Abstract
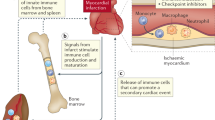
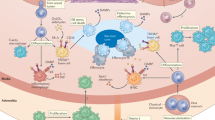
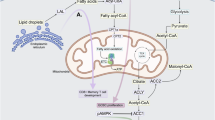
Cardiovascular diseases (CVDs), including atherosclerosis, myocardial infarction and heart failure, are the leading causes of morbidity and mortality worldwide. Emerging evidence suggests a crucial role for immune cell dysfunction and inflammation in the progression of this complex set of diseases. Recent advances demonstrate that immune cells, tightly linked to CVD pathogenesis, are sensitive to environmental signals and respond by engaging immunometabolic networks that shape their behavior. Inflammatory cues and altered nutrient availability within atherosclerotic plaques or following ischemia synergize to elicit metabolic shifts in immune cells that influence the course of disease pathology. Understanding these metabolic adaptations and how they contribute to cellular dysfunction may reveal novel therapeutic approaches for the treatment of CVD. Here we provide a comprehensive summary of the metabolic reprogramming that occurs in immune cells and their progenitors during CVD, offering insights into the potential therapeutic interventions to mitigate disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Murphy, A. J. & Tall, A. R. Disordered hematopoiesis and athero-thrombosis. Eur. Heart J. 37, 1113–1121 (2016).
Murphy, A. J. & Febbraio, M. A. Immune-based therapies in cardiovascular and metabolic diseases: past, present and future. Nat. Rev. Immunol. 21, 669–679 (2021).
Bjorkegren, J. L. M. & Lusis, A. J. Atherosclerosis: recent developments. Cell 185, 1630–1645 (2022).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121, 4138–4149 (2011). Described a critical role for the cholesterol content of hematopoietic progenitor cells as a driver of myelopoiesis and atherosclerosis development.
Karunakaran, D. et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ. Res. 117, 266–278 (2015). Discovered that macrophage-derived foam cells require functional mitochondria for cholesterol efflux to occur.
Sarrazy, V. et al. Disruption of Glut1 in hematopoietic stem cells prevents myelopoiesis and enhanced glucose flux in atheromatous plaques of ApoE–/– mice. Circ. Res. 118, 1062–1077 (2016). Discovered that glucose uptake as mediated by GLUT1 in hematopoiteic progenitors facilitates myelopoiesis to excaerbate atherosclerosis.
Cai, S. et al. Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J. Clin. Invest. https://doi.org/10.1172/JCI159498 (2023).
Oburoglu, L. et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell 15, 169–184 (2014).
Chapman, N. M. & Chi, H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity 55, 14–30 (2022).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Ryan, D. G. & O’Neill, L. A. J. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 38, 289–313 (2020).
Swain, A. et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat. Metab. 2, 594–602 (2020).
Qin, W. et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol. 15, 983–991 (2019).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). A seminal work detailing links among glycolysis, succinate accumulation and pro-inflammatory macrophage activation.
Hooftman, A. et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature 615, 490–498 (2023).
Ferreira, A. V. et al. Dimethyl itaconate induces long-term innate immune responses and confers protection against infection. Cell Rep. 42, 112658 (2023).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Johnson, M. O. et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780–1795 (2018).
Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18, 1197–1206 (2017).
Bacigalupa, Z. A., Landis, M. D. & Rathmell, J. C. Nutrient inputs and social metabolic control of T cell fate. Cell Metab. 36, 10–20 (2024).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009). A seminal work on the central role of fatty acid metabolism in T cell fate determination.
Wang, F., Beck-Garcia, K., Zorzin, C., Schamel, W. W. & Davis, M. M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 17, 844–850 (2016).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Chua, B. A. et al. Hematopoietic stem cells preferentially traffic misfolded proteins to aggresomes and depend on aggrephagy to maintain protein homeostasis. Cell Stem Cell 30, 460–472 (2023).
Liang, R. et al. Restraining lysosomal activity preserves hematopoietic stem cell quiescence and potency. Cell Stem Cell 26, 359–376 (2020).
Nakamura-Ishizu, A., Ito, K. & Suda, T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell 54, 239–255 (2020).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Shirai, T. et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 (2016).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175 (2018).
Lauterbach, M. A. et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 51, 997–1011 (2019).
Baardman, J. et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat. Commun. 11, 6296 (2020).
