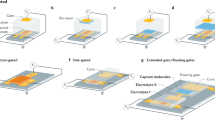
Abstract
Organic electrochemical transistors (OECTs) are electronic devices relying on electronic materials that are stable in aqueous environments. OECTs leverage ionic solutions for their operation, so OECTs are well-suited for interfacing with biological systems for electrophysiology and biochemical sensing, in particular, in point-of-care diagnostics, wearable and implantable technologies, and in organ-on-chip systems. The interface of OECTs with biological systems is a crucial parameter that determines the function and performance of the devices, influencing the design criteria, including the selection of materials and device form factor, geometry and architecture. The selected design features must enable seamless interaction with biological components while ensuring reliable and stable device performance in complex settings. In this Review, we investigate the biological interfaces of OECT-based biosensors, examining their complexity and length scale. We highlight interface designs with biomolecules, such as lipids, proteins and aptamers, as well as in vitro cell culture and the human body. Importantly, we explore strategies to improve each interface type and identify gaps in our current understanding that warrant further investigation.
Key points
-
Optimal bioelectronic interfacing requires efficient information transfer with minimal disruption to biological functions.
-
Organic electrochemical transistors (OECTs) can establish seamless bioelectronic interfaces with biomolecules, cells, lipid bilayers and the body to extract electrophysiological or biochemical signals.
-
OECT performance can be improved by optimizing the gate–electrolyte interface, the channel–electrolyte interface and the electrolyte.
-
Orientation, density and stable immobilization of recognition units and interference blockers on device surfaces are crucial for efficient biochemical sensing.
-
Intimate contact between OECT components and biological systems is the most important feature for efficient signal transduction, both in vivo and in vitro.
-
Standardized fabrication and development protocols are needed to engineer reproducible, stable and low-cost OECTs for widespread diagnostic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Andreou, C. et al. Resting-state connectivity in the prodromal phase of schizophrenia: insights from EEG microstates. Schizophr. Res. 152, 513–520 (2014).
Stevens, A. & Kircher, T. Cognitive decline unlike normal aging is associated with alterations of EEG temporo-spatial characteristics. Eur. Arch. Psychiatry Clin. Neurosci. 248, 259–266 (1998).
Nishida, K. et al. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer’s disease. Clin. Neurophysiol. 124, 1106–1114 (2013).
Strik, W. K., Dierks, T., Becker, T. & Lehmann, D. Larger topographical variance and decreased duration of brain electric microstates in depression. J. Neural Transm. 99, 213–222 (1995).
Stevens, A., Günther, W., Lutzenberger, W., Bartels, M. & Müller, N. Abnormal topography of EEG microstates in Gilles de la Tourette syndrome. Eur. Arch. Psychiatry Clin. Neurosci. 246, 310–316 (1996).
Kikuchi, M. et al. EEG microstate analysis in drug-naive patients with panic disorder. PLoS ONE 6, e22912 (2011).
Acharya, U. R., Vinitha Sree, S., Swapna, G., Martis, R. J. & Suri, J. S. Automated EEG analysis of epilepsy: a review. Knowl. Based Syst. 45, 147–165 (2013).
Zhai, X., Jelfs, B., Chan, R. H. M. & Tin, C. Self-recalibrating surface EMG pattern recognition for neuroprosthesis control based on convolutional neural network. Front. Neurosci. 11, 379 (2017).
Fraiwan, L. & Lweesy, K. in 2017 IEEE 13th International Colloquium on Signal Processing & its Applications (CSPA) 228–231 (IEEE, 2017).
Jirayucharoensak, S., Pan-Ngum, S. & Israsena, P. EEG-based emotion recognition using deep learning network with principal component based covariate shift adaptation. Sci. World J. 2014, 627892 (2014).
Yao, P. S., Zheng, S. F., Wang, F., Kang, D. Z. & Lin, Y. X. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J. Neurosurg. 128, 840–845 (2018).
Huynh, M. A., Nguyen, T.-H., Nguyen, T.-K. & Nguyen, N.-T. Convergence of implantable bioelectronics and brain–computer interfaces. ACS Appl. Electron. Mater. 5, 5777–5793 (2023).
Yao, G. et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 9, 5349 (2018).
Lorach, H. et al. Walking naturally after spinal cord injury using a brain–spine interface. Nature 618, 126–133 (2023).
