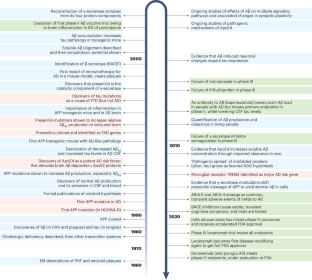
Abstract
Slowing neurodegenerative disorders of late life has lagged behind progress on other chronic diseases. But advances in two areas, biochemical pathology and human genetics, have now identified early pathogenic events, enabling molecular hypotheses and disease-modifying treatments. A salient example is the discovery that antibodies to amyloid ß-protein, long debated as a causative factor in Alzheimer’s disease (AD), clear amyloid plaques, decrease levels of abnormal tau proteins and slow cognitive decline. Approval of amyloid antibodies as the first disease-modifying treatments means a gradually rising fraction of the world’s estimated 60 million people with symptomatic disease may decline less or even stabilize. Society is entering an era in which the unchecked devastation of AD is no longer inevitable. This Perspective considers the impact of slowing AD and other neurodegenerative disorders on the trajectory of aging, allowing people to survive into late life with less functional decline. The implications of this moment for medicine and society are profound.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kang, J. et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 (1987).
Wasco, W. et al. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid β protein precursor. Proc. Natl Acad. Sci. USA 89, 10758–10762 (1992).
Wasco, W. et al. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer’s associated amyloid β protein precursor. Nat. Genet. 5, 95–100 (1993).
Sherrington, R. et al. Cloning of a novel gene bearing missense mutations in early onset familial Alzheimer disease. Nature 375, 754–760 (1995).
Rogaev, E. I. et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376, 775–778 (1995).
Vassar, R. et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999).
Sinha, S. et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402, 537–540 (1999).
Ghosh, A. K. et al. Design of potent inhibitors for human brain memapsin 2 (beta-secretase). J. Am. Chem. Soc. 122, 3522–3523 (2000).
Wolfe, M. S. et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517 (1999).
Struhl, G. & Greenwald, I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398, 522–525 (1999).
De Strooper, B. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Katzman, R. Editorial: the prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 33, 217–218 (1976).
Martens, Y. A. et al. ApoE cascade hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110, 1304–1317 (2022).
Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Prim. 7, 33 (2021).
Chen, X. & Holtzman, D. M. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 55, 2236–2254 (2022).
Alzheimer, A. Ueber eine eigenartige Erkrankung der Hirnrinde. Centralblatt Nervenheilkd. Psychiatr. 30, 177–179 (1907).
Davies, P. & Maloney, A. J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2, 1403 (1976).
White, P. et al. Neocortical cholinergic neurons in elderly people. Lancet 1, 668–671 (1977).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122, 1131–1135 (1984).
Masters, C. L. et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl Acad. Sci. USA 82, 4245–4249 (1985).
Levy, E. et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch-type. Science 248, 1124–1126 (1990).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706 (1991).
Haass, C. et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Brion, J., Passareiro, E., Nunez, J. & Flament-Durand, J. Mise en evidence immunologique de la protein tau au niveau des lesions de degenerescence neurofibrillaire de la maladie d’Alzheimer. Arch. Biol. 95, 229–235 (1985).
Nukina, N. & Ihara, Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J. Biochem. 99, 1541–1544 (1986).
Kosik, K. S., Joachim, C. L. & Selkoe, D. J. Microtubule-associated protein, tau, is a major antigenic component of paired helical filaments in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 83, 4044–4048 (1986).
Grundke-Iqbal, I. et al. Abnormal phosphorylation of the microtubule-associated protein t (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA 83, 4913–4917 (1986).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited FTDP-17. Nature 393, 702–705 (1998).
Strittmatter, W. J. et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 1977–1981 (1993).
Selkoe, D. J. Preventing Alzheimer’s disease. Science 337, 1488–1492 (2012).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012).
Prasher, V. P. et al. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 43, 380–383 (1998).
