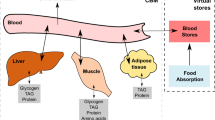
Abstract
Altered metabolic activity contributes to the pathogenesis of a number of diseases, including diabetes, heart failure, cancer, fibrosis and neurodegeneration. These diseases, and organismal metabolism more generally, are only partially recapitulated by cell culture models. Accordingly, it is important to measure metabolism in vivo. Over the past century, researchers studying glucose homeostasis have developed strategies for the measurement of tissue-specific and whole-body metabolic activity (pathway fluxes). The power of these strategies has been augmented by recent advances in metabolomics technologies. Here, we review techniques for measuring metabolic fluxes in intact mammals and discuss how to analyse and interpret the results. In tandem, we describe important findings from these techniques, and suggest promising avenues for their future application. Given the broad importance of metabolism to health and disease, more widespread application of these methods holds the potential to accelerate biomedical progress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F. & Whitehouse, C. M. Electrospray ionization—principles and practice. Mass Spectrom. Rev. 9, 37–70 (1990).
Tanaka, K. et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2, 151–153 (1988).
Wolfe, R. R. Tracers in Metabolic Research: Radioisotope and Stable Isotope/Mass Spectometry Methods (A.R. Liss, 1984).
McCabe, B. J. & Previs, S. F. Using isotope tracers to study metabolism: application in mouse models. Metab. Eng. 6, 25–35 (2004).
Fernández-García, J., Altea-Manzano, P., Pranzini, E. & Fendt, S.-M. Stable isotopes for tracing mammalian-cell metabolism in vivo. Trends Biochem. Sci. 45, 185–201 (2020).
Jain, M. et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336, 1040–1044 (2012).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Felig, P., Pozefsk, T., Marlis, E. & Cahill, G. F. Alanine: key role in gluconeogenesis. Science 167, 1003–1004 (1970).
Ivanisevic, J. et al. Arteriovenous blood metabolomics: a readout of intra-tissue metabostasis. Sci. Rep. 5, 12757 (2015).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Owen, O. E. et al. Brain metabolism during fasting. J. Clin. Invest. 46, 1589–1595 (1967).
Wilmore, D. W., Aulick, L. H., Mason, A. D. & Pruitt, B. A. Influence of the burn wound on local and systemic responses to injury. Ann. Surg. 186, 444–456 (1977).
Wilmore, D. W. et al. Effect of injury and infection on visceral metabolism and circulation. Ann. Surg. 192, 491–504 (1980).
Wahren, J., Felig, P., Ahlborg, G. & Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Invest. 50, 2715–2725 (1971).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Rennie, M. J. et al. Effect of exercise on protein turnover in man. Clin. Sci. 61, 627–639 (1981).
Taylor, R. et al. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J. Clin. Invest. 97, 126–132 (1996).
Wagenmakers, A. J. M. Tracers to investigate protein and amino acid metabolism in human subjects. Proc. Nutr. Soc. 58, 987–1000 (1999).
Ayala, J. E., Bracy, D. P., McGuinness, O. P. & Wasserman, D. H. Considerations in the design of hyperinsulinemic–euglycemic clamps in the conscious mouse. Diabetes 55, 390–397 (2006).
Hundal, R. S. et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 (2000).
Lee-Young, R. S. et al. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J. Biol. Chem. 284, 23925–23934 (2009).
Laughlin, M. H. & Armstrong, R. B. Muscular blood flow distribution patterns as a function of running speed in rats. Am. J. Physiol. Heart Circ. Physiol. 243, H296–H306 (1982).
Sjøberg, K. A., Rattigan, S., Hiscock, N., Richter, E. A. & Kiens, B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am. J. Physiol. Heart Circ. Physiol. 301, H450–H458 (2011).
Wei, K. et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97, 473–483 (1998).
Brown, R. P., Delp, M. D., Lindstedt, S. L., Rhomberg, L. R. & Beliles, R. P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13, 407–484 (1997).
Berne, R. M. Regulation of coronary blood flow. Physiol. Rev. 44, 1–29 (1964).
Høst, U. et al. Haemodynamic effects of eating: the role of meal composition. Clin. Sci. 90, 269–276 (1996).
Lang, C. H., Bagby, G. J., Ferguson, J. L. & Spitzer, J. J. Cardiac output and redistribution of organ blood flow in hypermetabolic sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 246, R331–R337 (1984).
Tabata, H., Kitamura, T. & Nagamatsu, N. Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab. Anim. 32, 143–148 (1998).
Ensinger, H., Weichel, T., Lindner, K. H., Grünert, A. & Ahnefeld, F. W. Effects of norepinephrine, epinephrine, and dopamine infusions on oxygen consumption in volunteers. Crit. Care Med. 21, 1502–1508 (1993).
