Abstract
Epidemiological studies have reported conflicting results regarding the association between maternal folic acid supplementation and the risk of congenital heart defects (CHDs). However, a meta-analysis of the association between maternal folic acid supplementation and CHDs in offspring has not been conducted. We searched the MEDLINE and EMBASE databases for articles cataloged between their inceptions and October 10, 2014 and identified relevant published studies that assessed the association between maternal folate supplementation and the risk of CHDs. Study-specific relative risk estimates were pooled using random-effects or fixed-effects models. Out of the 1,606 articles found in our initial literature searches, a total of 1 randomized controlled trial, 1 cohort study and 16 case-control studies were included in our final meta-analysis. The overall results of this meta-analysis provide evidence that maternal folate supplementation is associated with a significantly decreased risk of CHDs (RR = 0.72, 95% CI: 0.63–0.82). Statistically significant heterogeneity was detected (Q = 82.48, P < 0.001, I2 = 79.4%). We conducted stratified and meta-regression analyses to identify the origin of the heterogeneity among the studies and a Galbraith plot was generated to graphically assess the sources of heterogeneity. This meta-analysis provides a robust estimate of the positive association between maternal folate supplementation and a decreased risk of CHDs.
Similar content being viewed by others
Introduction
Congenital heart defects (CHDs) are the most common congenital malformations, affecting nearly 1% of live births worldwide1. CHDs represent approximately one-third of all congenital anomalies and are the leading cause of perinatal mortality2. Although tremendous breakthroughs in cardiovascular diagnostics and cardiothoracic surgery have been achieved over the past century, leading to increased survival for newborns with CHDs, the etiology of most congenital heart defects remains unknown.
Several chromosomal anomalies, certain maternal illnesses and prenatal exposures to specific therapeutic drugs are recognized risk factors. It is difficult to establish the role of a single factor because the cause of a defect is believed to be multifactorial in many cases; for example, some cases may result from a combination of environmental teratogens with genetic and chromosomal abnormalities3. A review published in 2007 provided a summary of the current literature on noninherited risk factors for CHDs4. CHDs comprise several distinct subtypes (e.g., conotruncal defects, artioventricular septal defect and septal defects) and there is a potential for etiologic heterogeneity. Thus, it is not surprising that studies that have examined individual categories of CHDs have come to different or even opposite conclusions.
More than a decade ago, the preventive effects of maternal folate supplementation on the occurrence and recurrence of neural tube defects was documented in several studies5,6. Primarily because the benefit of folic acid supplementation in preventing neural tube defects in women of childbearing age was shown to be conclusive, folic acid fortification of flour and grain products began in 19987. Maternal multivitamin supplements containing folic acid reduce the risk of neural tube defects and evidence suggests that maternal folic acid supplementation may also be associated with benefits for other reproductive outcomes, including the incidence of CHDs. Recently, there has been a steep increase in the number of maternal folic acid supplementation studies with the occurrence of CHDs as the primary health outcome; several studies have demonstrated positive associations, whereas others have not.
An increasing number of studies to date have focused on the association between maternal folic acid supplementation and the incidence of CHDs; however, the results have been ambiguous, perhaps due to inadequate sample sizes. Thus, we conducted a meta-analysis to quantitatively assess the effect of maternal folic acid supplementation on the risk of CHDs.
Results
Study characteristics
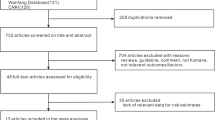
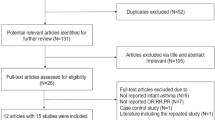
Our literature search strategy generated 1,606 citations. Of these, 18 were used in the final analysis, representing 18,500 incident cases (Figure 1). All of the studies were published between 1995 and 2013. These studies included 1 randomized controlled trial8, 1 cohort study9 and 16 case-control studies10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. The main characteristics of the included studies are presented in Table 1. As shown in Table 1, 9 studies were conducted in the United States, 8 in Europe and 1 in China. In the 16 case-control studies, the number of cases investigated varied from 77 to 3,278 and the number of control subjects ranged from 250 to 38,151.
