Featured
-
-
Article
| Open Access Fast three-color single-molecule FRET using statistical inference
Fast three-color single-molecule FRET using statistical inference
Three-colour FRET is a powerful tool to study macromolecular conformational dynamics, but is temporally limited due to the experimental complexity. Here the authors develop experimental and analytical methods for probing submillisecond-time scale dynamics using single continuous-wave excitation.
- Janghyun Yoo
- , Jae-Yeol Kim
- & Hoi Sung Chung
-
Article
| Open Access Non-cooperative 4E-BP2 folding with exchange between eIF4E-binding and binding-incompatible states tunes cap-dependent translation inhibition
Non-cooperative 4E-BP2 folding with exchange between eIF4E-binding and binding-incompatible states tunes cap-dependent translation inhibition
Phosphorylation of eIF4E binding proteins (4E-BPs) controls their folding and regulates cap-dependent translation. Here, the authors show that phosphorylation of the C-terminal disordered region stabilizes the non-cooperatively folded 4E-BP domain to an eIF4E binding-incompatible state to control translation.
- Jennifer E. Dawson
- , Alaji Bah
- & Julie D. Forman-Kay
-
Article
| Open Access Extent of N-terminus exposure of monomeric alpha-synuclein determines its aggregation propensity
Extent of N-terminus exposure of monomeric alpha-synuclein determines its aggregation propensity
In Parkinson’s disease (PD) the monomeric protein alpha-synuclein (aSyn) misfolds and aggregates into insoluble fibrils. Here the authors use NMR measurements and hydrogen–deuterium exchange mass spectrometry and find that the more solvent exposed the N-terminus of aSyn is, the more aggregation prone its conformation becomes, and further show how PD mutations and post translational modifications influence the extent of the N-terminus solvent exposure.
- Amberley D. Stephens
- , Maria Zacharopoulou
- & Gabriele S. Kaminski Schierle
-
Article
| Open Access Complex microparticle architectures from stimuli-responsive intrinsically disordered proteins
Complex microparticle architectures from stimuli-responsive intrinsically disordered proteins
The production of microparticles with complex geometries for biotechnological use historically requires sophisticated fabrication techniques. Here, the authors create complex particle geometries by exploiting the metastable region of the phase diagram of thermally responsive intrinsically disordered proteins within microdroplets.
- Stefan Roberts
- , Vincent Miao
- & Ashutosh Chilkoti
-
Article
| Open Access Liquid-liquid phase separation and extracellular multivalent interactions in the tale of galectin-3
Liquid-liquid phase separation and extracellular multivalent interactions in the tale of galectin-3
Galectin-3 consists of an unstructured N-terminal domain (NTD) and a structured carbohydrate-recognition domain and agglutinates neutrophils and glycosylated molecules in the extracellular milieu. Here the authors combine biophysical and biochemical experiments with NMR measurements and show that the galectin-3 NTD undergoes liquid-liquid phase separation (LLPS) and agglutinates other molecules through this process.
- Yi-Ping Chiu
- , Yung-Chen Sun
- & Jie-rong Huang
-
Article
| Open Access Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness
Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness
Antibody-mediated immune responses rely on antigen recognition by the B cell antigen receptor (BCR) and SLP65 is a key scaffold protein mediating BCR signaling. Here authors show that effective B cell activation requires tripartite phase separation of SLP65, CIN85, and lipid vesicles.
- Leo E. Wong
- , Arshiya Bhatt
- & Christian Griesinger
-
Article
| Open Access Atomic-level insight into mRNA processing bodies by combining solid and solution-state NMR spectroscopy
Atomic-level insight into mRNA processing bodies by combining solid and solution-state NMR spectroscopy
Processing bodies are membrane less organelles that contain enzymes involved in mRNA turnover, among them enhancer of decapping 3 (Edc3). Here the authors use solid- and solution-state NMR spectroscopy to characterize the structural organization and dynamics of Edc3 and find that its interactions with RNA and between the different Edc3 domains are largely preserved in the phase-separated state.
- Reinier Damman
- , Stefan Schütz
- & Marc Baldus
-
Article
| Open Access Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response
Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response
The autophagy protein p62 undergoes liquid-liquid phase separation but how this is regulated is unclear. Here, the authors report that the histone chaperone DAXX interacts with p62 in the cytoplasm to drive its phase separation.
