Abstract
Background
Whether use of SGLT2 inhibitors reduces the risk of cardiovascular and kidney events in people who contracted SARS-CoV-2 infection is not clear.
Methods
We used the healthcare databases of the United States Department of Veterans Affairs to build a cohort of 107,776 participants on antihyperglycemic therapy and had SARS-CoV-2 infection between March 01, 2020 and June 10, 2023. Within them, 11,588 used SGLT2 inhibitors and 96,188 used other antihyperglycemics. We examined the risks of major adverse cardiovascular events (MACE)—a composite of death, myocardial infarction and stroke, and major adverse kidney events (MAKE)—a composite of death, eGFR decline > 50%, and end stage kidney disease after balancing baseline characteristics between groups through inverse probability weighting. Survival analyses were conducted to generate hazard ratio (HR) and absolute risk reduction per 100 person-years (ARR).
Results
Over a median follow up of 1.57 (IQR: 1.05–2.49) years, compared to the control group, SGLT2 inhibitors use is associated with reduced risk of MACE (HR 0.82 (0.77, 0.88), ARR 1.73 (1.21, 2.25)) and reduced risk of MAKE (HR 0.75 (0.71, 0.80), ARR 2.62 (2.13, 3.11)). Compared to the control group, SGLT2 inhibitors use is associated with reduced risk of the secondary outcomes of hospitalization (HR 0.94 (0.90, 0.98), ARR 1.06 (1.36, 1.76)), anemia (HR 0.71 (0.65, 0.76), ARR 2.43 (1.95, 2.90)), and acute kidney injury (HR 0.84 (0.79, 0.89), ARR 1.86 (1.29, 2.42)).
Conclusions
Among people with SARS-CoV-2 infection on antihyperglycemic therapy, compared to those on other antihyperglycemics, those on SGLT2 inhibitors have less risk of adverse cardiovascular and kidney outcomes.
Plain language summary
SARS-CoV-2 infection leads to significant increase in risk of heart and kidney problems both shortly after infection and in the long-term. In this study, we evaluated whether SGLT2 inhibitors could reduce the risk of major adverse heart and kidney events in people with SARS-CoV-2 infection. SGLT2 inhibitors are a type of medication used to treat diabetes by lowering the amount of sugar in the blood. We compared a large group of people during and after SARS-CoV-2 infection and found that those who were using SGLT2 inhibitors had less major adverse heart and kidney problems than those who were using other types of sugar-lowering medications. Our findings could be useful for optimizing approaches to reduce risk of heart and kidney problems among people with diabetes and SARS-CoV-2 infection.
Similar content being viewed by others
Introduction
SARS-CoV-2 infection is associated with increased risk of adverse cardiovascular and kidney outcomes in both the acute and post-acute phase of the COVID-19 illness1,2,3,4,5. SGLT2 inhibitors have been shown in multiple randomized trials to reduce the risk of major adverse cardiovascular events (MACE) (death, myocardial infarction and stroke) and major adverse kidney events (MAKE) (death, eGFR decline > 50% and end-stage kidney disease (ESKD))6,7,8,9. Whether use of SGLT2 inhibitors reduces the risk of cardiovascular and kidney events in people who contracted SARS-CoV-2 infection is not clear.
The DARE-19 randomized placebo-controlled trial enrolled 1250 patients with cardiometabolic risk factors who were hospitalized with COVID-19. The results of this trial showed that treatment with dapagliflozin (vs placebo) yielded a hazard ratio of 0.80 (95% CI 0.58–1.10) for the composite outcome of organ dysfunction or death; and the hazard ratio for death was 0.77 (95% CI 0.52–1.16). Both risk estimates were favorable yet imprecise and statistically non-significant—likely due to low power10. The RECOVERY trial reported that in 4271 adults hospitalized with COVID-19, empagliflozin was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death11.
Both DARE-19 and RECOVERY exclusively enrolled people who were hospitalized with COVID-19 (who do not represent the majority of people with COVID-19), examined only acute outcomes at 28 days, and did not evaluate cardiovascular or kidney outcomes.
