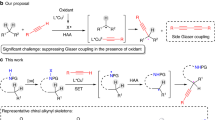
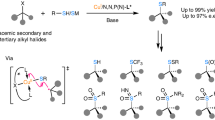
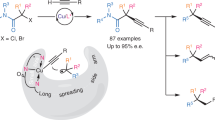
Abstract
Transition-metal-catalysed enantioconvergent cross-coupling reactions of highly reactive alkyl radicals often suffer from reduced chemoselectivity, mainly due to side reactions with closed-shell reactants. A strategy to overcome this challenge has yet to be identified, posing substantial limitations on the synthetic utility of this method. Here we report a method for enantioconvergent radical carbon–carbon cross-coupling of highly reactive cyclopropyl radicals with terminal alkynes, using redox state-tuned copper catalysis, under mild conditions. Key to this method is the use of hard chiral N,N,N-ligands in combination with Cu(II) salts of hard ligands/counterions, which results in elevated concentrations of Cu(II) species and thus enhanced cross-coupling reactions. This protocol not only exhibits a broad substrate scope across a wide range of both racemic cyclopropyl halide and terminal alkyne coupling partners but also provides access to useful yet synthetically challenging enantioenriched cyclopropane building blocks.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text and Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2264730 (20), 2267172 (78), 2267173 (86) and 2264731 (88). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
de Meijere, A., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More (Wiley, 2014).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Dong, X.-Y., Li, Z.-L., Gu, Q.-S. & Liu, X.-Y. Ligand development for copper-catalyzed enantioconvergent radical cross-coupling of racemic alkyl halides. J. Am. Chem. Soc. 144, 17319–17329 (2022).
Leifert, D. & Studer, A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 59, 74–108 (2020).
Hanson, P., Rowell, S. C., Walton, P. H. & Timms, A. W. Promotion of Sandmeyer hydroxylation (homolytic hydroxydediazoniation) and hydrodediazoniation by chelation of the copper catalyst: bidentate ligands. Org. Biomol. Chem. 2, 1838–1855 (2004).
Fattahi, A., Lis, L. & Kass, S. R. Phenylcyclopropane energetics and characterization of its conjugate base: phenyl substituent effects and the C–H bond dissociation energy of cyclopropane. J. Org. Chem. 81, 9175–9179 (2016).
Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds (CRC Press, 2003).
Cui, X. & Zhang, X. P. in Contemporary Carbene Chemistry (eds Moss, R. A. & Doyle, M. P.) 491–525 (Wiley, 2014).
Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Chemler, S. R., Karyakarte, S. D. & Khoder, Z. M. Stereoselective and regioselective synthesis of heterocycles via copper-catalyzed additions of amine derivatives and alcohols to alkenes. J. Org. Chem. 82, 11311–11325 (2017).
Lipp, A., Badir, S. O. & Molander, G. A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed. 60, 1714–1726 (2021).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Zhang, L. & Meggers, E. Steering asymmetric Lewis acid catalysis exclusively with octahedral metal-centered chirality. Acc. Chem. Res. 50, 320–330 (2017).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Li, B., Shi, J.-L. & Xia, Y. Diversified synthesis of all-carbon quaternary gem-difluorinated cyclopropanes via copper-catalyzed cross-coupling. Org. Lett. 25, 2674–2679 (2023).
McKinney, M. A., Anderson, S. W., Keyes, M. & Schmidt, R. Cyclopropyl halides. Electron transfer in the lithium aluminum hydride reduction of gem-dibromo and monobromocyclopropanes. Tetrahedron Lett. 23, 3443–3446 (1982).
Tanabe, Y., Wakimura, K.-i & Nishii, Y. Sequential and highly stereoselective intermolecular radical additions of 2,3-cis-disubstituted 1,1-dibromo- and 1-bromocyclopropanes to electron-deficient olefins. Tetrahedron Lett. 37, 1837–1840 (1996).
Cohen, Y., Cohen, A. & Marek, I. Creating stereocenters within acyclic systems by C–C bond cleavage of cyclopropanes. Chem. Rev. 121, 140–161 (2021).
Zhang, Z., Gao, Y., Chen, S. & Wang, J. Transition-metal-catalyzed polymerization of cyclopropenes. Chin. J. Org. Chem. 41, 1888–1896 (2021).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Chen, Y., Fields, K. B. & Zhang, X. P. Bromoporphyrins as versatile synthons for modular construction of chiral porphyrins: cobalt-catalyzed highly enantioselective and diastereoselective cyclopropanation. J. Am. Chem. Soc. 126, 14718–14719 (2004).