Xu, H. et al. First human results with the 256 channel intelligent micro implant eye (IMIE 256). Transl. Vis. Sci. Technol. 10, 14 (2021).
Battelino, T. et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 11, 42–57 (2023).
Thiebaud, P., de Rooij, N. F., Koudelka-Hep, M. & Stoppini, L. Microelectrode arrays for electrophysiological monitoring of hippocampal organotypic slice cultures. IEEE Trans. Biomed. Eng. 44, 1159–1163 (1997).
Preininger, M. K. et al. A human pluripotent stem cell model of catecholaminergic polymorphic ventricular tachycardia recapitulates patient-specific drug responses. Dis. Models Mech. 9, 927–939 (2016).
Millard, D. et al. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol. Sci. 164, 550–562 (2018).
Conant, G. et al. High-content assessment of cardiac function using heart-on-a-chip devices as drug screening model. Stem Cell Rev. Rep. 13, 335–346 (2017).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Kukhta, N. A., Marks, A. & Luscombe, C. K. Molecular design strategies toward improvement of charge injection and ionic conduction in organic mixed ionic–electronic conductors for organic electrochemical transistors. Chem. Rev. 122, 4325–4355 (2022).
He, Y., Kukhta, N. A., Marks, A. & Luscombe, C. K. The effect of side chain engineering on conjugated polymers in organic electrochemical transistors for bioelectronic applications. J. Mater. Chem. C 10, 2314–2332 (2022).
Paudel, P. R., Tropp, J., Kaphle, V., Azoulay, J. D. & Lüssem, B. Organic electrochemical transistors – from device models to a targeted design of materials. J. Mater. Chem. C 9, 9761–9790 (2021).
Li, P. & Lei, T. Molecular design strategies for high-performance organic electrochemical transistors. J. Polym. Sci. 60, 377–392 (2022).
Griggs, S., Marks, A., Bristow, H. & McCulloch, I. n-Type organic semiconducting polymers: stability limitations, design considerations and applications. J. Mater. Chem. C 9, 8099–8128 (2021).
Inal, S., Malliaras, G. G. & Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 8, 1767 (2017).
Ji, X., Lin, X. & Rivnay, J. Organic electrochemical transistors as on-site signal amplifiers for electrochemical aptamer-based sensing. Nat. Commun. 14, 1665 (2023).
Tan, S. T. M. et al. High-gain chemically gated organic electrochemical transistor. Adv. Funct. Mater. 31, 2010868 (2021).
Pappa, A. M. et al. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 4, eaat0911 (2018).
Tang, T. et al. Functional infectious nanoparticle detector: finding viruses by detecting their host entry functions using organic bioelectronic devices. ACS Nano 15, 18142–18152 (2021).
Xie, K. et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. eLife 9, e50345 (2020).
Zhong, Y., Saleh, A. & Inal, S. Decoding electrophysiological signals with organic electrochemical transistors. Macromol. Biosci. 21, e2100187 (2021).
Friedlein, J. T., McLeod, R. R. & Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 63, 398–414 (2018).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Spanu, A., Martines, L. & Bonfiglio, A. Interfacing cells with organic transistors: a review of in vitro and in vivo applications. Lab Chip 21, 795–820 (2021).
Marks, A., Griggs, S., Gasparini, N. & Moser, M. Organic electrochemical transistors: an emerging technology for biosensing. Adv. Mater. Interfaces 9, 2102039 (2022).
Wang, N., Yang, A., Fu, Y., Li, Y. & Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 52, 277–287 (2019).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Torricelli, F. et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. Methods Primers 1, 66 (2021).
Sarcina, L. et al. Controlling the binding efficiency of surface confined antibodies through the design of mixed self-assembled monolayers. Adv. Mater. Interfaces 10, 2300017 (2023).
Yeung, S. Y., Gu, X., Tsang, C. M., Tsao, S. W. & Hsing, I. M. Engineering organic electrochemical transistor (OECT) to be sensitive cell-based biosensor through tuning of channel area. Sens. Actuators A Phys. 287, 185–193 (2019).
Sarcina, L. et al. A stable physisorbed layer of packed capture antibodies for high-performance sensing applications. J. Mater. Chem. C 11, 9093–9106 (2023).
Ohayon, D. et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 19, 456–463 (2020).
Druet, V. et al. Operation mechanism of n-type organic electronic metabolite sensors. Adv. Electron. Mater. 8, 2200065 (2022).
