Featured
-
-
Article
| Open Access Photo-cycloaddition reactions of vinyldiazo compounds
Photo-cycloaddition reactions of vinyldiazo compounds
Heterocycles, highly prized by the pharmaceutical industry, are often constructed from diazo compounds. Here, the authors report that direct photolysis of vinyldiazo compounds followed by [3+2]-cycloaddition gives access to these motifs.
- Ming Bao
- , Klaudia Łuczak
- & Michael P. Doyle
-
Article
| Open Access Catalytic asymmetric [4 + 2] dearomative photocycloadditions of anthracene and its derivatives with alkenylazaarenes
Catalytic asymmetric [4 + 2] dearomative photocycloadditions of anthracene and its derivatives with alkenylazaarenes
While significant progress has been made in classical Diels-Alder reactions, the use of simple and inert feedstock chemicals under mild reaction conditions still remains challenging. Here the authors present the development of catalytic asymmetric [4 + 2] dearomative photocycloaddition involving anthracene and its derivatives with alkenylazaarenes.
- Dong Tian
- , Wenshuo Shi
- & Zhiyong Jiang
-
Article
| Open Access Cobalt-catalyzed cross-electrophile coupling of alkynyl sulfides with unactivated chlorosilanes
Cobalt-catalyzed cross-electrophile coupling of alkynyl sulfides with unactivated chlorosilanes
Transition-metal catalyzed cross-electrophile coupling (XEC) is a powerful tool for the construction of molecules but XEC between carbon electrophile and chlorosilanes to access organosilicon compounds remains underdeveloped. Here the authors disclose a highly efficient cobalt-catalyzed cross-electrophile alkynylation of a broad range of unactivated chlorosilanes with alkynyl sulfides as a stable and practical alkynyl electrophiles.
- Donghui Xing
- , Jinlin Liu
- & Liangbin Huang
-
Article
| Open Access Enantioselective nickel-catalyzed anodic oxidative dienylation and allylation reactions
Enantioselective nickel-catalyzed anodic oxidative dienylation and allylation reactions
Precision control of stereochemistry in radical reactions remains a formidable challenge. Here the authors demonstrate an electricity driven asymmetric Lewis acid catalysis to facilitate asymmetric dienylation and allylation reactions, resulting in the formation of all-carbon quaternary stereocenters.
- Qinglin Zhang
- , Jiayin Zhang
- & Chang Guo
-
Article
| Open Access Titanium catalyzed [2σ + 2π] cycloaddition of bicyclo[1.1.0]-butanes with 1,3-dienes for efficient synthesis of stilbene bioisosteres
Titanium catalyzed [2σ + 2π] cycloaddition of bicyclo[1.1.0]-butanes with 1,3-dienes for efficient synthesis of stilbene bioisosteres
Natural stilbenes show significant potential in the prevention and treatment of diseases due to their diverse pharmacological activities. Here the authors present a mild and effective Ti-catalyzed intermolecular radical-relay cycloaddition reaction with good regio- and trans-selectivity offering rapid access to structurally diverse stilbene bioisosteres.
- Yonghong Liu
- , Zhixian Wu
- & Lei Shi
-
Article
| Open Access Organocatalytic skeletal reorganization for enantioselective synthesis of S-stereogenic sulfinamides
Organocatalytic skeletal reorganization for enantioselective synthesis of S-stereogenic sulfinamides
The enantioselective synthesis of S-stereogenic sulfinamides has garnered considerable attention due to their unique structural and physicochemical properties but catalytic asymmetric synthesis of sulfinamides still remains challenging. Here, the authors present the synthesis of S-stereogenic sulfinamides through the peptide-mimic phosphonium salt-catalyzed asymmetric skeletal reorganization of simple prochiral and racemic sulfoximines.