Maternal folate supplementation and CHDs
The overall results of this meta-analysis provided evidence for a significant decrease in the risk of CHDs with maternal folate supplementation (RR = 0.72, 95% CI: 0.63–0.82; Figure 2). Statistically significant heterogeneity was detected (Q = 82.48, P < 0.001, I2 = 79.4%), with no publication bias (Begg's test: P = 0.198; Figure 3). The 18 study-specific relative risks ranged from a low of 0.69 (95% CI: 0.59–0.80, Q = 76.40, P = 0.000, I2 = 79.1%), after omission of the study by Malik et al.18, to a high of 0.74 (95% CI: 0.65–0.84, Q = 72.29, P = 0.000, I2 = 77.9%), after omission of the study by Li et al.17. In stratified analyses, the corresponding pooled RRs were not materially altered in any stratification (Figure 4, Table 2).
Relative risk (RR) estimates for the association between maternal folate supplementation and the risk of CHDs.
Meta-analysis random-effects estimates were used. The sizes of the squares reflect the weighting of the included studies. Bars represent 95% confidence intervals (CIs). The center of the diamond indicates the summary effect; the left and right points of the diamond indicate the 95% confidence interval.
Relative risk (RR) estimates for the association between maternal folate supplementation and the risk of individual subtypes of CHDs (CTD; ASD or VSD; and AVSD).
Meta-analysis random-effects estimates were used. The sizes of the squares reflect the weighting of the included studies. Bars represent 95% confidence intervals (CIs). The center of the diamond indicates the summary effect; the left and right points of the diamond indicate the 95% confidence interval.
Heterogeneity Analysis
To clarify the sources of heterogeneity, we conducted a sensitivity analysis. However, I2 did not decrease substantially when any individual study was removed. Subsequently, a meta-regression was performed using a Knapp-Hartung modification and we found that differences in geographical region may contribute to the heterogeneity we observed (P = 0.025). We further created a Galbraith plot to graphically assess the sources of heterogeneity (Supplementary Figure S1). A total of 8 studies were identified as the primary sources of heterogeneity. Once the outlying studies were excluded, the heterogeneity was effectively removed (I2 = 31.9%); however, the corresponding pooled RRs were not materially altered (RR = 0.78, 95% CI: 0.69–0.89).
Discussion
To our knowledge, this is the first quantitative meta-analysis to evaluate the association between maternal folate supplementation and the risk of CHDs. Overall, the findings of our meta-analysis suggest that maternal folate supplementation is significantly associated with a decreased risk of CHDs (RR = 0.72, 95% CI: 0.63–0.82). Moreover, these results were consistent across most of the subgroup analyses (Table 2).
Although the specific biological mechanisms underlying the relationship between maternal folate supplementation and the risk of CHDs remain to be determined, some relevant evidence has been published to date. It has been hypothesized that impaired folate and/or homocysteine metabolism interferes with the development of the heart, possibly by affecting neural crest cells. Methylenetetrahydrofolate reductase (MTHFR), which is a critical folate-metabolizing enzyme, plays an important role in processing amino acids. A C→T substitution is commonly found at position 677 in the MTHFR enzyme and results in a substitution of valine for alanine; this substitution causes impaired folate binding and reduced activity of the MTHFR enzyme25. The effect of the MTHFR 677TT genotype on homocysteine levels is more pronounced with low folate status26. In 1999, Kapusta et al.27 were the first to link maternal hyperhomocysteinemia to an increased risk for CHDs. More recently, Hobbs et al. studied mothers whose children were born with CHDs and identified homocysteine, S-adenosylhomocysteine and methionine levels as the most important biomarkers predictive of the presence of CHDs28. Hernandez-Diaz et al.29 showed that periconceptional intake of medications acting as folic acid antagonists, including anti-epileptic agents and dihydrofolate reductase inhibitors, doubled the risk of cardiovascular defects. In vitro studies found that impaired folate and homocysteine metabolism affects neural crest cell formation and migration30,31. Tang et al.32 demonstrated that impaired folic acid transport results in extensive apoptotic cell death in the developing heart; apoptotic cells were shown to be concentrated in the truncus arteriosus and interventricular septum and were thus anatomically restricted to the two regions in which most congenital heart defects are found. Folic acid may play a role in the migration of the cardiac neural crest cells that contribute to the formation of the truncus arteriosus and its division into the pulmonary artery and aorta and thus likely affects the generation of conotruncal defects in particular33,34. The precise effects of folate supplementation on cardiac morphogenesis are unclear, so it is important to corroborate this hypothesis with evidence from clinical and population-based studies.