Zhang, Y. et al. Visualizing the solid–liquid interface of conjugated copolymer films using fluorescent liposomes. ACS Appl. Bio Mater. 1, 1348–1354 (2018).
Ohayon, D. et al. Interactions of catalytic enzymes with n-type polymers for high-performance metabolite sensors. ACS Appl. Mater. Interfaces 15, 9726–9739 (2023).
Koklu, A. et al. Microfluidics integrated n-type organic electrochemical transistor for metabolite sensing. Sens. Actuators B Chem. 329, 129251 (2021).
Song, Y., Wagner, J. & Katz, H. E. The behavior of carboxylated and hydroxylated polythiophene as bioreceptor layer: anti-human IgG and human IgG interaction detection based on organic electrochemical transistors. Electrochem. Sci. Adv. 2, e2100166 (2022).
Song, Y. et al. Nanoscale bioreceptor layers comprising carboxylated polythiophene for organic electrochemical transistor-based biosensors. ACS Appl. Nano Mater. 4, 13459–13468 (2021).
Fenoy, G. E. et al. “Clickable” organic electrochemical transistors. JACS Au 2, 2778–2790 (2022).
Fenoy, G. E. et al. Interface engineering of “clickable” organic electrochemical transistors toward biosensing devices. ACS Appl. Mater. Interfaces 15, 10885–10896 (2023).
Parlak, O., Keene, S. T., Marais, A., Curto, V. F. & Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 4, eaar2904 (2018).
Koklu, A. et al. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-β detection. ACS Nano 15, 8130–8141 (2021).
Macchia, E. et al. A handheld intelligent single-molecule binary bioelectronic system for fast and reliable immunometric point-of-care testing. Sci. Adv. 8, eabo0881 (2022).
Chen, L., Wu, J., Yan, F. & Ju, H. Monose-modified organic electrochemical transistors for cell surface glycan analysis via competitive recognition to enzyme-labeled lectin. Microchim. Acta 188, 252 (2021).
Chen, L. et al. Organic electrochemical transistors for the detection of cell surface glycans. ACS Appl. Mater. Interfaces 10, 18470–18477 (2018).
Peruzzi, C. et al. Interfacing aptamers, nanoparticles and graphene in a hierarchical structure for highly selective detection of biomolecules in OECT devices. Sci. Rep. 11, 9380 (2021).
Ding, L. et al. Turning on high-sensitive organic electrochemical transistor-based photoelectrochemical-type sensor over modulation of Fe-MOF by PEDOT. Adv. Funct. Mater. 32, 2202735 (2022).
Liu, H. et al. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 7, eabg8387 (2021).
Guo, K. et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 5, 666–677 (2021).
Guo, K. et al. SpyDirect: a novel biofunctionalization method for high stability and longevity of electronic biosensors. Adv. Sci. https://doi.org/10.1002/advs.202306716 (2023).
Figueroa-Miranda, G. et al. Polyethylene glycol-mediated blocking and monolayer morphology of an electrochemical aptasensor for malaria biomarker detection in human serum. Bioelectrochemistry 136, 107589 (2020).
Lee, D. et al. Ionic contrast across a lipid membrane for Debye length extension: towards an ultimate bioelectronic transducer. Nat. Commun. 12, 3741 (2021).
de Oliveira Filho, J. I., Faleiros, M. C., Ferreira, D. C., Mani, V. & Salama, K. N. Empowering electrochemical biosensors with AI: overcoming interference for precise dopamine detection in complex samples. Adv. Intell. Syst. 5, 2300227 (2023).
Ramuz, M., Hama, A., Rivnay, J., Leleux, P. & Owens, R. M. Monitoring of cell layer coverage and differentiation with the organic electrochemical transistor. J. Mater. Chem. B 3, 5971–5977 (2015).
Jimbo, Y. et al. An organic transistor matrix for multipoint intracellular action potential recording. Proc. Natl Acad. Sci. USA 118, e2022300118 (2021).
Decataldo, F. et al. Organic electrochemical transistors as versatile tool for real-time and automatized viral cytopathic effect evaluation. Viruses 14, 1155 (2022).
Traberg, W. C. et al. Organic electronic platform for real-time phenotypic screening of extracellular-vesicle-driven breast cancer metastasis. Adv. Healthc. Mater. 12, 2301194 (2023).