Yet, it is now widely recognized that people with SARS-CoV-2 infection—including those who were hospitalized and non-hospitalized during the acute phase of the infection — experience increased risk of adverse cardiovascular and kidney events in the acute and post-acute phase of the disease and that the risk may remain elevated even a year after infection3,4,12,13.
Whether use of SGLT2 inhibitors reduces risk of adverse cardiovascular and kidney outcomes in people with SARS-CoV-2 infection is still not yet known. Addressing this question will inform prevention and treatment approaches of the adverse cardiovascular and kidney consequences of SARS-CoV-2 infection.
In this study, we used the electronic health records of the US Department of Veterans Affairs and identified 11,588 users of SGLT2 inhibitors and 96,188 users of other antihyperglycemics who had SARS-CoV-2 between March 01, 2020 and June 10, 2023. We then applied inverse probability weighting to balance the health and demographic characteristics between antihyperglycemics users who received SGLT2 inhibitors vs those who did not (the control group) and evaluated whether treatment with SGLT2 inhibitors was associated with reduced risk of MACE (defined as composite of death, myocardial infarction and stroke) and MAKE (defined as composite of death, eGFR decline > 50%, and end stage kidney disease (ESKD)). In this study, we find that among people with SARS-CoV-2 infection on antihyperglycemic therapy, compared to those on other antihyperglycemics, those on SGLT2 inhibitors have less risk of MACE (Hazard ratio (HR) 0.82 (0.77, 0.88), absolute risk reduction per 100 person-years (ARR)1.73 (1.21, 2.25)) and MAKE (HR 0.75 (0.71, 0.80), ARR 2.62 (2.13, 3.11)). We conclude that among people with SARS-CoV-2 infection on antihyperglycemic therapy, compared to those on other antihyperglycemics, those on SGLT2 inhibitors have less risk of adverse cardiovascular and kidney outcomes.
Methods
Setting
The study was conducted using data from the US Department of Veterans Affairs (VA) healthcare databases—which operates 1293 healthcare facilities including 171 medical centers and 1112 outpatient sites. As the largest integrated healthcare system in the US, the VA provides comprehensive healthcare services to veterans of the US armed forces. These services encompass preventive and health maintenance care, outpatient and inpatient hospital care, mental healthcare, home healthcare, primary and specialty care, geriatric and extended care, as well as provision of pharmaceuticals, medical equipment and prosthetics.
Data sources
The healthcare databases of the US Department of Veterans Affairs were utilized in this study. These databases include information collected during patients’ routine healthcare encounters and are updated daily. The data domains include outpatient and inpatient diagnoses, pharmacy, and laboratory results. Vaccination status was collected from the VA COVID-19 Shared Data Resource. The Area Deprivation Index (ADI)—a composite measure of income, education, employment, and housing—served as a summary measure of contextual disadvantage at the participants’ residential locations14.
Cohort
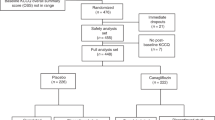
We present a flowchart of cohort construction in Fig. 1. We enrolled 749,551 users of VA health care system who had a positive SARS-CoV-2 test result between March 01, 2020 and June 10, 2023. The date of first positive test was set to be T0. We further selected participants who used antihyperglycemics at the date of infection based on prescription records (N = 143,396). We then removed participants who had end stage kidney disease or had an eGFR < 30 mL/min/1.73m2 (N = 128,984). Participants with incident use of SGLT2 inhibitor within one year before T0 were selected into the SGLT2 inhibitors group (N = 11,588) and participants without use of SGLT2 inhibitors before T0 were selected into the control group (N = 96,188). Participants were followed until July 10, 2023.
Exposure
The exposure group was defined as participants who initiated SGLT2 inhibitors within 1 year prior to the date of infection and continued using SGLT2 inhibitors at the date of infection. The control group comprised participants without a history of SGLT2 inhibitors use, and were using any other antihyperglycemic besides SGLT2 inhibitors at the date of infection.
Outcomes
In this study, we evaluated the risk of MACE, defined as a composite of death, myocardial infarction and stroke; and also risk of MAKE, defined as a composite of death, eGFR decline > 50%, and ESKD15,16,17. We also evaluated the risk of secondary outcomes, including each individual component of the composite outcomes (death, stroke, myocardial infarction, eGFR decline > 50%, and ESKD), along with hospitalization, anemia, and acute kidney injury (Supplementary Table 1). The risk of incident outcomes was assessed within participants who had no history of the outcome within the 3 years preceding T0.