- Zanjiao Liu
- , Siqiang Fang
- & Tianli Wang
-
Article
| Open Access Diastereoselective dearomatization of indoles via photocatalytic hydroboration on hydramine-functionalized carbon nitride
Diastereoselective dearomatization of indoles via photocatalytic hydroboration on hydramine-functionalized carbon nitride
Dearomative hydroboration of predominantly existing indole derivatives provides a straightforward strategy to synthesize boryl indolines, but developing eco-friendly methods for remains challenging. Here, the authors develop a method for heterogeneous photocatalytic trans-hydroboration of indole derivatives with NHC-borane.
- Qiao Zhang
- , Wengang Xu
- & Mingbo Wu
-
Article
| Open Access Observation of unusual outer-sphere mechanism using simple alkenes as nucleophiles in allylation chemistry
Observation of unusual outer-sphere mechanism using simple alkenes as nucleophiles in allylation chemistry
Allylic substitution reaction of alkenes has been well-developed, but it has limitations, partly due to the intrinsic predilection for an inner-sphere mechanism. Herein, the authors present an outer-sphere mechanism in Rh-catalyzed allylic substitution reaction of simple alkenes using gem-difluorinated cyclopropanes as allyl surrogates.
- Yaxin Zeng
- , Han Gao
- & Ying Xia
-
Article
| Open Access A facile synthesis of α,β-unsaturated imines via palladium-catalyzed dehydrogenation
A facile synthesis of α,β-unsaturated imines via palladium-catalyzed dehydrogenation
α,β-unsaturated compounds serve as versatile synthons in organic chemistry, but the α,β-desaturation of aliphatic imines is challenging due to easy hydrolysis and preferential dimerization. Herein, the authors report the synthesis of α,β-unsaturated imines by employing a pre-fluorination and palladium-catalyzed dehydrogenation reaction sequence.
- Chunyang Zhao
- , Rongwan Gao
- & Junkai Fu
-
Article
| Open Access Para-selective nitrobenzene amination lead by C(sp2)-H/N-H oxidative cross-coupling through aminyl radical
Para-selective nitrobenzene amination lead by C(sp2)-H/N-H oxidative cross-coupling through aminyl radical
Direct radical C–H amination strategies have exhibited innovation, but challenges remain with C–H amination of electron-poor nitroarenes due to the essence of the electron-deficient nitrogen radical. Herein, the authors report a transition metal-free dehydrogenative C(sp2)-H/N-H cross-coupling between electron-poor nitroarenes and amines.
- Zhen Zhang
- , Shusheng Yue
- & Hu Cai
-
Article
| Open Access Introduction of the difluoromethyl group at the meta- or para-position of pyridines through regioselectivity switch
Introduction of the difluoromethyl group at the meta- or para-position of pyridines through regioselectivity switch
The direct C−H-difluoromethylation of pyridines represents a highly efficient economic way to access azines. However, the direct meta-difluoromethylation of pyridines remains elusive. Here, the authors demonstrate switchable meta- as well as para-C−H difluoromethylation of pyridines through radical processes by using oxazino pyridine intermediates.
- Pengwei Xu
- , Zhe Wang
- & Armido Studer
-
Article
| Open Access Late-stage benzenoid-to-troponoid skeletal modification of the cephalotanes exemplified by the total synthesis of harringtonolide
Late-stage benzenoid-to-troponoid skeletal modification of the cephalotanes exemplified by the total synthesis of harringtonolide
Many natural products exist as families of structurally similar molecules, and therefore developing skeletal modifications of common intermediates offers flexible and powerful approaches for target synthesis. Here, the authors report a single-atom insertion into the framework of the benzenoid subfamily, providing access to the troponoid congeners.
- Stefan Wiesler
- , Goh Sennari
- & Richmond Sarpong
-
Article
| Open Access Asymmetric C–H Dehydrogenative Alkenylation via a Photo-induced Chiral α‑Imino Radical Intermediate
Asymmetric C–H Dehydrogenative Alkenylation via a Photo-induced Chiral α‑Imino Radical Intermediate
The direct alkenylation with simple alkenes stands out as the most ideal yet challenging strategy for obtaining high-valued desaturated alkanes. Herein, the authors present a direct asymmetric dehydrogenative α-C(sp3)-H alkenylation of carbonyls based on synergistic photoredox-cobalt-chiral primary amine catalysis under visible light.