Ruparelia, N. et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur. Heart J. 36, 1923–1934 (2015).
Panizzi, P. et al. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J. Am. Coll. Cardiol. 55, 1629–1638 (2010).
DeBerge, M. et al. Hypoxia-inducible factors individually facilitate inflammatory myeloid metabolism and inefficient cardiac repair. J. Exp. Med. https://doi.org/10.1084/jem.20200667 (2021).
Wang, F. et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 19, 2331–2344 (2017).
Doddapattar, P. et al. Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circ. Res. 130, 1289–1305 (2022). Discovered that the deletion of a glycolytic intermediate, PKM2, in myeloid cells enhances the clearance of apoptotic cells in the plaque.
Nishizawa, T. et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 7, 356–365 (2014).
Parathath, S. et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 109, 1141–1152 (2011).
Aarup, A. et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, 1782–1790 (2016).
Freigang, S. et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 14, 1045–1053 (2013).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Baardman, J. et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 25, 2044–2052 (2018).
Rayner, K. J. et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 (2010).
Ouimet, M. et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Invest. 125, 4334–4348 (2015).
Park, D. et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature 477, 220–224 (2011).
Zhang, S. et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 29, 443–456 (2019).
Morioka, S. et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718 (2018).
Schilperoort, M., Ngai, D., Katerelos, M., Power, D. A. & Tabas, I. PFKFB2-mediated glycolysis promotes lactate-driven continual efferocytosis by macrophages. Nat. Metab. 5, 431–444 (2023).
Fond, A. M., Lee, C. S., Schulman, I. G., Kiss, R. S. & Ravichandran, K. S. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J. Clin. Invest. 125, 2748–2758 (2015).
Yurdagul, A. Jr et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 31, 518–533 (2020).
Ampomah, P. B. et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 4, 444–457 (2022).
Chia, S. et al. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am. J. Cardiol. 103, 333–337 (2009).
Papandreou, I., Cairns, R. A., Fontana, L., Lim, A. L. & Denko, N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 (2006).
Fossati, G. et al. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 170, 1964–1972 (2003).
Horckmans, M. et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 38, 187–197 (2017).
Wang, J. et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116 (2017).
Sreejit, G. et al. Retention of the NLRP3 inflammasome-primed neutrophils in the bone marrow is essential for myocardial infarction-induced granulopoiesis. Circulation 145, 31–44 (2022).
Sreejit, G. et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 141, 1080–1094 (2020).
Pruenster, M. et al. E-selectin-mediated rapid NLRP3 inflammasome activation regulates S100A8/S100A9 release from neutrophils via transient gasdermin D pore formation. Nat. Immunol. https://doi.org/10.1038/s41590-023-01656-1 (2023).
Doring, Y., Soehnlein, O. & Weber, C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 120, 736–743 (2017).
Westerterp, M. et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation 138, 898–912 (2018).
Silvestre-Roig, C. et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019).
Dhawan, U. K. et al. Hypercholesterolemia impairs clearance of neutrophil extracellular traps and promotes inflammation and atherosclerotic plaque progression. Arterioscler. Thromb. Vasc. Biol. 41, 2598–2615 (2021).
Pierini, L. M. et al. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 278, 10831–10841 (2003).
Murphy, A. J. et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 1333–1341 (2011).
Wong, S. L. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 21, 815–819 (2015).
Amini, P. et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 9, 2958 (2018).
Wang, L. et al. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front. Immunol. 9, 3076 (2018).
Nagareddy, P. R. et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 17, 695–708 (2013).
Flynn, M. C. et al. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ. Res. 127, 877–892 (2020).
Angeli, V. et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 (2004).
Shamshiev, A. T. et al. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8α-negative dendritic cells and protective Th1 type immunity. J. Exp. Med. 204, 441–452 (2007).
Gautier, E. L. et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 119, 2367–2375 (2009).
Westerterp, M. et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 25, 1294–1304 (2017).
Roman, M. J. et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2399–2406 (2003).
Bobryshev, Y. V. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur. Heart J. 26, 1700–1704 (2005).
Van der Borght, K. et al. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Rep. 18, 3005–3017 (2017).
Lee, J. S. et al. Conventional dendritic cells impair recovery after myocardial infarction. J. Immunol. 201, 1784–1798 (2018).