Covariates
Baseline covariates that may affect the exposure and the outcomes were ascertained within three years before T0 based on literature review and prior knowledge1,3,15,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32, where covariates status closest to T0 were used. We selected demographic factors including age, self-declared race (white, Black, and other), self-declared sex, ADI, health factors including COVID-19 vaccine status (unvaccinated, received 1 or 2 doses vaccine, boosted), body mass index (BMI), smoking status (never, former, and current), and use of long-term care. We also selected covariates representing comorbidities including eGFR, low-density lipoprotein cholesterol (LDL), systolic and diastolic blood pressure, cancer, cardiovascular disease, hyperlipidemia, peripheral artery disease, chronic lung disease, dementia, acute kidney injury, acute pancreatitis, venous thromboembolism, immune dysfunction, and albuminuria. Medication use including history use of ACE/ARB, calcium channel blockers, beta blockers, diuretics, statins were also included as covariates. To adjust for antihyperglycemics use at date of infection, we also accounted for the use of metformin, DPP4 inhibitors, GLP-1RAs, sulfonylureas, thiazolidinediones, insulin and other antihyperglycemics including alpha or amylin or meglitinide at the date of infection. In addition, we adjusted for outpatient COVID-19 treatments, including COVID-19 antivirals including nirmatrelvir, molnupiravir, and remdesivir and COVID-19 monoclonal antibody medications. Healthcare utilization including number of outpatient and inpatient encounters, number of blood panel tests, number of outpatient prescriptions, number of HbA1c tests and number of Medicare outpatient and inpatient encounters and pandemic related characteristics represented by week of the T0 were also adjusted. All missing continuous variables (2.76% of eGFR, 3.07% of BMI, 0.08% of blood pressure and 0.65% of LDL) were imputed based on fully conditional specification method conditioning on all covariates and assigned values based on predictive mean matching33. Continuous variables were transformed into restricted cubic spline functions in the process of modeling34.
Statistical analysis
Baseline characteristics of those on SGLT2 inhibitors and those in the control group were reported. Differences of the baseline characteristics between the two groups were assessed using absolute standardized differences where a value of less than 0.1 was considered evidence of good balance35.
Inverse probability weighting was used to balance the differences in baseline characteristics between SGLT2 inhibitors and the control group. Multivariate logistic regressions were constructed to estimate the probability of belonging to the SGLT2 inhibitors group (the propensity score). We then constructed the weighting toward the SGLT2 inhibitors group by assigning weights of 1 for those in the SGLT2 inhibitors group and weights of propensity score/(1-propensity score) for those in the control group36. Weighted Cox survival models were employed to estimate the association between SGLT2 inhibitors and outcomes. Event rates and absolute risk reductions were estimated based on the survival probabilities of the two groups generated from the survival model. To estimate the risk of incident outcomes, outcome-specific propensity score models and survival models were conducted among participants with no history of the evaluated outcome.
We further examined the risk of MACE and MAKE across various subgroups including age ( ≤ 60 and > 60 years), sex (male and female), race (white and Black), vaccination status (unvaccinated, received 1 or 2 doses of vaccine, boosted), status of hospitalization during acute phase of infection, metformin use, insulin use, cardiovascular disease, BMI ( > 30 and ≤ 30 kg/m2) and eGFR ( ≥ 60 and < 60 ml/min/1.73m2).
To examine the robustness of our findings, we conducted multiple sensitivity analyses. These included (1) applying an overlap weighting method instead of the inverse probability weighting method used in the primary approach37; (2) using doubly robust adjustment for covariates in the weighted survival model, instead of solely balancing based on the weighting in the primary approach38; (3) redefining the exposure group as those who initiated SGLT2 inhibitors within 180 days and, separately, within 90 days before the infection, to proxy incident use instead of defining the exposure group as those who initiated SGLT2 inhibitors within 1 year before infection as in the primary approach; (4) additionally adjusting for healthcare utilization factors such as the number of outpatient and inpatient visits, the number of laboratory tests, and the number of prescriptions received during follow-up, instead of only adjusting for baseline characteristics as in the primary approach; (5) additionally adjusting for time-varying HbA1c values during follow-up, instead of only adjusting for baseline HbA1c as in the primary approach; (6) conducted per-protocol analyses based on inverse probability of censoring weight where the protocol for the SGLT2 inhibitors group was defined as continued use of SGLT2 inhibitors during follow up; and the protocol for the control group, the protocol was defined as non-use of SGLT2 inhibitors during follow up, whereas the primary analyses employed intention to treat approach39.