- Zongbin Jia
- , Liang Cheng
- & Sanzhong Luo
-
Article
| Open Access Rapid and scalable photocatalytic C(sp2)–C(sp3) Suzuki−Miyaura cross-coupling of aryl bromides with alkyl boranes
Rapid and scalable photocatalytic C(sp2)–C(sp3) Suzuki−Miyaura cross-coupling of aryl bromides with alkyl boranes
In recent years a growing demand for drug design approaches that incorporate a higher number of sp3-hybridized carbons fuelled the development of innovative cross-coupling strategies to reliably introduce aliphatic fragments. Here, the authors present a powerful approach for the light-mediated B-alkyl Suzuki−Miyaura crosscoupling between alkyl boranes and aryl bromides.
- Ting Wan
- , Luca Capaldo
- & Timothy Noël
-
Article
| Open Access Deciphering complexity in Pd–catalyzed cross-couplings
Deciphering complexity in Pd–catalyzed cross-couplings
Knowledge about the full reaction signature, such as the complete profile of products and side-products is important in accelerating discovery chemistry. Here, the authors report a methodology using high-throughput experimentation and multivariate data analysis to examine Palladium- catalyzed cross-coulpling reactions.
- George E. Clarke
- , James D. Firth
- & Ian J. S. Fairlamb
-
Article
| Open Access “On-Water” accelerated dearomative cycloaddition via aquaphotocatalysis
“On-Water” accelerated dearomative cycloaddition via aquaphotocatalysis
Developing water compatible synthetic methods for Sulfur(VI) fluoride exchange (SuFEx) chemistry is of paramount importance. Here the authors demonstrate a catalytic dearomative [2+2] photocycloaddition that significantly speeds up the synthesis of heterocyclic alkyl SuFEx hubs, leveraging the unique reactivity at the water-oil interface.
- Soo Bok Kim
- , Dong Hyeon Kim
- & Han Yong Bae
-
Article
| Open Access One pot conversion of phenols and anilines to aldehydes and ketones exploiting α gem boryl carbanions
One pot conversion of phenols and anilines to aldehydes and ketones exploiting α gem boryl carbanions
For the carbonylation reactions to synthesize aldehyde, ester, amide, and other carbonyl derivatives, a transition metal-free and CO- free reaction was challenging but highly demanded. Here, the authors report a transition metal-free and CO- free method for deoxygenetive/denitrogenetive conversion of phenols and anilines to aldehydes and ketones.
- Kanak Kanti Das
- , Debasis Aich
- & Santanu Panda
-
Article
| Open Access Electrochemical C−H deuteration of pyridine derivatives with D2O
Electrochemical C−H deuteration of pyridine derivatives with D2O
Despite the established C − H deuteration of pyridine derivatives synthetic methods with good D-incorporation, under milder conditions with easy operation procedures is still on the way. Here, the authors develop a straightforward, metal-free, and acid-/base-free electrochemical C4-selective C − H deuteration of pyridine derivatives with high selectivity and excellent D-incorporation at room temperature, which use D2O as economic and convenient D-source.
- Zhiwei Zhao
- , Ranran Zhang
- & Youai Qiu
-
Article
| Open Access Enantioselective synthesis of chiral α,α-dialkyl indoles and related azoles by cobalt-catalyzed hydroalkylation and regioselectivity switch
Enantioselective synthesis of chiral α,α-dialkyl indoles and related azoles by cobalt-catalyzed hydroalkylation and regioselectivity switch
Catalytic and enantioselective construction of chiral α,α-dialkyl indoles represents an important yet challenging objective to be developed. Herein the authors describe a cobalt catalyzed enantioselective anti-Markovnikov alkene hydroalkylation via the remote stereocontrol for the synthesis of α,α-dialkyl indoles and other N-heterocycles.