- Yi Yang
- , Thea L. Willis
- & Shouqing Luo
-
Article
| Open Access The prion-like domain of Drosophila Imp promotes axonal transport of RNP granules in vivo
The prion-like domain of Drosophila Imp promotes axonal transport of RNP granules in vivo
The physiological role of prion-like domains (PLDs) within RNA-binding proteins is not well understood. Here, authors show in Drosophila that the PLD in the protein Imp is required for localization of ribonucleoprotein granules to axons and axonal remodelling.
- Jeshlee Vijayakumar
- , Charlène Perrois
- & Florence Besse
-
Article
| Open Access Tau local structure shields an amyloid-forming motif and controls aggregation propensity
Tau local structure shields an amyloid-forming motif and controls aggregation propensity
The biophysical mechanisms of how disease-associated tau mutations drive amyloid formation are not well understood. Here the authors use biophysical approaches, cell models and MD simulations and find that the intrinsically disordered repeat domain of tau encodes a metastable local structure and perturbations through mutations and proline isomerization cause an aggregation phenotype in vitro and in cells.
- Dailu Chen
- , Kenneth W. Drombosky
- & Lukasz A. Joachimiak
-
Article
| Open Access Disordered RNA chaperones can enhance nucleic acid folding via local charge screening
Disordered RNA chaperones can enhance nucleic acid folding via local charge screening
RNA chaperones, such as the hepatitic C virus (HCV) core protein, are proteins that aid in the folding of nucleic acids. Here authors use single‐molecule spectroscopy and simulation to show that the HCV core protein acts as a flexible macromolecular counterion which facilitates nucleic acid folding.
- Erik D. Holmstrom
- , Zhaowei Liu
- & Benjamin Schuler
-
Article
| Open Access Side chain to main chain hydrogen bonds stabilize a polyglutamine helix in a transcription factor
Side chain to main chain hydrogen bonds stabilize a polyglutamine helix in a transcription factor
Polyglutamine (polyQ) tracts are low-complexity regions and their expansion is linked to certain neurodegenerative diseases. Here the authors combine experimental and computational approaches to find that the length of the androgen receptor polyQ tract correlates with its helicity and show that the polyQ helical structure is stabilized by hydrogen bonds between the Gln side chains and main chain carbonyl groups.
- Albert Escobedo
- , Busra Topal
- & Xavier Salvatella
-
Article
| Open Access Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms
Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms
Amyloid beta (Aβ42) peptides form heterogeneous mixtures of aggregates, which are closely linked to Alzheimer’s disease. This study shows how different types of Aβ42 aggregates are associated with distinct mechanisms of toxicity
- Suman De
- , David C. Wirthensohn
- & David Klenerman
-
Article
| Open Access A dual role in regulation and toxicity for the disordered N-terminus of the toxin GraT
A dual role in regulation and toxicity for the disordered N-terminus of the toxin GraT
The Pseudomonas putida toxin GraT and antitoxin GraA form a type II toxin-antoxin module. Here the authors present the crystal structures of the GraA dimer, GraTA and GraA-DNA complexes and show that GraT contains a functionally important N-terminal intrinsic disordered region that prevents the binding of the GraTA complex to the operator.
- Ariel Talavera
- , Hedvig Tamman
- & Remy Loris
-
Article
| Open Access The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger
The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger
The microalgal pyrenoid has been reported to behave as a phase-separated liquid compartment. Here the authors demonstrate that the CO2-fixing enzyme Rubisco and the linker protein EPYC1 are necessary and sufficient to bring about a liquid-liquid phase separation that recapitulates the pyrenoid’s liquid-like behavior.
- Tobias Wunder
- , Steven Le Hung Cheng
- & Oliver Mueller-Cajar
-
Article
| Open Access Compositional adaptability in NPM1-SURF6 scaffolding networks enabled by dynamic switching of phase separation mechanisms
Compositional adaptability in NPM1-SURF6 scaffolding networks enabled by dynamic switching of phase separation mechanisms
The nucleolus is a membrane-less organelle and both Nucleophosmin (NPM1) and Surfeit locus protein 6 (SURF6) are abundant proteins within the nucleolus. Here the authors employ biophysical methods to study the properties of NPM1-S6N droplets and provide insights into the role of SURF6 in maintaining and modulating the liquid-like structure of the nucleolus.