In this study, 95% CI of the hazard ratio that does not cross 1 and 95% CI of the absolute risk reduction that does not cross 0 were considered statistically significant. Data management and analyses were performed with SAS Enterprise Guide, version 8.3 (SAS Institute, Cary, NC). Data visualizations were performed in R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval
This study used data from the U.S. Department of Veterans Affairs healthcare database. This research project was reviewed and approved by the Institutional Review Board (IRB) of the VA Saint Louis Health Care System (Protocol number 1606333). The Institutional Review Board waived the need to obtain informed consent from veterans whose data is included in the healthcare database.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
The study enrolled 107,776 participants who had a positive SARS-CoV-2 test. Within them, 11,588 participants were in the SGLT2 inhibitors group and 96,188 participants were in the control group of other antihyperglycemics. Baseline demographic and health characteristics of the SGLT2 inhibitors and the control groups before and after inverse probability weighting are presented in Supplementary Data 1 and Supplementary Data 2, respectively. Assessment of the absolute standardized mean differences (SMDs) of the demographic and health characteristics between the SGLT2 inhibitors and the control groups after weighting yielded SMDs below 0.1 – indicating good balance (Supplementary Data 2, Supplementary Fig. 1).
Over the follow up period (median 1.57 (IQR: 1.05–2.49) years) which corresponded to 184,563 person-years of follow up, compared to the control group, SGLT2 inhibitors use was associated with reduced risk MACE (HR 0.82 (0.77, 0.88), ARR 1.73 (1.21, 2.25)); the point estimates for the individual components of MACE were HR 0.76 (0.71, 0.81), ARR 1.96 (1.52, 2.40) for death, HR 0.92 (0.81, 1.04). ARR 0.18 (−0.08, 0.44) for myocardial infarction, and HR 0.93 (0.82, 1.04), ARR 0.19 (−0.10, 0.47) for stroke (Figs. 2, 3, Supplementary Table 2).
a Major adverse cardiovascular events (MACE); b Major adverse kidney events (MAKE). MACE was a composite of death, myocardial infarction and stroke. MAKE was a composite of death, eGFR decline > 50%, and end stage kidney disease. Cumulative incident functions presented for SGLT2 inhibitors (purple) and control group (red). Shaded areas are 95% confidence intervals.
n for SGLT2 inhibitors = 11,588, n for control group = 96,188. a Major adverse cardiovascular events (MACE) and its components including death, myocardial infarction and stroke; b Major adverse kidney events (MAKE) and its components including death, eGFR decline > 50%, and end stage kidney disease; c secondary outcomes including hospitalization, anemia and acute kidney injury. Adjusted hazard ratios and 95% confidence intervals are presented. Length of the bar represents the risk reduction per 100 persons at 180 days and associated 95% confidence intervals are also shown.
Compared to the control group, SGLT2 inhibitors use was associated with reduced risk of MAKE (HR 0.75 (0.71, 0.80), ARR 2.62 (2.13, 3.11)). SGLT2 inhibitors use was associated with reduced risk of the individual components of MAKE including death (HR 0.76 (0.71, 0.81), ARR 1.96 (1.52, 2.40)), eGFR decline > 50% (HR 0.73 (0.66, 0.81), ARR 0.98 (0.70, 1.26)), and ESKD (HR 0.69 (0.58, 0.81), ARR 0.44 (0.27, 0.62)) (Figs. 2, 3, Supplementary Table 3).