Lemere, C. A. et al. Sequence of deposition of heterogeneous amyloid β-peptides and Apo E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 3, 16–32 (1996).
Mann, D. M. & Iwatsubo, T. Diffuse plaques in the cerebellum and corpus striatum in Down’s syndrome contain amyloid beta protein (Aβ) only in the form of Aβ42(43). Neurodegeneration 5, 115–120 (1996).
Rovelet-Lecrux, A. et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 38, 24–26 (2006).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Castellano, J. M. et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57 (2011).
Zhang, Q. et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat. Commun. 11, 4799 (2020).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Kakuda, N. et al. γ-Secretase activity is associated with braak senile plaque stages. Am. J. Pathol. 190, 1323–1331 (2020).
Kakuda, N. et al. Altered gamma-secretase activity in mild cognitive impairment and Alzheimer’s disease. EMBO Mol. Med. 4, 344–352 (2012).
Huang, Z. et al. Proteomics of resilience to Alzheimer’s disease identifies brain regional soluble Aβ levels, actin filament processes, and response to injury. Preprint at bioRxiv https://doi.org/10.1101/2022.10.09.511430 (2022).
Glenner, G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (first of two parts). N. Engl. J. Med. 302, 1283–1292 (1980).
Selkoe, D. J. The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498 (1991).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Karran, E. & De Strooper, B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21, 306–318 (2022).
Haass, C. & Selkoe, D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 20, e3001694 (2022).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112, 415–422 (2003).
Serrano-Pozo, A. et al. Beneficial effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. Brain 133, 1312–1327 (2010).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Lord, A. et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 36, 425–434 (2009).
Salloway, S. et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73, 2061–2070 (2009).
Sperling, R. A. et al. Trial of solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 389, 1096–1107 (2023).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer Disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Johnson, K. et al. Biomarker assessments from Clarity AD: downstream implications of targeting protofibrils and tau as a predictive biomarker. In 16th Clinical Trialson Alzheimer’s Disease (ed. Shobha, D.) S9–S10 (CTAD, 2023).
Rafii, M. S. et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 19, 1227–1233 (2023).
Boxer, A. L. & Sperling, R. Accelerating Alzheimer’s therapeutic development: the past and future of clinical trials. Cell 186, 4757–4772 (2023).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10, 362–377 (2023).
Rosenberg, A., Mangialasche, F., Ngandu, T., Solomon, A. & Kivipelto, M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to world-wide FINGERS. J. Prev. Alzheimers Dis. 7, 29–36 (2020).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Ohki, Y. et al. Phenylpiperidine-type gamma-secretase modulators target the transmembrane domain 1 of presenilin 1. EMBO J. 30, 4815–4824 (2011).
Crump, C. J., Johnson, D. S. & Li, Y. M. Development and mechanism of γ-secretase modulators for Alzheimer’s disease. Biochemistry 52, 3197–3216 (2013).
Wagner, S. L. et al. Soluble γ-secretase modulators selectively inhibit the production of the 42-amino acid amyloid beta peptide variant and augment the production of multiple carboxy-truncated amyloid beta species. Biochemistry 53, 702–713 (2014).
Johnson, D. S., Li, Y.-M., Pettersson, M. & St George-Hyslop, P. H. Structural and chemical biology of presenilin complexes. Cold Spring Harb. Perspect. Med. 7, a024067 (2017).
Liu, L., Lauro, B. M., Wolfe, M. S. & Selkoe, D. J. Hydrophilic loop 1 of presenilin-1 and the APP GxxxG transmembrane motif regulate gamma-secretase function in generating Alzheimer-causing Abeta peptides. J. Biol. Chem. 296, 100393 (2021).
McGowan, E. et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47, 191–199 (2005).
Kim, J. et al. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 27, 627–633 (2007).
Sturm, S. RG6289, A new γ-secretase modulator for the treatment of alzheimer’s disease: results from a phase I healthy volunteer study. In 16th Clinical Trials on Alzheimer’s Disease (ed. Shobha, D.) S23-24 (CTAD, 2023).