Zhu, S., Ruppel, J. V., Lu, H., Wojtas, L. & Zhang, X. P. Cobalt-catalyzed asymmetric cyclopropanation with diazosulfones: rigidification and polarization of ligand chiral environment via hydrogen bonding and cyclization. J. Am. Chem. Soc. 130, 5042–5043 (2008).
Xu, X. et al. Highly asymmetric intramolecular cyclopropanation of acceptor-substituted diazoacetates by Co(II)-based metalloradical catalysis: iterative approach for development of new-generation catalysts. J. Am. Chem. Soc. 133, 15292–15295 (2011).
Hu, Y. et al. Next-generation D2-symmetric chiral porphyrins for cobalt(II)-based metalloradical catalysis: catalyst engineering by distal bridging. Angew. Chem. Int. Ed. 58, 2670–2674 (2019).
Ebner, C. & Carreira, E. M. Cyclopropanation strategies in recent total syntheses. Chem. Rev. 117, 11651–11679 (2017).
Dian, L. & Marek, I. Asymmetric preparation of polysubstituted cyclopropanes based on direct functionalization of achiral three-membered carbocycles. Chem. Rev. 118, 8415–8434 (2018).
Talele, T. T. The ‘cyclopropyl fragment’ is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 59, 8712–8756 (2016).
Top Pharmaceuticals Poster. https://sites.arizona.edu/njardarson-lab/top200-posters/ (University of Arizona, 2022).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2017).
Lebel, H., Marcoux, J.-F., Molinaro, C. & Charette, A. B. Stereoselective cyclopropanation reactions. Chem. Rev. 103, 977–1050 (2003).
Mato, M., Franchino, A., García-Morales, C. & Echavarren, A. M. Gold-catalyzed synthesis of small rings. Chem. Rev. 121, 8613–8684 (2021).
Kulinkovich, O. G. & de Meijere, A. 1,n-Dicarbanionic titanium intermediates from monocarbanionic organometallics and their application in organic synthesis. Chem. Rev. 100, 2789–2834 (2000).
Doyle, M. P., Duffy, R., Ratnikov, M. & Zhou, L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 110, 704–724 (2010).
He, Y. et al. Recent advances in transition-metal-catalyzed carbene insertion to C–H bonds. Chem. Soc. Rev. 51, 2759–2852 (2022).
Wang, X. & Zhang, X. P. in Transition Metal-Catalyzed Carbene Transformations (eds Wang, J., Che, C.-M. & Doyle, M. P.) 25–66 (Wiley, 2022).
Vicente, R. C–C bond cleavages of cyclopropenes: operating for selective ring-opening reactions. Chem. Rev. 121, 162–226 (2021).
Sustac Roman, D. & Charette, A. B. in C–H Bond Activation and Catalytic Functionalization II (eds Dixneuf, P. H. & Doucet, H.) 91–114 (Springer, 2015).
Shao, Q., Wu, K., Zhuang, Z., Qian, S. & Yu, J.-Q. From Pd(OAc)2 to chiral catalysts: the discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C–H functionalization reactions. Acc. Chem. Res. 53, 833–851 (2020).
Lee, W.-C. C. & Zhang, X. P. Asymmetric radical cyclopropanation of alkenes. Trends Chem. 4, 850–851 (2022).
Lee, W.-C. C. & Zhang, X. P. Metalloradical catalysis: general approach for controlling reactivity and selectivity of homolytic radical reactions. Angew. Chem. Int. Ed. 63, e202320243 (2024).
Lee, W.-C. C. et al. Asymmetric radical cyclopropanation of dehydroaminocarboxylates: stereoselective synthesis of cyclopropyl α-amino acids. Chem 7, 1588–1601 (2021).
Wang, X. et al. Asymmetric radical process for general synthesis of chiral heteroaryl cyclopropanes. J. Am. Chem. Soc. 143, 11121–11129 (2021).
Wang, J., Xie, J., Lee, W.-C. C., Wang, D.-S. & Zhang, X. P. Radical differentiation of two ester groups in unsymmetrical diazomalonates for highly asymmetric olefin cyclopropanation. Chem Catal. 2, 330–344 (2022).