Compared to the control group, SGLT2 inhibitors was associated with reduced risk of the secondary outcomes of hospitalization (HR 0.94 (0.90, 0.98), ARR 1.06 (1.36, 1.76)) anemia (HR 0.71 (0.65, 0.76), ARR 2.43 (1.95, 2.90)), and AKI (HR 0.84 (0.79, 0.89), ARR 1.86 (1.29, 2.42)) (Fig. 3, Supplementary Table 4). SGLT2 inhibitors use was associated with reduced risk of the secondary outcome of hospitalization (HR 0.91 (0.84, 0.98)) during the acute phase (first 30 days) of SARS-CoV-2 infection. We examined the association between SGLT2 inhibitors use and the risks of MACE and MAKE in several subgroups including age ( ≤ 60 and > 60 years), sex (male and female), race (white and Black), vaccination status (unvaccinated, received 1 or 2 doses of vaccine, boosted), status of hospitalization during acute phase of SARS-CoV-2 infection, metformin use, insulin use, cardiovascular disease status, BMI ( > 30 and ≤ 30 kg/m2) and eGFR ( ≥ 60 and < 60 ml/min/1.73m2). Compared to the control group, SGLT2 inhibitors use was associated with reduced risk of the composite outcomes of MACE and MAKE in most subgroups (Fig. 4, Supplementary Tables 5–6).
n for SGLT2 inhibitors=11,588, n for control group= 96,188. Subgroups including age ( ≤ 60 (n for SGLT2 inhibitor = 2822, n for control group = 21,867) and > 60 years (n for SGLT2 inhibitor = 8766, n for control group = 74,321)), sex (male (n for SGLT2 inhibitor = 11,024, n for control group = 89,593) and female (n for SGLT2 inhibitor = 564, n for control group = 6595), race (white (n for SGLT2 inhibitor = 8457, n for control group = 70,088 and Black (n for SGLT2 inhibitor = 2368, n for control group = 20,403), vaccination status (unvaccinated (n for SGLT2 inhibitor = 4026, n for control group = 45,887), received 1 or 2 doses of vaccine (n for SGLT2 inhibitor = 3382, n for control group = 25,271), boosted (n for SGLT2 inhibitor = 4180, n for control group = 25,030), status of hospitalization during acute phase of infection (n for SGLT2 inhibitor = 1759, n for control group = 14,725), status of non-hospitalization during acute phase of infection (n for SGLT2 inhibitor = 9829, n for control group = 81,463), metformin use (n for SGLT2 inhibitor = 5500, n for control group = 64,892), no metformin use (n for SGLT2 inhibitor =6088, n for control group =31,296), insulin use (n for SGLT2 inhibitor = 3499, n for control group = 30,825), no insulin use (n for SGLT2 inhibitor =8089, n for control group = 65,363), cardiovascular disease (n for SGLT2 inhibitor = 5999, n for control group = 35,418), no cardiovascular disease (n for SGLT2 inhibitor =5589, n for control group = 60,770), BMI ( > 30 (n for SGLT2 inhibitor =7496, n for control group = 61,396) and ≤ 30 kg/m2 (n for SGLT2 inhibitor = 4092, n for control group = 34,792)) and eGFR ( ≥ 60 (n for SGLT2 inhibitor =7929, n for control group = 71,288) and < 60 ml/min/1.73m2 (n for SGLT2 inhibitor =3659, n for control group = 24,900)).
Sensitivity analyses
Multiple sensitivity analyses were conducted to assess the robustness of our findings. (1) We applied an overlap weighting method instead of the inverse probability weighting method used in the primary approach; (2) we used doubly robust adjustment for covariates in the weighted survival model, instead of solely balancing based on the weighting in the primary approach; (3) we redefined the exposure group as those who initiated SGLT2 inhibitors within 180 days and, separately, within 90 days before SARS-CoV-2 infection, to proxy incident use instead of defining the exposure group as those who initiated SGLT2 inhibitors within 1 year before infection as in the primary approach; (4) we additionally adjusted for healthcare utilization factors such as the number of outpatient and inpatient visits, the number of laboratory tests, and the number of prescriptions received during follow-up, instead of only adjusting for baseline characteristics as in the primary approach. (5) We additionally adjusted for time-varying HbA1c values during follow-up, instead of only adjusting for baseline HbA1c as in the primary approach; (6) We conducted per-protocol analyses based on inverse probability of censoring weight where the protocol for the SGLT2 inhibitors group was defined as continued use of the SGLT2 inhibitors during follow up and the protocol for the control group was defined as non-use SGLT2 inhibitors during follow up, whereas intention to treat analyses were used in the primary approach. Results from all sensitivity analyses were consistent with our main findings (Supplementary Table 7).