Guo, X. et al. Organic electrochemical transistor for in situ detection of H2O2 released from adherent cells and its application in evaluating the in vitro cytotoxicity of nanomaterial. Anal. Chem. 92, 908–915 (2020).
Jackman, J. A. & Cho, N.-J. Supported lipid bilayer formation: beyond vesicle fusion. Langmuir 36, 1387–1400 (2020).
Lieberth, K. et al. Monitoring reversible tight junction modulation with a current-driven organic electrochemical transistor. Adv. Mater. Technol. 6, 2000940 (2021).
Pitsalidis, C. et al. Biomimetic electronic devices for measuring bacterial membrane disruption. Adv. Mater. 30, e1803130 (2018).
Cavassin, P. et al. Organic transistors incorporating lipid monolayers for drug interaction studies. Adv. Mater. Technol. 5, 1900680 (2020).
Krauss, G. et al. Polydiketopyrrolopyrroles carrying ethylene glycol substituents as efficient mixed ion-electron conductors for biocompatible organic electrochemical transistors. Adv. Funct. Mater. 31, 2010048 (2021).
Simon, D. T., Gabrielsson, E. O., Tybrandt, K. & Berggren, M. Organic bioelectronics: bridging the signaling gap between biology and technology. Chem. Rev. 116, 13009–13041 (2016).
Gu, X. et al. Organic electrochemical transistor arrays for in vitro electrophysiology monitoring of 2D and 3D cardiac tissues. Adv. Biosyst. 3, e1800248 (2019).
Gu, X., Yao, C., Liu, Y. & Hsing, I.-M. 16-Channel organic electrochemical transistor array for in vitro conduction mapping of cardiac action potential. Adv. Healthc. Mater. 5, 2345–2351 (2016).
Hempel, F. et al. PEDOT:PSS organic electrochemical transistor arrays for extracellular electrophysiological sensing of cardiac cells. Biosens. Bioelectron. 93, 132–138 (2017).
Liang, Y. et al. Tuning channel architecture of interdigitated organic electrochemical transistors for recording the action potentials of electrogenic cells. Adv. Funct. Mater. 29, 1902085 (2019).
Abarkan, M. et al. Vertical organic electrochemical transistors and electronics for low amplitude micro-organ signals. Adv. Sci. 9, 2105211 (2022).
Yao, C., Li, Q., Guo, J., Yan, F. & Hsing, I.-M. Rigid and flexible organic electrochemical transistor arrays for monitoring action potentials from electrogenic cells. Adv. Healthc. Mater. 4, 528–533 (2015).
Ramuz, M. et al. Optimization of a planar all-polymer transistor for characterization of barrier tissue. ChemPhysChem 16, 1210–1216 (2015).
Du, W. et al. Improving the compatibility of diketopyrrolopyrrole semiconducting polymers for biological interfacing by lysine attachment. Chem. Mater. 30, 6164–6172 (2018).
Bonafè, F. et al. AC amplification gain in organic electrochemical transistors for impedance-based single cell sensors. Nat. Commun. 13, 5423 (2022).
Kalluri, R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 3, 422–433 (2003).
Hempel, F. et al. PEDOT:PSS organic electrochemical transistors for electrical cell-substrate impedance sensing down to single cells. Biosens. Bioelectron. 180, 113101 (2021).
Chen, L., Wu, J., Yan, F. & Ju, H. A facile strategy for quantitative sensing of glycans on cell surface using organic electrochemical transistors. Biosens. Bioelectron. 175, 112878 (2021).
Zhang, Y. et al. Supported lipid bilayer assembly on PEDOT:PSS films and transistors. Adv. Funct. Mater. 26, 7304–7313 (2016).
Zhang, Y. et al. Lipid bilayer formation on organic electronic materials. J. Mater. Chem. C 6, 5218–5227 (2018).
Kawan, M. et al. Monitoring supported lipid bilayers with n-type organic electrochemical transistors. Mater. Horiz. 7, 2348–2358 (2020).
Richards, M. J. et al. Membrane protein mobility and orientation preserved in supported bilayers created directly from cell plasma membrane blebs. Langmuir 32, 2963–2974 (2016).
Pappa, A.-M. et al. Optical and electronic ion channel monitoring from native human membranes. ACS Nano 14, 12538–12545 (2020).