Although the potential role of folic acid in the prevention of neural tube defects was reported as early as 1980, public health campaigns have resulted in preconception supplementation in only one-third of pregnant women35, partly because one-half of all pregnancies are unplanned. Presently, widely publicized recommendations by various authorities suggest that women should supplement their diet with daily doses of at least 0.4 mg of folate (4 mg for women at higher risk) to reduce the risk of delivering a child with neural tube defects (NTDs)36. In many centers, women are advised to begin taking prenatal vitamin supplements when they decide to attempt to conceive. The optimal dose of periconceptional folic acid supplements to prevent CHD cannot be deduced from our study or previous studies because most women had taken supplements containing at least 0.4 mg. Whether a lower or higher dose would be more effective is difficult to explore because 0.4 mg is the level that has been advised for preventing NTDs. There is growing evidence that because mothers are becoming heavier (i.e., because maternal BMIs are increasing), the recommended daily dose of folate will need to be increased to maintain a similar preventive effect37.
Some limitations of our study must be taken into account. First, 1 randomized controlled trial, 1 cohort study and 16 case-control studies were included in our meta-analysis and we extracted our raw data primarily from several case-control studies, which were susceptible to selection and information biases. In addition, our meta-analysis was limited to studies published in English, so our results may have been affected by a lack of data from studies published in other languages. Thus, our conclusions must be considered carefully. Second, although no evidence of publication bias was found, heterogeneity was among the studies included in the analysis and this heterogeneity may affect the interpretation of the overall results. In this study, we conducted sensitivity analyses to explore the sources of this heterogeneity by removing one study at a time from our pooled analysis. However, heterogeneity could not be fully removed by the exclusion of any individual study. Moreover, study results may vary with geographical regions, publication periods, sample sizes, CHD subtypes and other risk factors. Thus, we performed meta-regression and subgroup analyses to further investigate the sources of the heterogeneity that we observed. We found that the heterogeneity in the study results could partly be attributed to the geographical region in which the studies that we examined were conducted. We also created a Galbraith plot to assess the heterogeneity that we observed and to identify potential outlying studies. A total of 8 studies were identified to be the primary contributors to the heterogeneity in the analysis. After excluding these outlying studies, the heterogeneity was effectively removed, whereas the corresponding pooled RRs were not materially altered, indicating that the overall results regarding maternal folate supplementation were statistically stable.
Our study has several important strengths. First, to our knowledge, this is the first meta-analysis to report an association between maternal folate supplementation and the risk of CHDs. Moreover, our literature search was conducted using multiple databases and the references from the retrieved articles were carefully examined to find any additional studies that may have been of interest. Thus, our study included data for 18,500 cases, which is enough to have sufficient statistical power to investigate the potential association between maternal folate supplementation and the risk of CHDs. Another strength of our study is that although heterogeneity exists in our meta-analysis, we conducted a number of sensitivity, subgroup and Galbraith plot analyses and found that our results were stable.
In summary, this study provides evidence that maternal folate supplementation is positively associated with a decreased risk of CHDs. However, more prospective studies, particularly in developing countries, are needed to further investigate the association between maternal folate supplementation and the risk of CHDs, especially with regard to the different subtypes of CHDs.