- Jiangtao Ren
- , Zheng Sun
- & Zhihui Shao
-
Article
| Open Access Decarboxylative stereoretentive C–N coupling by harnessing aminating reagent
Decarboxylative stereoretentive C–N coupling by harnessing aminating reagent
Accessing chiral alkylamines is highly important due to their ubiquity in bioactive molecules. Here, the authors present the development of stereoretentive decarboxylative amidation of carboxylic acids using dioxazolone under ambient conditions.
- Jeonguk Kweon
- , Bumsu Park
- & Sukbok Chang
-
Article
| Open Access Carbon–nitrogen transmutation in polycyclic arenol skeletons to access N-heteroarenes
Carbon–nitrogen transmutation in polycyclic arenol skeletons to access N-heteroarenes
Developing skeletal editing tools, especially to realize the single-atom transmutation in a ring system without altering the ring size is challenging. Here, the authors introduce a skeletal editing strategy that enables polycyclic arenols to be readily converted into N-heteroarenes through carbon–nitrogen transmutation.
- Hong Lu
- , Yu Zhang
- & Hao Wei
-
Article
| Open Access Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes
Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes
The widespread use of organoboronic acids has prompted the development of synthetic methodologies to meet the demands on structural diversity and functional group tolerance. Herein, the authors disclose a divergent radical dechloroborylation reaction enabled by dinuclear gold catalysis with visible light irradiation.
- Cheng-Long Ji
- , Hongliang Chen
- & Jin Xie
-
Article
| Open Access Stereospecific syn-dihalogenations and regiodivergent syn-interhalogenation of alkenes via vicinal double electrophilic activation strategy
Stereospecific syn-dihalogenations and regiodivergent syn-interhalogenation of alkenes via vicinal double electrophilic activation strategy
Restricted reaction mechanism of anti-dihalogenation of alkenes makes it challenging to alter the diastereochemical course into the complementary syn-dihalogenation process. Here, the authors report a conceptually distinctive strategy for the simultaneous double electrophilic activation of the two alkene carbons from the same side to realize syn-dihalogenation.
- Hyeon Moon
- , Jungi Jung
- & Won-jin Chung
-
Article
| Open Access Diastereo-divergent synthesis of chiral hindered ethers via a synergistic calcium(II)/gold(I) catalyzed cascade hydration/1,4-addition reaction
Diastereo-divergent synthesis of chiral hindered ethers via a synergistic calcium(II)/gold(I) catalyzed cascade hydration/1,4-addition reaction
Developing an efficient method for the stereocontrolled synthesis of all stereoisomers of chiral hindered ethers is highly desirable but challenging. Here, the authors report an asymmetric cascade reaction catalyzed by a bimetallic catalytic system with control over the configuration of the stereocenters of tetra-aryl substituted ethers.
- Xiangfeng Lin
- , Xia Mu
- & Can Li
-
Article
| Open Access Switchable chemoselective aryne reactions between nucleophiles and pericyclic reaction partners using either 3-methoxybenzyne or 3-silylbenzyne
Switchable chemoselective aryne reactions between nucleophiles and pericyclic reaction partners using either 3-methoxybenzyne or 3-silylbenzyne
Due to the electrophilic nature of arynes, it is very challenging to control chemoselectivity, when substrates possess multiple competing reaction sites. Here, the authors demonstrate that chemoselective control between two major types of benzyne transformation is accomplished by varying the 3-substituent on aryne intermediate.
- Hongcheng Tan
- , Shuxin Yu
- & Yang Li
-
Article
| Open Access Catalytic 1,1-diazidation of alkenes
Catalytic 1,1-diazidation of alkenes
Compared to well-developed catalytic 1,2-diazidation of alkenes, the corresponding catalytic 1,1-diazidation of alkenes has not been realized. Here, the authors report an efficient approach for catalytic 1,1-diazidation of alkenes by redox-active selenium catalysis.