Basit, F., Mathan, T., Sancho, D. & de Vries, I. J. M. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front. Immunol. 9, 2489 (2018).
Clement, C. C. et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity 54, 721–736 (2021).
Shaw, M. K. et al. T-cells specific for a self-peptide of ApoB-100 exacerbate aortic atheroma in murine atherosclerosis. Front. Immunol. 8, 95 (2017).
Lu, H. et al. High glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cells. Cardiovasc. Diabetol. 12, 80 (2013).
Barrachina, M. N. et al. Efficient megakaryopoiesis and platelet production require phospholipid remodeling and PUFA uptake through CD36. Nat. Cardiovasc. Res. 2, 746–763 (2023).
de Jonckheere, B. et al. Critical shifts in lipid metabolism promote megakaryocyte differentiation and proplatelet formation. Nat. Cardiovasc. Res. 2, 835–852 (2023).
Dhenge, A., Limbkar, K., Melinkeri, S., Kale, V. P. & Limaye, L. Arachidonic acid and docosahexanoic acid enhance platelet formation from human apheresis-derived CD34+ cells. Cell Cycle 16, 979–990 (2017).
Kraakman, M. J. et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Invest. 127, 2133–2147 (2017).
Helou, M. A., Sisler, I., Ning, Y. & Liu, H. Is obesity alone associated with increased blood cell counts in otherwise healthy children? Blood 118, 3135–3135 (2011).
Kelly, K. L. et al. De novo lipogenesis is essential for platelet production in humans. Nat. Metab. 2, 1163–1178 (2020).
Valet, C. et al. Adipocyte fatty acid transfer supports megakaryocyte maturation. Cell Rep. 32, 107875 (2020).
Nayak, M. K. et al. The metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood 137, 1658–1668 (2021).
Flora, G. D. et al. Mitochondrial pyruvate dehydrogenase kinases contribute to platelet function and thrombosis in mice by regulating aerobic glycolysis. Blood Adv. 7, 2347–2359 (2023).
Wu, D. et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018).
Lepropre, S. et al. AMPK–ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood 132, 1180–1192 (2018).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Hofmann, U. & Frantz, S. Role of T cells in myocardial infarction. Eur. Heart J. 37, 873–879 (2016).
Bensinger, S. J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008). An early demonstration of the importance of LXR signaling and lipid metabolism in modulating T cell expansion and acquired immunity.
Armstrong, A. J., Gebre, A. K., Parks, J. S. & Hedrick, C. C. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 184, 173–183 (2010).
Gaddis, D. E. et al. Atherosclerosis impairs naive CD4 T-cell responses via disruption of glycolysis. Arterioscler. Thromb. Vasc. Biol. 41, 2387–2398 (2021).
Gerriets, V. A. et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46, 1970–1983 (2016).
Taleb, S. et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2691–2698 (2007).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Cheng, H. Y. et al. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest. 126, 3236–3246 (2016).
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Bazioti, V. et al. T cell cholesterol efflux suppresses apoptosis and senescence and increases atherosclerosis in middle aged mice. Nat. Commun. 13, 3799 (2022).
Tyrrell, D. J. et al. Clonally expanded memory CD8+ T cells accumulate in atherosclerotic plaques and are pro-atherogenic in aged mice. Nat. Aging https://doi.org/10.1038/s43587-023-00515-w (2023).
Quinn, K. M. et al. Metabolic characteristics of CD8+ T cell subsets in young and aged individuals are not predictive of functionality. Nat. Commun. 11, 2857 (2020).
Wagner, A. et al. Metabolic modeling of single TH17 cells reveals regulators of autoimmunity. Cell 184, 4168–4185 (2021).
O’Brien, K. L. et al. De novo polyamine synthesis supports metabolic and functional responses in activated murine NK cells. Eur. J. Immunol. 51, 91–102 (2021).
Ron-Harel, N. et al. T cell activation depends on extracellular alanine. Cell Rep. 28, 3011–3021 (2019).
Martinez, N. et al. Chromatin decondensation and T cell hyperresponsiveness in diabetes-associated hyperglycemia. J. Immunol. 193, 4457–4468 (2014).
Desdin-Mico, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Ouyang, J., Wang, H. & Huang, J. The role of lactate in cardiovascular diseases. Cell Commun. Signal. 21, 317 (2023).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 54, 1561–1577 (2021).
Mauro, C. et al. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metab. 25, 593–609 (2017).