Methods
Literature search
A computerized literature search was conducted by two independent investigators (Feng and Tong) using the MEDLINE and EMBASE databases to find articles catalogs from the inceptions of these databases through October 10, 2014. We searched for relevant studies using the following search strategy: (“Multivitamins” OR “Vitamin” OR “Folate” OR “Folic Acid”) AND (“abnormalities” OR “birth defects” OR “congenital anomaly” OR “malformations” OR “congenital malformations” OR “congenital heart defect” OR “Heart Abnormality” OR “Malformation of heart” or “CHD”) AND (“maternal” OR “mother” OR “periconceptional” OR “pregnant” OR “gestation”). In addition, we searched for studies that investigated a broad range of environmental teratogens and CHDs and examined the relevant references and review articles that were found. We were thus able to identify relevant information found in other related studies. We followed published quality standards for conducting and reporting meta-analyses38.
Eligibility Criteria
We selected articles that (1) were original epidemiologic studies (i.e., case–control, cohort or RCT), (2) examined the association between periconceptional folic acid use and either CHDs overall or any one of the CHD subtypes in infants, (3) were published in the English language, (4) reported RRs (i.e., risk ratios or odds ratios) and associated 95% confidence intervals (CIs) or provided raw data from which these measures could be calculated and (5) defined CHDs or one of the CHD subtypes as an outcome. Articles that reported results from more than one population were considered to consist of separate studies, with 1 study for each population investigated. When multiple articles were found to examine the same study, we included in our study the article with the most applicable information and the largest number of cases. We excluded non-peer-reviewed articles, experimental animal studies, ecological assessments, correlation studies and mechanistic studies.
Data extraction
Data extraction was conducted separately by two reviewers (Feng and Wang) working independently. If differences of opinion arose, these were resolved by discussion between the two reviewers. The studies that met the inclusion criteria were reviewed to retrieve relevant information. Relevant information included author names, the year of publication, the geographic region in which the study was conducted, the period in which data were collected, the study design, the sample size, case classification information, exposure and outcome assessments, adjusted estimates and their corresponding 95% CIs and confounding factors that were controlled for by matching cases or adjustments in the data analysis. When no adjusted estimates were available, we extracted a crude estimate. If no estimate of relative risk was provided in a given study, we recalculated odds ratios or risk ratios and 95% CIs from the raw data presented in the study using standard equations.
To assess study quality, we used a 9-star system based on the Newcastle-Ottawa Scale39. Our system judges a study based on three broad characteristics: the selection of study groups, the comparability of study groups and the ascertainment of the exposure or outcome of interest for case-control and cohort studies, respectively. We defined a high quality study as one with a quality score greater than or equal to 7.
Statistical analysis
We used study-specific relative risks as summary statistics for the association between maternal folate supplementation and CHD risk. To simplify the procedure, an RR was used to represent all reported study-specific results from cohort studies and an OR was used to represent results from case-control studies. Cochran's Q and I2 statistics were used to test for heterogeneity among studies40. If there was evidence of heterogeneity (P < 0.05 or I2≧56%), a random-effects model was used, which provided a more appropriate summary estimate for heterogeneous study-specific estimates. If the study revealed no evidence of heterogeneity, a fixed-effects analysis was conducted and an inverse variance weighting was applied to calculate summary RR estimates41.
We conducted subgroup analyses based on study design (i.e., RCT or cohort versus case-control studies), geographical region (i.e., USA, Europe and China), number of cases (i.e., ≤1,000 versus >1,000), publication period (i.e., before 2010 versus 2010 or after), primary focus of the study (i.e., whether the title or abstract refers to folate supplementation as the focus of the study, yes versus no) and study quality (i.e., low versus high quality). We evaluated heterogeneity between subgroups by meta-regression analysis. A P-value less than 0.05 for the meta-regression analysis was considered to indicate a significant difference between subgroups. Finally, we conducted sensitivity analyses to explore whether a specific study strongly influenced the results, by excluding one study at a time.
Publication bias was assessed via visual inspection of a funnel plot for asymmetry using both Egger's linear regression42 and Begg's rank correlation43 methods. For both tests, significant statistical publication bias was defined to be indicated by a P-value of <0.05. All statistical analyses were performed using STATA software (version 11.0; StataCorp, College Station, Texas, USA).
Change history
24 July 2015
The version of this Article previously published quoted an incorrect email address for Xuming Mo. Correspondence and request for materials should also be addressed to mohsuming15@sina.com. This has now been corrected in the HTML and PDF versions of the Article.