- Wangzhen Qiu
- , Lihao Liao
- & Xiaodan Zhao
-
Article
| Open Access Pathway-divergent coupling of 1,3-enynes with acrylates through cascade cobalt catalysis
Pathway-divergent coupling of 1,3-enynes with acrylates through cascade cobalt catalysis
The development of divergent reaction pathways controlled by different ligands is a critical goal. Here the authors describe a cobalt-catalyzed strategy for cascade coupling of 1,3-enynes with two molecules of acrylates through three reaction modes.
- Heng Wang
- , Xiaofeng Jie
- & Fanke Meng
-
Article
| Open Access Dehydroxylative radical N-glycosylation of heterocycles with 1-hydroxycarbohydrates enabled by copper metallaphotoredox catalysis
Dehydroxylative radical N-glycosylation of heterocycles with 1-hydroxycarbohydrates enabled by copper metallaphotoredox catalysis
N-Glycosylated heterocycles play important roles in biological systems and drug development, but the synthesis heavily relies on ionic N-glycosylation. Herein, the authors report a dehydroxylative radical method for synthesizing N-glycosides by leveraging copper metallaphotoredox catalysis.
- Da-Peng Liu
- , Xiao-Sen Zhang
- & Xiang-Guo Hu
-
Article
| Open Access Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
The synthesis of sequence-regulated oligosulfates has not yet been established due to the difficulties in precise reactivity control. Here, the authors report a multi-directional divergent iterative method to furnish oligosulfates based on a chain homologation approach, in which the fluorosulfate unit is regenerated.
- Min Pyeong Kim
- , Swatilekha Kayal
- & Sung You Hong
-
Article
| Open Access Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes
Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes
Developing facile and direct synthesis routes for enantioselective construction of cyclic π-conjugated molecules is crucial but the chirality orginiating from the distorted structure around heptagon-containing polyarenes is largely overlooked. Herein the authors present a highly enantioselective synthesis for fabrication of all carbon heptagon-containing polyarenes via palladium-catalyzed carbene-based cross–coupling of benzyl bromides and N-arylsulfonylhydrazones.
- Huan Zhang
- , Chuan-Jun Lu
- & Ren-Rong Liu
-
Article
| Open Access Noncovalent interaction with a spirobipyridine ligand enables efficient iridium-catalyzed C–H activation
Noncovalent interaction with a spirobipyridine ligand enables efficient iridium-catalyzed C–H activation
Exploitation of noncovalent interactions has received much attention for the design of metal catalysts. However, because of the weak nature, CH-π interactions have been less utilized for the control of organic reactions. Here, the authors report that the CH-π interaction can be used to kinetically accelerate catalytic C-H activation of arenes.
- Yushu Jin
- , Boobalan Ramadoss
- & Laurean Ilies
-
Article
| Open Access Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes
Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes
Planar chiral [2.2]paracyclophanes have a wide range of applications in asymmetric synthesis and materials science. However, they are accessed via time-consuming chiral separations or kinetic resolution approaches. Here, the authors report a simple, metal-free protocol for organocatalytic desymmetrization of prochiral diformyl[2.2]paracyclophanes.
- Vojtěch Dočekal
- , Filip Koucký
- & Jan Veselý
-
Article
| Open Access Carbon dioxide capture and functionalization by bis(N-heterocyclic carbene)-borylene complexes
Carbon dioxide capture and functionalization by bis(N-heterocyclic carbene)-borylene complexes
Bis(N-heterocyclic carbene)-borylene complexes are capable of capturing and functionalizing CO2, but stable single-site-boron-carbon dioxide adducts are rarely reported. Here, the authors report the synthesis of a stable borylene-CO2 complex as well as the functionalization of the captured CO2.