Ke, J. et al. Metalloradical activation of in situ-generated α-alkynyldiazomethanes for asymmetric radical cyclopropanation of alkenes. J. Am. Chem. Soc. 144, 2368–2378 (2022).
Lee, W.-C. C., Wang, D.-S., Zhu, Y. & Zhang, X. P. Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism. Nat. Chem. 15, 1569–1580 (2023).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Zhang, X. & Tan, C.-H. Stereospecific and stereoconvergent nucleophilic substitution reactions at tertiary carbon centers. Chem 7, 1451–1486 (2021).
An, L., Tong, F.-F., Zhang, S. & Zhang, X. Stereoselective functionalization of racemic cyclopropylzinc reagents via enantiodivergent relay coupling. J. Am. Chem. Soc. 142, 11884–11892 (2020).
Müller, D. S. & Marek, I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 45, 4552–4566 (2016).
Onneken, C. et al. Light-enabled deracemization of cyclopropanes by Al-salen photocatalysis. Nature 621, 753–759 (2023).
Boche, G. & Walborsky, H. M. in Cyclopropane Derived Reactive Intermediates (eds Patai, S. & Rappoport, Z.) 117–173 (Wiley, 1990).
Gabbey, A. L., Scotchburn, K. & Rousseaux, S. A. L. Metal-catalysed C–C bond formation at cyclopropanes. Nat. Rev. Chem. 7, 548–560 (2023).
Rubin, M., Rubina, M. & Gevorgyan, V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem. Rev. 107, 3117–3179 (2007).
Moragas, T. & Martin, R. Nickel-catalyzed reductive carboxylation of cyclopropyl motifs with carbon dioxide. Synthesis 48, 2816–2822 (2016).
Mao, R., Balon, J. & Hu, X. Decarboxylative C(sp3)–O cross-coupling. Angew. Chem. Int. Ed. 57, 13624–13628 (2018).
Salgueiro, D. C., Chi, B. K., Guzei, I. A., Garcίa-Reynaga, P. & Weix, D. J. Control of redox-active ester reactivity enables a general cross-electrophile approach to access arylated strained rings. Angew. Chem. Int. Ed. 61, e202205673 (2022).
Chen, T.-G. et al. Building C(sp3)-rich complexity by combining cycloaddition and C–C cross–coupling reactions. Nature 560, 350–354 (2018).
West, M. S., Gabbey, A. L., Huestis, M. P. & Rousseaux, S. A. L. Ni-catalyzed reductive cross-coupling of cyclopropylamines and other strained ring NHP esters with (hetero)aryl halides. Org. Lett. 24, 8441–8446 (2022).
DeCicco, E. M. et al. Decarboxylative cross-electrophile coupling of (hetero)aromatic bromides and NHP esters. J. Org. Chem. 88, 12329–12340 (2023).
Liao, J. et al. Deaminative reductive cross-electrophile couplings of alkylpyridinium salts and aryl bromides. Org. Lett. 21, 2941–2946 (2019).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Mills, L. R., Monteith, J. J., dos Passos Gomes, G., Aspuru-Guzik, A. & Rousseaux, S. A. L. The cyclopropane ring as a reporter of radical leaving-group reactivity for Ni-catalyzed C(sp3)–O arylation. J. Am. Chem. Soc. 142, 13246–13254 (2020).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Wang, F.-L. et al. Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes. Nat. Chem. 14, 949–957 (2022).
Wang, L.-L. et al. A general copper-catalysed enantioconvergent radical Michaelis–Becker-type C(sp3)–P cross-coupling. Nat. Synth. 2, 430–438 (2023).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Tian, Y. et al. A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution. Nat. Chem. 16, 466–475 (2024).
Ryabchuk, P., Edwards, A., Gerasimchuk, N., Rubina, M. & Rubin, M. Dual control of the selectivity in the formal nucleophilic substitution of bromocyclopropanes en route to densely functionalized, chirally rich cyclopropyl derivatives. Org. Lett. 15, 6010–6013 (2013).
Sladojevich, F., Trabocchi, A., Guarna, A. & Dixon, D. J. A new family of cinchona-derived amino phosphine precatalysts: application to the highly enantio- and diastereoselective silver-catalyzed isocyanoacetate aldol reaction. J. Am. Chem. Soc. 133, 1710–1713 (2011).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Wu, X., Lei, C., Yue, G. & Zhou, J. Palladium-catalyzed direct cyclopropylation of heterocycles. Angew. Chem. Int. Ed. 54, 9601–9605 (2015).