Discussion
In this study, we enrolled 107,776 people with SARS-CoV-2 infection — including 11,588 users of SGLT2 inhibitors and 96,188 users of other antihyperglycemics — and followed them for a median of 1.57 (IQR: 1.05–2.49) years after infection which altogether corresponded to 184,563 person-years of follow up. Compared to the control group, use SGT2 inhibitors was associated with reduced risk of MACE and MAKE. SGLT2 inhibitors use was also associated with reduced risk of hospitalization, anemia and AKI. Altogether, the findings that suggest that among people on antihyperglycemic therapy who contract SARS-CoV-2 infection, use of SGLT2 inhibitors was associated with cardiovascular and kidney protective effects. These findings may help inform choice of antihyperglycemic therapy.
People with diabetes have increased risk of adverse cardiovascular and kidney events6,8. SARS-CoV-2 itself is associated with increased risks of diabetes26,40, cardiovascular and kidney events for at least a year after SARS-CoV-2 infection and reinfection12,26,29,31,40,41,42. Evidence also suggests that the adverse health effects of SARS-CoV-2 may be even more pronounced in people with comorbidities including diabetes29. Our results may help aid in management decisions and choice of antihyperglycemic therapy to maintain cardiovascular and kidney health in people with both diabetes and SARS-CoV-2.
Both the DARE-19 and the RECOVERY trial were built on the hypothesis that SGLT2 inhibitors may reduce the risk of acute adverse health outcomes in people hospitalized for SARS-CoV-2 infection10,11. Both showed non-statistically significant results. Both trials focused exclusively on hospitalized individuals and only examined acute outcomes (within 28 days). Our subgroup analyses according to hospitalization status during the acute phase of the infection show that the salutary association of SGLT2 inhibitors with both MACE and MAKE was weaker among those hospitalized than non-hospitalized – which may explain the results of these two trials.
Our results are consistent with the large body of evidence showing protective cardiac and kidney benefits of SGLT2 inhibitors in people who require antihyperglycemic therapy8. Evidence suggests that SGLT2 inhibitors may provide protective cardiorenal effects through various mechanisms beyond glucose control43,44,45. SGLT2 inhibitors also reduce the risk of acute kidney disease; whether and to what extent the reduction in risks of MACE and MAKE is mediated by reduction in risk of acute kidney disease (a risk factor for both MACE and MAKE) should be evaluated in future studies. However, the question of whether those for whom antihyperglycemic therapy may not be indicated would benefit from initiation of SGLT2 inhibitors remains to be addressed (e.g., whether those at high risk of cardiovascular and kidney events following SARS-CoV-2 infection (sans diabetes and other established indications for SGLT2 inhibitors) may derive benefit from initiation of SGLT2 inhibitors to lessen the risk of cardiovascular and kidney disease post-COVID is yet to be investigated)46,47,48.
This study also has strengths. It was conducted using real-world data from the VA and incorporated information across multiple data domains, including demographics, diagnoses, laboratory tests, medications, vital signs, healthcare utilization, and contextual factors. The study was conducted within the VA, which provides prescription benefits to study participants, thereby reducing biases related to financial considerations (i.e. cost of SGLT2 inhibitors). We examined and reported the risk of MACE and MAKE on both the relative and absolute scales – the latter provides quantitative estimates of risk reduction on the absolute scale which may help decision-making by patients, healthcare providers and policy makers. The robustness of our approach was assessed through multiple sensitivity analyses, which yielded consistent results.