- Jun Fan
- , An-Ping Koh
- & Cheuk-Wai So
-
Article
| Open Access Paired electrocatalysis unlocks cross-dehydrogenative coupling of C(sp3)-H bonds using a pentacoordinated cobalt-salen catalyst
Paired electrocatalysis unlocks cross-dehydrogenative coupling of C(sp3)-H bonds using a pentacoordinated cobalt-salen catalyst
Cross-dehydrogenative coupling (CDC) of C-H bonds is an ideal approach for C-C bond construction but suffers from low selectivity of similar C-H bonds. Here, the authors describe a highly selective paired electrocatalysis strategy towards CDC combining hydrogen evolution reaction catalysis with hydride transfer catalysis.
- Ke Liu
- , Mengna Lei
- & Sheng Zhang
-
Article
| Open Access Nickel-catalyzed switchable arylative/endo-cyclization of 1,6-enynes
Nickel-catalyzed switchable arylative/endo-cyclization of 1,6-enynes
Divergent functionalizations of pi bonds allow for synthetic chemists to move from simplicity to complexity. Here, the authors report a nickel-catalyzed switchable arylation/cyclization of 1,6-enynes in which the nature of the ligand dictates the regioselectivity of alkyne arylation, while the electrophilic trapping reagents determine the selectivity of the cyclization mode.
- Wenfeng Liu
- , Wei Li
- & Wangqing Kong
-
Article
| Open Access Umpolung reactivity of strained C–C σ-bonds without transition-metal catalysis
Umpolung reactivity of strained C–C σ-bonds without transition-metal catalysis
Umpolung reactions typically focus on carbonyls or imine derivatives. Here, the authors report the umpolung reaction of C–C σ-bonds in bicyclo[1.1.0]butanes (BCBs) with electrophilic alkenes, yielding various cyclobutenes or conjugated dienes.
- Dachang Bai
- , Xiuli Guo
- & Junbiao Chang
-
Article
| Open Access Trialkoxysilane-Induced Iridium-Catalyzed para-Selective C–H Bond Borylation of Arenes
Trialkoxysilane-Induced Iridium-Catalyzed para-Selective C–H Bond Borylation of Arenes
Controlling regioselectivity is one of the most challenging aspects for the construction of aryl boron compounds, and there are few practical and general strategies for such transformations. Here, the authors report an iridium-catalyzed trialkoxysilane protecting group-assisted regioselective C–H borylation with various aromatic compounds.
- Guodong Ju
- , Zhibin Huang
- & Yingsheng Zhao
-
Article
| Open Access Electroreduction of unactivated alkenes using water as hydrogen source
Electroreduction of unactivated alkenes using water as hydrogen source
The reduction of unactivated alkyl alkenes is a difficult challenge in organic chemistry. Here, the authors present a silicon-mediated electroreduction of alkyl alkenes, using water as a hydrogen source, enabled by [Fe]-H.
- Yanwei Wang
- , Qian Wang
- & Youai Qiu
-
Article
| Open Access Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides
Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides
The direct reductive coupling of alkyl alcohol and aryl halide enables efficient access to valuable compounds, but the asymmetric pattern remains unknown. Here the authors describe the enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohol and aryl bromide in the presence of an NHC activating agent.
- Li-Li Zhang
- , Yu-Zhong Gao
- & Ze-Peng Yang
-
Article
| Open Access Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
While the homo N-N coupling of two NH moieties to form the hydrazide N-N bond is well developed, the crossdehydrogenative hetero N-N coupling remains unevolved. Here the authors present an efficient, PhI(OAc)2-mediated intermolecular N-N cross-coupling of primary benzamides with primary and secondary amines.
- Subban Kathiravan
- , Prakriti Dhillon
- & Ian A. Nicholls
-
Article
| Open Access Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Carbon isotope labelling of bioactive molecules is essential for accessing the pharmacokinetic and pharmacodynamic properties of new drug entities. Here, the authors propose an electrochemical isotope-labelling protocol which enables the use of near-stoichiometric 14CO2, facilitating late-stage and single-step carbon-14 labelling of pharmaceuticals and representative precursors.
