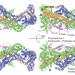 Structure and control of the actin regulatory WAVE complex
Structure and control of the actin regulatory WAVE complex
In cells, WAVE protein, a central regulator of actin dynamics during cell motility, is constitutively incorporated into WAVE regulatory complex (WRC), is normally present in an inactive state and can be activated by a number of inputs. These authors present the structure and mechanistic analysis of WRC. The combined data reveal how the WAVE protein is inhibited within the WRC complex and provide mechanisms for WRC activation at the plasma membrane.
- Zhucheng Chen
- , Dominika Borek
- & Michael K. Rosen
-
Letter |
 Structures of APC/CCdh1 with substrates identify Cdh1 and Apc10 as the D-box co-receptor
Structures of APC/CCdh1 with substrates identify Cdh1 and Apc10 as the D-box co-receptor
The anaphase promoting complex/cyclosome (APC/C) is a large multimeric ubiquitin E3 ligase that regulates the eukaryotic cell cycle in processes such as chromatid segregation and completion of mitosis. It catalyses the polyubiquitylation of a diverse array of mitotic regulatory proteins and targets them for proteasomal degradation. Target selection also involves a co-activator protein (either Cdc20 or Cdh1) together with core APC/C subunits. Here, a cryo-EM structure of APC/CChd1 bound to a D-box peptide substrate is presented. The structure provides important insight into the recognition and catalytic mechanism of APC/C substrates.
- Paula C. A. da Fonseca
- , Eric H. Kong
- & David Barford
-
Books & Arts |
 History: Franklin, centre stage
History: Franklin, centre stage
Josie Glausiusz enjoys a play capturing the zeal and backstabbing in the race to discover DNA's structure.
- Josie Glausiusz
-
Article |
 Cap binding and immune evasion revealed by Lassa nucleoprotein structure
Cap binding and immune evasion revealed by Lassa nucleoprotein structure
The first crystal structure for an arenavirus nucleoprotein is solved, revealing some new functions. The C-terminal domain has 3′ to 5′ exonuclease activity, and it is confirmed that Lassa virus nucleoprotein is capable of cleaving short RNAs and suppressing virus-induced interferon induction. The N-terminal domain contains a unique cap-binding feature, which has implications for understanding the distinctive cap-snatching mechanism of arenaviruses.
- Xiaoxuan Qi
- , Shuiyun Lan
- & Changjiang Dong
-
Article |
 Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA
Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA
tRNAs are synthesized in a premature form that requires trimming of the 5′ and 3′ ends and modification of specific nucleotides. RNase P, a complex containing a long catalytic RNA and a protein cofactor, catalyses the cleavage that generates the mature 5′ end. Here, the structure of RNase P bound to mature tRNAPhe is solved. Recognition of the leader sequence and its mechanism of cleavage is determined by soaking an oligonucleotide corresponding to the premature 5′ end into the crystal.
- Nicholas J. Reiter
- , Amy Osterman
- & Alfonso Mondragón
-
-
Letter |
 The mechanism of retroviral integration from X-ray structures of its key intermediates
The mechanism of retroviral integration from X-ray structures of its key intermediates
Insertion of retrovirus genome into host genome to replicate is mediated by a tetramer of the virus-encoded integrase protein. The structure of a related integrase from prototype foamy virus bound to the cleaved viral DNA ends, a complex called the intasome, was previously revealed. These authors solve the structure of the intasome interacting with the target host DNA both before and after it is cleaved, revealing new details of the integration process that may help in designing improved inhibitors of HIV.
- Goedele N. Maertens
- , Stephen Hare
- & Peter Cherepanov
-
Letter |
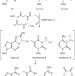 Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase
Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase
Mononuclear iron-containing oxygenases have many important roles in the cell, including the demethylation of DNA and histones. These authors crystallized the AlkB oxygenase in complex with various modified DNAs. By growing the crystals under anaerobic conditions and then exposing them to dioxygen to initiate oxidation, two different intermediates were trapped. A third type of intermediate was determined using additional computational analysis. These structures provide insight into how these enzymes perform oxidative demethylation.
- Chengqi Yi
- , Guifang Jia
- & Chuan He
-
News |
 HIV immunity is all in the amino acids
HIV immunity is all in the amino acids
Worldwide study implicates structural changes in a protein binding site
- Joseph Milton
-
Research Highlights |
Structural biology: A walk through the genome
-
News & Views |
 On stress and pressure
On stress and pressure
The protein angiotensinogen must undergo conformational changes to be cleaved into a precursor of the hormone angiotensin, which increases blood pressure. Oxidative stress seems to mediate this structural alteration. See Letter p.108
- Curt D. Sigmund
-
Letter |
 The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule
The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule
Mutations in ryanodine receptors can lead to severe genetic conditions in both cardiac and skeletal muscles. These authors report the X-ray crystal structure of a type 1 ryanodine receptor and pinpoint the exact locations of more than 50 disease-related mutations in the full-length receptor. The disease mutations seem to cause misfolding of an individual domain, to destabilize interactions between the three amino-terminal domains, or to otherwise affect one of the other domain interfaces.
- Ching-Chieh Tung
- , Paolo A. Lobo
- & Filip Van Petegem
-
Letter |
 The structural basis for membrane binding and pore formation by lymphocyte perforin
The structural basis for membrane binding and pore formation by lymphocyte perforin
Natural killer cells and cytotoxic T cells kill virus-infected and malignant cells, releasing the pore-forming protein perforin in the process. Perforin is required for the delivery of pro-apoptotic granzymes to the target cell. These authors present the crystal structure of a perforin monomer together with a cryo-electron microscopy reconstruction of the oligomeric pore. Perforin monomers within the pore are arranged with an inside-out orientation relative to the structurally homologous monomers of cholesterol-dependent cytolysins.
- Ruby H. P. Law
- , Natalya Lukoyanova
- & James C. Whisstock

 Crystal structure of metarhodopsin II
Crystal structure of metarhodopsin II