This study also has several limitations. The VA population is predominantly white and male, which may limit the generalizability of our findings. We evaluated effectiveness within those who had a positive SARS-CoV-2 test result; our study may not represent those infected but were not tested for SARS-CoV-2. Although we carefully designed our study and balanced characteristics across multiple data domains, biases including residual confounding and misclassification may not be ruled out. We relied on VA pharmacy records to define exposure. If participants in the control group received SGLT2 inhibitors outside of the VA, the observed difference between the two groups might be biased toward null. Because initiation or switching of antihyperglycemics occur rather infrequently around the time of SARS-CoV-2, this precluded us from developing an incident user design where exposure would be defined as incident use of SGLT2 inhibitors or other antihyperglycemics at the time of infection; instead, we evaluated the effect of current use of SGLT2 inhibitors within those who initiated this treatment within one year of SARS-CoV-2 infection. We focused on the outcomes of MACE and MAKE and did not explore the association between SGLT2 inhibitors and other adverse outcomes of COVID-19. We only examined the effect of the SGLT2 inhibitor class and did not evaluate the effect of each medication within this drug class. Different types of SGLT2 inhibitors may have different effects on the examined outcomes49. We evaluated the effect of SGLT2 inhibitors in people with SARS-CoV-2; our cohorts did not include a control group of participants without SARS-CoV-2 infection; consequently, we do not disentangle the effect of SGLT2 inhibitors on outcomes that are caused by SARS-CoV-2 from those caused by other pathways50. Due to the dynamic nature of the pandemic, including the mutation of SARS-CoV-2, changes in immunity levels in the population, and alterations in treatment plans for COVID-19, the underlying risk of the population may change and as a result, the effectiveness of SGLT2 inhibitors on risks of MACE and MAKE in people with SARS-CoV-2 infection may also change over time51.
In sum, among people with SARS-CoV-2 infection on antihyperglycemic therapy, those on SGLT2 inhibitors had less risk of MACE and MAKE and several secondary endpoints. These results suggest that SGLT2 inhibitors maintain their cardiovascular and kidney protective effects in people with SARS-CoV-2 infection. The findings may help guide use of antihyperglycemic therapy in people with SARS-CoV-2 infection.
Data availability
The data that support the findings of this study are available from the US Department of Veterans Affairs. Data from the US Department of Veterans Affairs must be securely stored behind VA firewall and only investigators approved by the VA could have access to the data. VA data are made freely available to researchers behind the VA firewall with an approved VA study protocol. For more information, please visit https://www.virec.research.va.gov or contact the VA Information Resource Center (VIReC) at VIReC@va.gov. The numerical data (source data) underlying Fig. 2 can be found in Supplementary Tables 2 and 3. The numerical data (source data) underlying Fig. 3 can be found in Supplementary Tables 2–4. The numerical data (source data) underlying Fig. 4 can be found in Supplementary Tables 2, 3, 5, and 6.
References
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Al-Aly, Z. & Topol, E. Solving the puzzle of Long Covid. Science 383, 830–832 (2024).
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590 (2022).
Bowe, B., Xie, Y., Xu, E. & Al-Aly, Z. Kidney outcomes in Long COVID. J. Am. Soc. Nephrol. 32, 2851–2862 (2021).
Al-Aly, Z. et al. Long Covid Science, Research and Policy. Nat. Med. 30, 2148–2164 (2024).
Neuen, B. L. et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab. 2, 382–390 (2020).
Zelniker, T. A. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393, 31–39 (2019).
Neuen, B. L. et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2019).
Heerspink, H. J., Perkins, B. A., Fitchett, D. H., Husain, M. & Cherney, D. Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134, 752–772 (2016).
Kosiborod, M. N. et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diab. Endocrinol. 9, 586–594 (2021).
Horby, P. W. et al. Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv, 2023.2004.2013.23288469 (2023).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med 29, 2347–2357 (2023).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Kind, A. J. H. & Buckingham, W. R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med 378, 2456–2458 (2018).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diab. Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium-glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diab. Care 43, 2785–2795 (2020).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diab. Endocrinol. 11, 644–656 (2023).
Xie, Y., Bowe, B., Maddukuri, G. & Al-Aly, Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 371, m4677 (2020).
Bowe, B. et al. Acute kidney injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin. J. Am. Soc. Nephrol. 16, 14–25 (2021).
Xie, Y. & Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diab. Endocrinol. 10, 311–321 (2022).
Xie, Y. et al. Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with COVID-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with COVID-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of covid-19: cohort study. BMJ 381, e074572 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 183, 554–564 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diab. Endocrinol. 11, 120–128 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xie, Y. B. B., Gibson, A. K., McGill, J. B., Maddukuri, G., Al-Aly, Z. Clinical implications of estimated glomerular filtration rate dip following sodium-glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes Yan. J. Am. Heart Assoc. (2021).