References
Pierpont, M. E. et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115, 3015–3038 (2007).
Boneva, R. S. et al. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation 103, 2376–2381 (2001).
Brent, R. L. Environmental causes of human congenital malformations: the pediatrician's role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 113, 957–968 (2004).
Jenkins, K. J. et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115, 2995–3014 (2007).
Smithells, R. W. & Sheppard, S. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet 1, 647 (1980).
Laurence, K. M., James, N., Miller, M. H., Tennant, G. B. & Campbell, H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J (Clin Res Ed) 282, 1509–1511 (1981).
Food labeling: health claims and labeling statements; dietary fiber and cancer; antioxidant vitamins and cancer; omega-3 fatty acids and coronary heart disease; folate and neural tube defects; revocation. Food and Drug Administration, HHS. Final rule. Fed Regist 65, 58917–58918 (2000).
Czeizel, A. E. Periconceptional folic acid containing multivitamin supplementation. Eur J Obstet Gynecol Reprod Biol 78, 151–161 (1998).
Czeizel, A. E., Dobo, M. & Vargha, P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Res A Clin Mol Teratol 70, 853–861 (2004).
Bean, L. J. et al. Lack of maternal folic acid supplementation is associated with heart defects in Down syndrome: a report from the National Down Syndrome Project. Birth Defects Res A Clin Mol Teratol 91, 885–893 (2011).
Botto, L. D., Mulinare, J. & Erickson, J. D. Occurrence of congenital heart defects in relation to maternal mulitivitamin use. Am J Epidemiol 151, 878–884 (2000).
Correa, A., Botto, L., Liu, Y., Mulinare, J. & Erickson, J. D. Do multivitamin supplements attenuate the risk for diabetes-associated birth defects? Pediatrics 111, 1146–1151 (2003).
Csaky-Szunyogh, M., Vereczkey, A., Kosa, Z., Gerencser, B. & Czeizel, A. E. Risk and protective factors in the origin of conotruncal defects of heart--a population-based case-control study. Am J Med Genet A 161A, 2444–2452 (2013).
Csaky-Szunyogh, M., Vereczkey, A., Kosa, Z., Urban, R. & Czeizel, A. E. Association of maternal diseases during pregnancy with the risk of single ventricular septal defects in the offspring--a population-based case-control study. J Matern Fetal Neonatal Med 26, 738–747 (2013).
Csaky-Szunyogh, M., Vereczkey, A., Kosa, Z., Gerencser, B. & Czeizel, A. E. Risk factors in the origin of congenital left-ventricular outflow-tract obstruction defects of the heart: a population-based case-control study. Pediatc cardiol 35, 108–120 (2014).
Hobbs, C. A., MacLeod, S. L., Jill James, S. & Cleves, M. A. Congenital heart defects and maternal genetic, metabolic and lifestyle factors. Birth Defects Res A Clin Mol Teratol 91, 195–203 (2011).
Li, X. et al. The association between periconceptional folic acid supplementation and congenital heart defects: a case-control study in China. Prev Med 56, 385–389 (2013).
Malik, S. et al. Maternal smoking and congenital heart defects. Pediatrics 121, e810–816 (2008).
Obermann-Borst, S. A. et al. General maternal medication use, folic acid, the MDR1 C3435T polymorphism and the risk of a child with a congenital heart defect. Am J Obstet Gynecol 204 (2011).
Scanlon, K. S. et al. Preconceptional folate intake and malformations of the cardiac outflow tract. Baltimore-Washington Infant Study Group. Epidemiology 9, 95–98 (1998).
Shaw, G. M., O'Malley, C. D., Wasserman, C. R., Tolarova, M. M. & Lammer, E. J. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet 59, 536–545 (1995).
van Beynum, I. M. et al. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur Heart J 31, 464–471 (2010).
Vereczkey, A., Kosa, Z., Csaky-Szunyogh, M. & Czeizel, A. E. Isolated atrioventricular canal defects: birth outcomes and risk factors: a population-based Hungarian case-control study, 1980-1996. Birth Defects Res A Clin Mol Teratol 97, 217–224 (2013).