Dipalo, M. et al. Membrane poration mechanisms at the cell–nanostructure interface. Adv. Biosyst. 3, e1900148 (2019).
Spanu, A. et al. A three-dimensional micro-electrode array for in-vitro neuronal interfacing. J. Neural Eng. 17, 036033 (2020).
Santoro, F. et al. Revealing the cell–material interface with nanometer resolution by focused ion beam/scanning electron microscopy. ACS Nano 11, 8320–8328 (2017).
Nikolova, M. P. & Chavali, M. S. Recent advances in biomaterials for 3D scaffolds: a review. Bioact. Mater. 4, 271–292 (2019).
Pitsalidis, C. et al. Transistor in a tube: a route to three-dimensional bioelectronics. Sci. Adv. 4, eaat4253 (2018).
Moysidou, C.-M. et al. 3D bioelectronic model of the human intestine. Adv. Biol. 5, 2000306 (2021).
Del Agua, I. et al. Conducting polymer scaffolds based on poly(3,4-ethylenedioxythiophene) and xanthan gum for live-cell monitoring. ACS Omega 3, 7424–7431 (2018).
Pitsalidis, C. et al. Organic electronic transmembrane device for hosting and monitoring 3D cell cultures. Sci. Adv. 8, eabo4761 (2022).
Wang, L. & Lu, N. Conformability of a thin elastic membrane laminated on a soft substrate with slightly wavy surface. J. Appl. Mech. 83, 041007 (2016).
Ren, G. et al. A laser-induced graphene-based flexible and all-carbon organic electrochemical transistor. J. Mater. Chem. C 11, 4916–4928 (2023).
Kanakamedala, S. K., Alshakhouri, H. T., Agarwal, M. & DeCoster, M. A. A simple polymer based electrochemical transistor for micromolar glucose sensing. Sens. Actuators B Chem. 157, 92–97 (2011).
Gualandi, I. et al. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep. 6, 33637 (2016).
Dai, Y. et al. Stretchable redox-active semiconducting polymers for high-performance organic electrochemical transistors. Adv. Mater. 34, e2201178 (2022).
Yang, C.-Y. et al. Low-power/high-gain flexible complementary circuits based on printed organic electrochemical transistors. Adv. Electron. Mater. 8, 2100907 (2022).
Zabihipour, M. et al. High yield manufacturing of fully screen-printed organic electrochemical transistors. npj Flex. Electron. 4, 15 (2020).
Yang, A. et al. Wearable organic electrochemical transistor array for skin-surface electrocardiogram mapping above a human heart. Adv. Funct. Mater. 33, 2215037 (2023).
Zhang, S. et al. Hydrogel-enabled transfer-printing of conducting polymer films for soft organic bioelectronics. Adv. Funct. Mater. 30, 1906016 (2020).
Qing, X. et al. Wearable fiber-based organic electrochemical transistors as a platform for highly sensitive dopamine monitoring. ACS Appl. Mater. Interfaces 11, 13105–13113 (2019).
Wu, H. et al. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 8, 2001938 (2021).
Lee, W. et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 4, eaau2426 (2018).
Wang, J. et al. Nanomesh organic electrochemical transistor for comfortable on-skin electrodes with local amplifying function. ACS Appl. Electron. Mater. 2, 3601–3609 (2020).
Li, Y., Wang, N., Yang, A., Ling, H. & Yan, F. Biomimicking stretchable organic electrochemical transistor. Adv. Electron. Mater. 5, 1900566 (2019).
Li, Y., Zhang, S., Li, X., Unnava, V. R. N. & Cicoira, F. Highly stretchable PEDOT:PSS organic electrochemical transistors achieved via polyethylene glycol addition. Flex. Print. Electron. 4, 044004 (2019).
Lee, H. et al. Ultrathin organic electrochemical transistor with nonvolatile and thin gel electrolyte for long-term electrophysiological monitoring. Adv. Funct. Mater. 29, 1906982 (2019).
Liu, D. et al. Intrinsically stretchable organic electrochemical transistors with rigid-device-benchmarkable performance. Adv. Sci. 9, 2203418 (2022).
Jo, Y. J. et al. Biocompatible and biodegradable organic transistors using a solid-state electrolyte incorporated with choline-based ionic liquid and polysaccharide. Adv. Funct. Mater. 30, 1909707 (2020).