Xie, Y., Bowe, B. & Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 12, 6571 (2021).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 28, 2406–2415 (2022).
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022).
Xie, Y., Xu, E. & Al-Aly, Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 376, e068993 (2022).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis (Springer, 2001).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Thomas, L. E., Li, F. & Pencina, M. J. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 323, 2417–2418 (2020).
Funk, M. J. et al. Doubly robust estimation of causal effects. Am. J. Epidemiol. 173, 761–767 (2011).
Hernan, M. A. & Hernandez-Diaz, S. Beyond the intention-to-treat in comparative effectiveness research. Clin. Trials 9, 48–55 (2012).
Al-Aly, Z. & Cao, B. The enduring effect of the COVID-19 pandemic on diabetes. Lancet Diab. Endocrinol. 12, 508–510 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med 28, 2398–2405 (2022).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Chen, X., Hocher, C. F., Shen, L., Krämer, B. K. & Hocher, B. Reno- and cardioprotective molecular mechanisms of SGLT2 inhibitors beyond glycemic control: from bedside to bench. Am. J. Physiol. Cell Physiol. 325, C661–c681 (2023).
Xiong, Y. et al. Regulation of SARS CoV-2 host factors in the kidney and heart in rats with 5/6 nephrectomy-effects of salt, ARB, DPP4 inhibitor and SGLT2 blocker. BMC Nephrol. 23, 117 (2022).
Chu, C. et al. Head-to-head comparison of two SGLT-2 inhibitors on AKI outcomes in a rat ischemia-reperfusion model. Biomed. Pharmacother. 153, 113357 (2022).
Al-Aly, Z. Diabetes after SARS-CoV-2 infection. Lancet Diab. Endocrinol. 11, 11–13 (2023).
Al-Aly, Z. Prevention of long COVID: progress and challenges. Lancet Infect. Dis. 23, 776–777 (2023).
Al-Aly, Z., Agarwal, A., Alwan, N. & Luyckx, V. A. Long COVID: long-term health outcomes and implications for policy and research. Nat. Rev. Nephrol. 19, 1–2 (2023).
Chu, C., Lu, Y. P., Yin, L. & Hocher, B. The SGLT2 inhibitor empagliflozin might be a new approach for the prevention of acute kidney injury. Kidney Blood Press Res. 44, 149–157 (2019).
Chu, C. et al. Comparison of infection risks and clinical outcomes in patients with and without SARS-CoV-2 lung infection under renin-angiotensin-aldosterone system blockade: Systematic review and meta-analysis. Br. J. Clin. Pharm. 87, 2475–2492 (2021).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron Eras. N. Engl. J. Med. 391, 515–525 (2024).
Acknowledgements
This study used data from the VA COVID-19 Shared Data Resource. This research was funded by the United States Department of Veterans Affairs (ZAA). The contents do not represent the views of the VA or the US government.
Author information
Authors and Affiliations
Contributions
Z.A.A., T.C., and Y.X. contributed to the development of the study concept and design. Z.A.A., T.C., and Y.X. contributed to data analysis and interpretation of results. Z.A.A., T.C., and Y.X. drafted the manuscript. Z.A.A., T.C., and Y.X. contributed to critical revision of the manuscript. Z.A.A. provided administrative, technical, and material support, as well as supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the report. The corresponding author attests that all the listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
Y.X. and Z.A.-A. report consulting (uncompensated) for Pfizer. Y.X. reports consulting for Guidepoint. Z.A.-A. reports consulting for Gilead Sciences and Tonix Pharmaceuticals. All other authors declare no competing interests.Funding/Support: This study used data from the US Department of Veterans Affairs (VA). This research was funded by the VA (Z.A.A.). Role of the Funder/Sponsor: The VA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, T., Xie, Y. & Al-Aly, Z. Adverse cardiovascular and kidney outcomes in people with SARS-CoV-2 treated with SGLT2 inhibitors. Commun Med 4, 179 (2024). https://doi.org/10.1038/s43856-024-00599-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00599-4