Ghosal, S. et al. Mouse handling limits the impact of stress on metabolic endpoints. Physiol. Behav. 150, 31–37 (2015).
Schoenheimer, R. & Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism. 3. The role of the fat tissues. J. Biol. Chem. 111, 175–181 (1935).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Searle, G. L., Strisower, E. H. & Chaikoff, I. L. Glucose pool and glucose space in the normal and diabetic dog. Am. J. Physiol. 176, 190–194 (1954).
Searle, G. L., Strisower, E. H. & Chaikoff, I. L. Determination of rates of glucose oxidation in normal and diabetic dogs by a technique involving continuous Injection of C14-glucose. Am. J. Physiol. 185, 589–594 (1956).
Ayala, J. E. et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Models Mech. 3, 525–534 (2010).
Sherwin, R. S. et al. A model of the kinetics of insulin in man. J. Clin. Invest. 53, 1481–1492 (1974).
Stanley, W. C. et al. Lactate extraction during net lactate release in legs of humans during exercise. J. Appl. Physiol. 60, 1116–1120 (1986).
Okajima, F., Chenoweth, M., Rognstad, R., Dunn, A. & Katz, J. Metabolism of 3H- and 14C-labelled lactate in starved rats. Biochem. J. 194, 525–540 (1981).
Donovan, C. M. & Brooks, G. A. Endurance training affects lactate clearance, not lactate production. Am. J. Physiol. Endocrinol. Metab. 244, E83–E92 (1983).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Bergman, B. C. et al. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. Endocrinol. Metab. 277, E81–E92 (1999).
Bergman, B. C. et al. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 87, 1684–1696 (1999).
Sahlin, K. Lactate production cannot be measured with tracer techniques. Am. J. Physiol. Endocrinol. Metab. 252, E439–E440 (1987).
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005).
Landau, B. R. et al. Glycerol production and utilization in humans: sites and quantitation. Am. J. Physiol. Endocrinol. Metab. 271, E1110–E1117 (1996).
Klein, S., Young, V. R., Blackburn, G. L., Bistrian, B. R. & Wolfe, R. R. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J. Clin. Invest. 78, 928–933 (1986).
Perry, R. J. et al. Leptin mediates a glucose–fatty acid cycle to maintain glucose homeostasis in starvation. Cell 172, 234–248.e17 (2018).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429.e4 (2019).
Matthews, D. E. et al. Regulation of leucine metabolism in man: a stable isotope study. Science 214, 1129–1131 (1981).
Waterlow, J. C. Whole-body protein turnover in humans—past, present, and future. Annu. Rev. Nutr. 15, 57–92 (1995).
Wolfe, R. R., Goodenough, R. D., Burke, J. F. & Wolfe, M. H. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann. Surg. 197, 163–171 (1983).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688.e4 (2020).
Sprinson, D. B. & Rittenberg, D. The rate of interaction of the ammo acids of the diet with the tissue proteins. J. Biol. Chem. 180, 715–726 (1949).
Golden, S., Chenoweth, M., Dunn, A., Okajima, F. & Katz, J. Metabolism of tritium- and 14C-labeled alanine in rats. Am. J. Physiol. Endocrinol. Metab. 241, E121–E128 (1981).
Katz, J., Okajima, F., Chenoweth, M. & Dunn, A. The determination of lactate turnover in vivo with 3H- and 14C-labelled lactate. The significance of sites of tracer administration and sampling. Biochem. J. 194, 513–524 (1981).
Rendina, A. R., Hermes, J. D. & Cleland, W. W. Use of multiple isotope effects to study the mechanism of 6-phosphogluconate dehydrogenase. Biochemistry 23, 6257–6262 (1984).
Zhang, Z., Chen, L., Liu, L., Su, X. & Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. J. Am. Chem. Soc. 139, 14368–14371 (2017).
Schoenheimer, R. & Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism. 9. The conversion of stearic acid into palmitic acid in the organism. J. Biol. Chem. 120, 155–165 (1937).
Lau, A. N. et al. Dissecting cell-type-specific metabolism in pancreatic ductal adenocarcinoma. eLife 9, e56782 (2020).
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870.e5 (2019).
Zhang, L. et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 3, 402–413 (2019).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Mayers, J. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Brunt, E. M. et al. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 1, 15080 (2015).
Hudgins, L. C. et al. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J. Lipid Res. 41, 595–604 (2000).
Lambert, J. E., Ramos–Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Lu, W. et al. Metabolite measurement: pitfalls to avoid and practices to follow. Annu. Rev. Biochem. 86, 277–304 (2017).
TeSlaa, T. et al. The source of glycolytic intermediates in mammalian tissues. Cell Metab. 33, 367–378 (2021).