Keene, S. T. et al. Wearable organic electrochemical transistor patch for multiplexed sensing of calcium and ammonium ions from human perspiration. Adv. Healthc. Mater. 8, 1901321 (2019).
Uguz, I. et al. Autoclave sterilization of PEDOT:PSS electrophysiology devices. Adv. Healthc. Mater. 5, 3094–3098 (2016).
Granelli, R. et al. High-performance bioelectronic circuits integrated on biodegradable and compostable substrates with fully printed mask-less organic electrochemical transistors. Small 18, 2108077 (2022).
Campana, A., Cramer, T., Simon, D. T., Berggren, M. & Biscarini, F. Electrocardiographic recording with conformable organic electrochemical transistor fabricated on resorbable bioscaffold. Adv. Mater. 26, 3874–3878 (2014).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Wu, M. et al. Ultrathin, soft, bioresorbable organic electrochemical transistors for transient spatiotemporal mapping of brain activity. Adv. Sci. 10, 2300504 (2023).
Uguz, I. & Shepard, K. L. Spatially controlled, bipolar, cortical stimulation with high-capacitance, mechanically flexible subdural surface microelectrode arrays. Sci. Adv. 8, eabq6354 (2022).
Lee, W. et al. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl Acad. Sci. USA 114, 10554–10559 (2017).
Uguz, I. et al. Flexible switch matrix addressable electrode arrays with organic electrochemical transistor and pn diode technology. Nat. Commun. 15, 533 (2024).
Donahue, M. J. et al. High-performance vertical organic electrochemical transistors. Adv. Mater. 30, e1705031 (2018).
Uguz, I. et al. Complementary integration of organic electrochemical transistors for front-end amplifier circuits of flexible neural implants. Sci. Adv. 10, eadi9710 (2024).
Cea, C. et al. Integrated internal ion-gated organic electrochemical transistors for stand-alone conformable bioelectronics. Nat. Mater. 22, 1227–1235 (2023).
Zhao, Z., Spyropoulos, G. D., Cea, C., Gelinas, J. N. & Khodagholy, D. Ionic communication for implantable bioelectronics. Sci. Adv. 8, eabm7851 (2022).
Wu, X. et al. Fiber-shaped organic electrochemical transistors for biochemical detections with high sensitivity and stability. Sci. China Chem. 63, 1281–1288 (2020).
Diacci, C. et al. Diurnal in vivo xylem sap glucose and sucrose monitoring using implantable organic electrochemical transistor sensors. iScience 24, 101966 (2021).
Bischak, C. G., Flagg, L. Q. & Ginger, D. S. Ion exchange gels allow organic electrochemical transistor operation with hydrophobic polymers in aqueous solution. Adv. Mater. 32, e2002610 (2020).
Gentile, F. et al. A biomimetic, biocompatible OECT sensor for the real-time measurement of concentration and saturation of ions in plant sap. Adv. Electron. Mater. 8, 2200092 (2022).
Stavrinidou, E. et al. Electronic plants. Sci. Adv. 1, e1501136 (2015).
Inal, S. Turning tissues into conducting matter. Science 379, 758–759 (2023).
Strakosas, X. et al. Metabolite-induced in vivo fabrication of substrate-free organic bioelectronics. Science 379, 795–802 (2023).
Arya, S. S., Dias, S. B., Jelinek, H. F., Hadjileontiadis, L. J. & Pappa, A.-M. The convergence of traditional and digital biomarkers through AI-assisted biosensing: a new era in translational diagnostics? Biosens. Bioelectron. 235, 115387 (2023).
Land, K. J., Boeras, D. I., Chen, X.-S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019).
Tian, X. et al. Pushing OECTs toward wearable: development of a miniaturized analytical control unit for wireless device characterization. Anal. Chem. 94, 6156–6162 (2022).
Acknowledgements
For this Review, we used large language models, including ChatGPT (OpenAI) and Claude (Anthropic), to correct the grammar and wording of the text. All text in the manuscript was written by the authors, and large language models were used solely for style corrections with no input in the content or meaning.
Author information
Authors and Affiliations
Contributions
A.S. and S.I. developed the concept of the article. A.S. worked with all authors to write the article. All authors contributed to the article’s research and discussion. S.I. did final edits and revisions with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Haungxian Ju and Paschalis Gkoupidenis for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleh, A., Koklu, A., Uguz, I. et al. Bioelectronic interfaces of organic electrochemical transistors. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00180-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00180-7