- Mylene C. Ferrolino
- , Diana M. Mitrea
- & Richard W. Kriwacki
-
Article
| Open Access Transition path times of coupled folding and binding reveal the formation of an encounter complex
Transition path times of coupled folding and binding reveal the formation of an encounter complex
How interactions between binding partners form or break is hidden in the transition paths from the encounter to the formation of a stable complex. Here authors use single‐molecule spectroscopy to measure the transition path times for the association of two intrinsically disordered proteins that form a folded dimer upon binding and identify a metastable encounter complex.
- Flurin Sturzenegger
- , Franziska Zosel
- & Benjamin Schuler
-
Article
| Open Access Diffusion-limited association of disordered protein by non-native electrostatic interactions
Diffusion-limited association of disordered protein by non-native electrostatic interactions
Intrinsically disordered proteins (IDPs) usually fold during binding to target proteins which involves the formation of a transient complex (TC). Here authors use single-molecule FRET to show that the lifetime of TC for IDP binding is very long due to the stabilization by non-native electrostatic interactions, which makes fast association possible.
- Jae-Yeol Kim
- , Fanjie Meng
- & Hoi Sung Chung
-
Article
| Open Access Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing
Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing
Many proteins which sense membrane curvature contain intrinsically disordered domains. Here the authors use Monte Carlo simulations combined with experimental approaches and report that disordered domains are potent sensors of membrane curvature.
- Wade F. Zeno
- , Upayan Baul
- & Jeanne C. Stachowiak
-
Article
| Open Access Controllable protein phase separation and modular recruitment to form responsive membraneless organelles
Controllable protein phase separation and modular recruitment to form responsive membraneless organelles
Designer organelles with new biochemical functionalities are of great interest in synthetic biology and cellular engineering. Here the authors present a single-protein-based platform for generating synthetic membraneless compartments that is capable of enzymatically-triggered alterations to phase behavior and of recruiting and concentrating cargo proteins.
- Benjamin S. Schuster
- , Ellen H. Reed
- & Daniel A. Hammer
-
Article
| Open Access Conformational switching within dynamic oligomers underpins toxic gain-of-function by diabetes-associated amyloid
Conformational switching within dynamic oligomers underpins toxic gain-of-function by diabetes-associated amyloid
Toxic gain-of-function by islet amyloid polypeptide (IAPP) is thought to be mediated by membrane poration. Here the authors develop diluted-FRET to show that changes in pore structure correlate with onset of toxicity inside insulin secreting cells.
- Melissa Birol
- , Sunil Kumar
- & Andrew D. Miranker
-
Article
| Open Access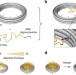 DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex
DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex
FG-Nups are disordered proteins in the nuclear pore complex (NPC) where they selectively control nuclear transport. Here authors build NPC-mimics based on DNA origami rings which attach a certain numbers of Nups to analyse those nanopores by cryoEM and conductance measurements.
- Philip Ketterer
- , Adithya N. Ananth
- & Cees Dekker
-
Article
| Open Access Self-interaction of NPM1 modulates multiple mechanisms of liquid–liquid phase separation
Self-interaction of NPM1 modulates multiple mechanisms of liquid–liquid phase separation
The nucleolus is a membrane-less organelle formed through liquid–liquid phase separation (LLPS). Here the authors use biophysical methods and show that the nucleolar protein nucleophosmin (NPM1) also undergoes LLPS through homotypic, inter-NPM1 interactions and discuss implications for the ribosome biogenesis process.
- Diana M. Mitrea
- , Jaclyn A. Cika
- & Richard W. Kriwacki
-
Article
| Open Access Discovery and characterization of stable and toxic Tau/phospholipid oligomeric complexes
Discovery and characterization of stable and toxic Tau/phospholipid oligomeric complexes
The Alzheimer protein Tau interacts with biological membranes, but the role of these interactions in regulating Tau function in health and disease remains unexplored. Here, the authors report on the discovery and characterization of neurotoxic oligomeric protein/phospholipid complexes.