Bai, J.-F., Yasumoto, K., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral 1,4-enynes through organocatalytic alkenylation of propargyl alcohols with trialkenylboroxines. Angew. Chem. Int. Ed. 58, 8898–8901 (2019).
Xia, H.-D. et al. Photoinduced copper-catalyzed asymmetric decarboxylative alkynylation with terminal alkynes. Angew. Chem. Int. Ed. 59, 16926–16932 (2020).
Sagadevan, A., Ragupathi, A. & Hwang, K. C. Photoinduced copper-catalyzed regioselective synthesis of indoles: three-component coupling of arylamines, terminal alkynes, and quinones. Angew. Chem. Int. Ed. 54, 13896–13901 (2015).
Zhang, Y. et al. Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 22, 1490–1494 (2020).
Harayama, T., Fukushi, H., Ogawa, K. & Yoneda, F. An efficient method for preparing gem-dimethylcycloplopanes from gem-dibromocyclopropanes. Chem. Pharm. Bull. 33, 3564–3566 (1985).
Cohen, T., Dietz, A. G. Jr. & Miser, J. R. A simple preparation of phenols from diazonium ions via the generation and oxidation of aryl radicals by copper salts. J. Org. Chem. 42, 2053–2058 (1977).
Teator, A. J., Lastovickova, D. N. & Bielawski, C. W. Switchable polymerization catalysts. Chem. Rev. 116, 1969–1992 (2016).
Bunschoten, R. P. et al. Mechanistic basis of the Cu(OAc)2 catalyzed azide–ynamine (3 + 2) cycloaddition reaction. J. Am. Chem. Soc. 146, 13558–13570 (2024).
Chen, S.-J., Krska, S. W. & Stahl, S. S. Copper-catalyzed benzylic C–H cross-coupling enabled by redox buffers: expanding synthetic access to three-dimensional chemical space. Acc. Chem. Res. 56, 3604–3615 (2023).
Bakhoda, A. et al. Three-coordinate copper(II) alkynyl complex in C–C bond formation: the sesquicentennial of the Glaser coupling. J. Am. Chem. Soc. 142, 18483–18490 (2020).
Acknowledgements
Financial support from the National Natural Science Foundation of China (numbers 22025103, 92256301 and 22331006 to X.-Y.L.; 22271133 to Q.-S.G.; 22201127 to L.L.), the National Key R&D Program of China (numbers 2021YFF0701604 and 2021YFF0701704, to X.-Y.L.), the Guangdong Innovative Program (number 2019BT02Y335, to X.-Y.L.), the Guangdong Major Project of Basic and Applied Basic Research (number 2023B0303000020, to X.-Y.L.), the New Cornerstone Science Foundation through the Xplorer Prize (to X.-Y.L.), the Shenzhen Science and Technology Program (numbers KQTD20210811090112004, to X.-Y.L. and Q.-S.G.; JCYJ20220818100600001, to X.-Y.L.), the Shenzhen Key Laboratory of Cross-Coupling Reactions (number ZDSYS20220328104200001, to X.-Y.L.), High-Level of Special Funds (number G03050K003, to X.-Y.L.) and the High-Level Key Discipline Construction Project (number G030210001, to X.-Y.L.) is gratefully acknowledged. We appreciate the assistance of SUSTech Core Research Facilities.
Author information
Authors and Affiliations
Contributions
Z.G., L.L., W.W. and L.T. designed the experiments and analysed the data. Z.G., L.L., W.W. and N.-Y.Y. performed the experiments. J.-R.L. designed and performed the DFT calculations. L.L., Z.-L.L., Q.-S.G. and X.-Y.L. wrote the paper. Q.-S.G. and X.-Y.L. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17 and Tables 1–13, experimental procedures, synthetic procedures, characterization data, density functional theory (DFT) calculations and mechanistic discussion.
Supplementary Data 1
Crystallographic data for compound 20; CCDC reference 2264730.
Supplementary Data 2
Crystallographic data for compound 78; CCDC reference 2267172.
Supplementary Data 3
Crystallographic data for compound 86; CCDC reference 2267173.
Supplementary Data 4
Crystallographic data for compound 88; CCDC reference 2264731.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Z., Liu, L., Liu, JR. et al. Copper-catalysed synthesis of chiral alkynyl cyclopropanes using enantioconvergent radical cross-coupling of cyclopropyl halides with terminal alkynes. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00654-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00654-x