Rynearson, K. D. et al. Preclinical validation of a potent γ-secretase modulator for Alzheimer’s disease prevention. J. Exp. Med. 218, e20202560 (2021).
Edwards, A. L. et al. Exploratory tau biomarker results from a multiple ascending-dose study of BIIB080 in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 80, 1344–1352 (2023).
Frenkel, D. et al. A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann. Neurol. 63, 591–601 (2008).
He, W. G. D. & Kowal, P. U.S. Census Bureau, International Population Reports P95/16-1, An Aging World: 2015 (US Government Publishing Office, 2016).
US Census Bureau. 2014 National Population Projections: Downloadable Files (2014).
Rajan, K. B. et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 17, 1966–1975 (2021).
Yaffe, K. et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Br. Med. J. 347, f7051 (2013).
Plassman, B. L. et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29, 125–132 (2007).
Alzheimer’s Association. Alzheimer’s Disease Facts and Figures 2024 (Alzheimer’s Association, 2024).
CDC WONDER Online Database: About Underlying Cause of Death, 1999–2020 (US Centers for Disease Control, accessed 9 December 2022).
US Burden of Disease Collaborators Collaborators et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA 319, 1444–1472 (2018).
Brunnstrom, H. R. & Englund, E. M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 16, 488–492 (2009).
Arrighi, H. M., Neumann, P. J., Lieberburg, I. M. & Townsend, R. J. Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis. Assoc. Disord. 24, 90–95 (2010).
Tejada-Vera, B. Mortality from Alzheimer’s disease in the United States: data for 2000 and 2010. NCHS Data Brief 1–8 (2013).
Schrijvers, E. M. et al. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78, 1456–1463 (2012).
Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars (Alzheimer’s Association, 2015).
Zissimopoulos, J., Crimmins, E. & St Clair, P. The value of delaying Alzheimer’s disease onset. Forum Health Econ. Policy 18, 25–39 (2014).
Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures (Alzheimer’s Association, 2024).
Hall, K. S. et al. Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement. 5, 227–233 (2009).
Dufour, A. B., Shaffer, M. L., D’Agata, E. M., Habtemariam, D. & Mitchell, S. L. Survival after suspected urinary tract infection in individuals with advanced dementia. J. Am. Geriatr. Soc. 63, 2472–2477 (2015).
Nicolle, L. E. Urinary tract infection in long-term-care facility residents. Clin. Infect. Dis. 31, 757–761 (2000).
Lin, P. J., Fillit, H. M., Cohen, J. T. & Neumann, P. J. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 9, 30–38 (2013).
Skaria, A. P. The economic and societal burden of Alzheimer disease: managed care considerations. Am. J. Manag. Care 28, S188–S196 (2022).
Albert, S. M. et al. Hospitalization and Alzheimer’s disease: results from a community-based study. J. Gerontol. A Biol. Sci. Med Sci. 54, M267–M271 (1999).
Fillit, H., Hill, J. W. & Futterman, R. Health care utilization and costs of Alzheimer’s disease: the role of co-morbid conditions, disease stage, and pharmacotherapy. Fam. Med. 34, 528–535 (2002).
Fanning, S. et al. Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for parkinson treatment. Mol. Cell. 73, 1001–1014(2019).
Nuber, S. et al. A stearoyl-coenzyme a dDesaturase inhibitor prevents multiple Parkinson disease phenotypes in α-synuclein mice. Ann. Neurol. 89, 74–90 (2021).
Author information
Authors and Affiliations
Contributions
D.J.S. is the only contributor to the piece.
Corresponding author
Ethics declarations
Competing interests
D.J.S. is a director of and consultant to Prothena Biosciences and an ad hoc consultant to Eisai.
Peer review
Peer review information
Nature Aging thanks Wiesje van der Flier, Todd Golde, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selkoe, D.J. The advent of Alzheimer treatments will change the trajectory of human aging. Nat Aging 4, 453–463 (2024). https://doi.org/10.1038/s43587-024-00611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00611-5