Sage, A. P., Tsiantoulas, D., Binder, C. J. & Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 16, 180–196 (2019).
Hilgendorf, I. et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129, 1677–1687 (2014).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Chou, M. Y. et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 119, 1335–1349 (2009).
Muri, J., Thut, H., Bornkamm, G. W. & Kopf, M. B1 and marginal zone B cells but not follicular B2 cells require Gpx4 to prevent lipid peroxidation and ferroptosis. Cell Rep. 29, 2731–2744 (2019).
Morgan, P. K. et al. A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility. Nat. Cell Biol. 26, 645–659 (2024).
Lorenzo, C. et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature 589, 287–292 (2021).
Rosser, E. C. & Mauri, C. The emerging field of regulatory B cell immunometabolism. Cell Metab. 33, 1088–1097 (2021).
Bibby, J. A. et al. Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate. Nat. Commun. 11, 3412 (2020).
Luo, W. et al. SREBP signaling is essential for effective B cell responses. Nat. Immunol. 24, 337–348 (2023). A recent demonstration that lipid homeostasis controls the quality and longevity of B cell immunity.
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science https://doi.org/10.1126/science.aan4673 (2019).
Edgar, L. et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144, 961–982 (2021).
Olivares, R., Ducimetiere, P. & Claude, J. R. Monocyte count: a risk factor for coronary heart disease? Am. J. Epidemiol. 137, 49–53 (1993).
van der Valk, F. M. et al. Increased haematopoietic activity in patients with atherosclerosis. Eur. Heart J. 38, 425–432 (2017).
Gomes, A. L., Carvalho, T., Serpa, J., Torre, C. & Dias, S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood 115, 3886–3894 (2010).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Gu, Q. et al. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science 363, 1085–1088 (2019).
Westerterp, M. et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206 (2012).
Lee, M. K. S. et al. Defective AMPK regulation of cholesterol metabolism accelerates atherosclerosis by promoting HSPC mobilization and myelopoiesis. Mol. Metab. 61, 101514 (2022).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Brandts, J. & Ray, K. K. Novel and future lipid-modulating therapies for the prevention of cardiovascular disease. Nat. Rev. Cardiol. 20, 600–616 (2023).
Kornberg, M. D. et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 (2018). A seminal exploration of the beneficial therapeutic effects of targeting immunometabolic pathways with dimethyl fumarate (a methyl ester of the TCA cycle intermediate fumarate) in chronic inflammatory disease.
Weng, J. H. et al. Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat. Metab. 3, 513–522 (2021).
DeBerge, M., Chaudhary, R., Schroth, S. & Thorp, E. B. Immunometabolism at the heart of cardiovascular disease. JACC Basic Transl. Sci. 8, 884–904 (2023).
Engelen, S. E., Robinson, A. J. B., Zurke, Y. X. & Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat. Rev. Cardiol. 19, 522–542 (2022).
Ussher, J. R. & Drucker, D. J. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 20, 463–474 (2023).
Packer, M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat. Rev. Cardiol. 20, 443–462 (2023).
Borcherding, N. & Brestoff, J. R. The power and potential of mitochondria transfer. Nature 623, 283–291 (2023).
Borcherding, N. et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 34, 1499–1513 (2022). New concept to transfer functional mitochondria into macrophages. This could be done in HSCs, which would reduce atherosclerosis-related myelopoiesis.
Palsson-McDermott, E. M. & O’Neill, L. A. J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 30, 300–314 (2020).
Acknowledgements
A.J.F. is supported by funding from Diabetes Australia. J.N. is supported by funding from the NHMRC (GNT202152). N.L.G. is supported by funding from the NHMRC (GNT2017335) and ARC (DP230102412). A.K. is supported by funding from the NHMRC (GNT2017420) and the Australia Research Council Discovery Grant. A.J.M. is supported by funding from the NHMRC (GNT1194329).
Author information
Authors and Affiliations
Contributions
A.J.F., J.N., N.L.G., A.K. and A.J.M. contributed equally to the writing and revision of the article. A.J.F. prepared the draft figures that were used as a guide for the final images.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Federica Marelli-Berg, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fleetwood, A.J., Noonan, J., La Gruta, N. et al. Immunometabolism in atherosclerotic disorders. Nat Cardiovasc Res 3, 637–650 (2024). https://doi.org/10.1038/s44161-024-00473-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00473-5