- Gabriel M. F. Batista
- , Ruth Ebenbauer
- & Troels Skrydstrup
-
Article
| Open Access Reduction of Li+ within a borate anion
Reduction of Li+ within a borate anion
Chemical reduction of alkali cations to their metals is extremely challenging. Here, the authors synthesized a series of redox-active borate anions stabilized by bipyridine ligands which can reduce lithium ions generating elemental lithium metal and borate radicals.
- Haokun Li
- , Jiachen Yao
- & Zhenpin Lu
-
Article
| Open Access A concise and scalable chemoenzymatic synthesis of prostaglandins
A concise and scalable chemoenzymatic synthesis of prostaglandins
Prostaglandins are of interest to synthetic chemists due to their biological activities. Here, the authors present a concise chemoenzymatic synthesis method for several representative prostaglandins, achieved in 5 to 7 steps, via the common intermediate bromohydrin, a radical equivalent of Corey lactone.
- Yunpeng Yin
- , Jinxin Wang
- & Jian Li
-
Article
| Open Access Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
P-stereogenic heterocycles are privileged chiral ligands and bioactive compounds, but the catalytic asymmetric synthesis of P-stereogenic phosphindane derivatives is challenging. Here, the authors report a catalytic kinetic resolution of phosphindole oxides via rhodium-catalyzed diastereo- and enantioselective conjugate addition to access enantiopure Pstereogenic phosphindane and phosphindole derivatives.
- Long Yin
- , Jiajia Li
- & Dong Guo
-
Article
| Open Access Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Chemically modified nucleobases and oligonucleotides are essential in several fields but introducing functional groups into nucleobases requires laborious chemical synthesis. Here, the authors report site-selective alkylation at the C8-position of guanines in guanosine, GMP, GDP, and GTP, as well as late-stage alkylation of RNA/DNA oligonucleotides through photomediated Minisci reaction.
- Ruoqian Xie
- , Wanlu Li
- & Gang Chen
-
Article
| Open Access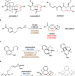 Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Medium-sized-ring compounds are challenging synthetic targets in organic chemistry. Here the authors report an intramolecular dearomative [5 + 4] cycloaddition of naphthalene-derived vinylcyclopropanes to construct these molecules under visible-light irradiation and a proper triplet photosensitizer.
- Min Zhu
- , Yuan-Jun Gao
- & Shu-Li You
-
Article
| Open Access Synthesis of meta-carbonyl phenols and anilines
Synthesis of meta-carbonyl phenols and anilines
Phenols and anilines are of extreme importance for medicinal chemistry and material science but the selective preparation of meta-substituted phenols and anilines remains challenging. Here the authors report an efficient copper-catalyzed dehydrogenation strategy to exclusively synthesize meta-carbonyl phenols and anilines from carbonyl substituted cyclohexanes.
- Bao-Yin Zhao
- , Qiong Jia
- & Yong-Qiang Wang
-
Article
| Open Access N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
Planar-chiral cyclophanes are important for drug discovery but the enantioselective synthesis of planar-chiral cyclophanes remains challenging. Here the authors describe an N-heterocyclic carbene-catalyzed asymmetric construction of planar-chiral cyclophanes.
- Jiayan Li
- , Ziyang Dong
- & Changgui Zhao
-
Article
| Open Access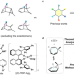 Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Combining data science and organic synthesis to achieve the rapid and precise creation of complex molecules while controlling multiple selectivities is an emerging trend, but few successful examples are reported. Here, the authors develop an artificial neural network regression model using bond orbital data to predict chemical reactivities.
- Shingo Harada
- , Hiroki Takenaka
- & Tetsuhiro Nemoto

 Atroposelective synthesis of biaxial bridged eight-membered terphenyls via a Co/SPDO-catalyzed aerobic oxidative coupling/desymmetrization of phenols
Atroposelective synthesis of biaxial bridged eight-membered terphenyls via a Co/SPDO-catalyzed aerobic oxidative coupling/desymmetrization of phenols