Werler, M. M., Hayes, C., Louik, C., Shapiro, S. & Mitchell, A. A. Multivitamin supplementation and risk of birth defects. Am J Epidemiol 150, 675–682 (1999).
Williams, L. J., Correa, A. & Rasmussen, S. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res A Clin Mol Teratol 70, 59–64 (2004).
Jacques, P. F., Bostom, A. G., Williams, R. R., Ellison, R. C. & Eckfeldt, J. H. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase and plasma homocysteine concentrations. Circulation 93, 7–9 (1996).
Kapusta, L., Haagmans, M. L., Steegers, E. A., Cuypers, M. H. & Blom, H. J. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr 135, 773–774 (1999).
Hobbs, C., Cleves, M. A., Melnyk, S., Zhao, W. & James, J. S. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr 81, 147–153 (2005).
Hernandez-Diaz, S., Werler, M. M., Walker, A. M. & Mitchell, A. A. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343, 1608–1614 (2000).
Boot, M. J. et al. Folic acid and homocysteine affect neural crest and neuroepithelial cell outgrowth and differentiation in vitro. Dev Dyn 227, 301–308 (2003).
Tierney, B. J., Ho, T., Reedy, M. V. & Brauer, P. R. Homocysteine inhibits cardiac neural crest cell formation and morphogenesis in vivo. Dev Dyn 229, 63–73 (2004).
Tang, L. S., Wlodarczyk, B. J., Santillano, D. R., Miranda, R. C. & Finnell, R. H. Developmental consequences of abnormal folate transport during murine heart morphogenesis. Birth Defects Res A Clin Mol Teratol 70, 449–458 (2004).
van Beynum, I. M. et al. Maternal MTHFR 677C>T is a risk factor for congenital heart defects: effect modification by periconceptional folate supplementation. Eur Heart J 27, 981–987 (2006).
Wild, J., Sutcliffe, M., Schorah, C. J. & Levene, M. I. Prevention of neural-tube defects. Lancet 350, 30–31 (1997).
Holmes, L., Harris, J., Oakley, G. P., Jr & Friedman, J. M. Teratology Society Consensus Statement on use of folic acid to reduce the risk of birth defects. Teratology 55, 381 (1997).
Ray, J. G., Wyatt, P. R., Vermeulen, M. J., Meier, C. & Cole, D. E. Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet Gynecol 105, 261–265 (2005).
Mojtabai, R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol 19, 1029–1036 (2004).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses.comparison. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Ma 3, 2013. (2013).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560 (2003).
Woolf, B. On estimating the relation between blood group and disease. Ann Hum Genet 19, 251–253 (1955).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Author information
Authors and Affiliations
Contributions
X.M.M. conceived and designed the experiments. Y.F., S.W. and X.M.M. performed the experiments. Y.F., S.W., R.S.C., X.T. and Z.Y.W. analyzed the data. R.S.C. and Z.Y.W. contributed software, hardware and analysis tools. Y.F. and S.W. wrote the paper. X.M.M. will provide access to the full-text article.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figure S1
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Feng, Y., Wang, S., Chen, R. et al. Maternal Folic Acid Supplementation and the Risk of Congenital Heart Defects in Offspring: A Meta-Analysis of Epidemiological Observational Studies. Sci Rep 5, 8506 (2015). https://doi.org/10.1038/srep08506
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08506
This article is cited by
-
Single-cell analysis reveals the spatial-temporal expression of genes associated with esophageal malformations
Scientific Reports (2024)
-
Evaluating agreement between evidence from randomised controlled trials and cohort studies in nutrition: a meta-research replication study
European Journal of Epidemiology (2024)
-
Association between MTHFR C677T variant and risk for congenital heart defects in Egyptian children: a case–control study including meta-analysis based on 147 cases and 143 controls
Egyptian Journal of Medical Human Genetics (2023)
-
Association of MTR gene polymorphisms with the occurrence of non-syndromic congenital heart disease: a case–control study
Scientific Reports (2023)
-
Periconceptional maternal folate supplementation impacts a diverse range of congenital malformations
Pediatric Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.