- Nadine Ait-Bouziad
- , Guohua Lv
- & Hilal A. Lashuel
-
Article
| Open Access Silk micrococoons for protein stabilisation and molecular encapsulation
Silk micrococoons for protein stabilisation and molecular encapsulation
Silk fibres currently used in biotechnology are chemically reconstituted silk fibroins (RSF), which are more stable than native silk fibroin (NSF) but possess different biophysical properties. Here, the authors use microfluidic droplets to encapsulate and store NSF, preserving their native structure.
- Ulyana Shimanovich
- , Francesco S. Ruggeri
- & Tuomas P. J. Knowles
-
Article
| Open Access Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core
Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core
Huntington's disease is caused by a polyglutamine stretch expansion in the first exon of huntingtin. Here, the authors use infrared spectroscopy and solid-state NMR and show that polymorphic huntingtin exon1 fibres differ in their flanking regions but not their core polyglutamine amyloid structures.
- Hsiang-Kai Lin
- , Jennifer C. Boatz
- & Patrick C. A. van der Wel
-
Article
| Open Access Phosphorylation induces sequence-specific conformational switches in the RNA polymerase II C-terminal domain
Phosphorylation induces sequence-specific conformational switches in the RNA polymerase II C-terminal domain
The RNA polymerase II C-terminal domain acts as a hub to coordinate transcription and nascent mRNA processing. Here the authors identify a phosphorylation-dependent switch in thetrans-to-cisisomerization of proline in the CTD heptad repeats that make those repeats susceptible to further modifications by regulatory enzymes.
- Eric B. Gibbs
- , Feiyue Lu
- & Scott A. Showalter
-
Article
| Open Access An allosteric conduit facilitates dynamic multisite substrate recognition by the SCFCdc4 ubiquitin ligase
An allosteric conduit facilitates dynamic multisite substrate recognition by the SCFCdc4 ubiquitin ligase
The WD40 domain of the SCFCdc4ubiquitin ligase targets substrates via multiple phosphorylated degron motifs. The authors define a second degron-binding WD40 pocket that imparts a negative allosteric effect on binding to the primary pocket, and thereby enables the dynamic exchange of bound degrons.
- Veronika Csizmok
- , Stephen Orlicky
- & Julie D. Forman-Kay
-
Article
| Open Access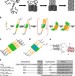 Rapid α-oligomer formation mediated by the Aβ C terminus initiates an amyloid assembly pathway
Rapid α-oligomer formation mediated by the Aβ C terminus initiates an amyloid assembly pathway
The elucidation of amyloid nucleation mechanisms remains challenging as early oligomeric intermediates are transient and difficult to distinguish. Here the authors use Aβ- polyglutamine hybrid peptides designed to slow and limit amyloid maturation to provide insights into the structures of Aβ self-assembly intermediates.
- Pinaki Misra
- , Ravindra Kodali
- & Ronald Wetzel
-
Article
| Open Access Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose)
Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose)
Intrinsically disordered proteins can phase separate from the soluble intracellular space. Here the authors show that the nucleic acid-mimicking biopolymer poly(ADP-ribose) (PAR) nucleates intracellular liquid demixing and orchestrates the earliest cellular responses to DNA breakage.
- Matthias Altmeyer
- , Kai J. Neelsen
- & Jiri Lukas
-
Article
| Open Access Cooperative folding of intrinsically disordered domains drives assembly of a strong elongated protein
Cooperative folding of intrinsically disordered domains drives assembly of a strong elongated protein
Staphylococcal biofilm formation is promoted by the surface protein SasG. Here, the authors characterize the structure and remarkable mechanical strength of the repeat region of SasG, and show how elongation is achieved by obligate folding of the disordered regions within the repeating units.
- Dominika T. Gruszka
- , Fiona Whelan
- & Jane Clarke
-
Article
| Open Access Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins
Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins
Hydration water plasticizes protein structures and is essential for their biological functions, such as enzymatic catalysis. Here, the authors use neutron scattering and molecular dynamics simulations to study hydration water at the dynamical transition of folded and disordered proteins.
- Giorgio Schirò
- , Yann Fichou
- & Martin Weik

 Molecular basis of host-adaptation interactions between influenza virus polymerase PB2 subunit and ANP32A
Molecular basis of host-adaptation interactions between influenza virus polymerase PB2 subunit and ANP32A