Previs, S. F. & Kelley, D. E. Tracer-based assessments of hepatic anaplerotic and TCA cycle flux: practicality, stoichiometry, and hidden assumptions. Am. J. Physiol. Endocrinol. Metab. 309, E727–E735 (2015).
Patgiri, A. et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD+ imbalance. Nat. Biotechnol. 38, 309–313 (2020).
Cham, C. M. & Gajewski, T. F. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 174, 4670–4677 (2005).
Flores, A. et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 19, 1017–1026 (2017).
Schell, J. C. et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 19, 1027–1036 (2017).
Ryan, D. J., Spraggins, J. M. & Caprioli, R. M. Protein identification strategies in MALDI imaging mass spectrometry: a brief review. Curr. Opin. Chem. Biol. 48, 64–72 (2019).
Abu-Remaileh, M. et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337.e11 (2016).
Lee, W. D., Mukha, D., Aizenshtein, E. & Shlomi, T. Spatial-fluxomics provides a subcellular-compartmentalized view of reductive glutamine metabolism in cancer cells. Nat. Commun. 10, 1351 (2019).
Orth, J. D., Thiele, I. & Palsson, B. Ø. What is flux balance analysis? Nat. Biotechnol. 28, 245–248 (2010).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012).
Lu, H. et al. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism. Nat. Commun. 10, 3586 (2019).
Blank, L. M., Kuepfer, L. & Sauer, U. Large-scale 13C-flux analysis reveals mechanistic principles of metabolic network robustness to null mutations in yeast. Genome Biol. 6, R49 (2005).
Shlomi, T., Cabili, M. N., Herrgård, M. J., Palsson, B. Ø. & Ruppin, E. Network-based prediction of human tissue-specific metabolism. Nat. Biotechnol. 26, 1003–1010 (2008).
Kacser, H., Burns, J. A., Kacser, H. & Fell, D. A. The control of flux. Biochem. Soc. Trans. 23, 341–366 (1995).
Emwas, A.-H. M. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. In Metabonomics: Methods and Protocols Vol. 1277 (ed. Bjerrum, J. T.) 161–193 (Humana, 2015).
Su, X., Lu, W. & Rabinowitz, J. D.Metabolite spectral accuracy on orbitraps.Anal. Chem. 89, 5940–5948 (2017).
Bajad, S. U. et al. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. A 1125, 76–88 (2006).
Zhang, Y. et al. Comparing stable isotope enrichment by gas chromatography with time-of-flight, quadrupole time-of-flight, and quadrupole mass spectrometry. Anal. Chem. 93, 2174–2182 (2021).
Perseghin, G. et al. Increased glucose transport—phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N. Engl. J. Med. 335, 1357–1362 (1996).
Zabielski, P. et al. Comparison of different mass spectrometry techniques in the measurement of L-[ring-13C6]phenylalanine incorporation into mixed muscle proteins. J. Mass Spectrom. 48, 269–275 (2013).
Wolfe, R. R. Measurement of urea kinetics in vivo by means of a constant tracer infusion of di-15N-urea. Am. J. Physiol. Endocrinol. Metab. 240, E428–E434 (1981).
Institute of Medicine (US) Committee on Military Nutrition Research. Emerging Technologies for Nutrition Research: Potential for Assessing Military Performance Capability (US National Academies Press, 1997).
Emwas, A.-H. et al. NMR spectroscopy for metabolomics research. Metabolites 9, 123 (2019).
Lin, P., Lane, A. N. & Fan, T. W.-M. Stable isotope-resolved metabolomics by NMR. Methods Mol. Biol. 2037, 151–168 (2019).
Roden, M. et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 97, 2859–2865 (1996).
Befroy, D. E. et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat. Med. 20, 98–102 (2014).
Mason, G. F. et al. Simultaneous determination of the rates of the TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cereb. Blood Flow. Metab. 15, 12–25 (1995).
Landau, B. R. et al. 14C-labeled propionate metabolism in vivo and estimates of hepatic gluconeogenesis relative to Krebs cycle flux. Am. J. Physiol. 265, E636–E647 (1993).
Brindle, K. M. Imaging metabolism with hyperpolarized 13C-labeled cell substrates. J. Am. Chem. Soc. 137, 6418–6427 (2015).
Witney, T. H. & Brindle, K. M. Imaging tumour cell metabolism using hyperpolarized 13C magnetic resonance spectroscopy. Biochem. Soc. Trans. 38, 1220–1224 (2010).
Deh, K. et al. Dynamic volumetric hyperpolarized 13C imaging with multi-echo EPI. Magn. Reson. Med. 85, 978–986 (2021).
Kernstine, K. H. et al. Does tumor FDG-PET avidity represent enhanced glycolytic metabolism in non-small cell lung cancer? Ann. Thorac. Surg. 109, 1019–1025 (2020).
Chen, D. L. et al. Increased T cell glucose uptake reflects acute rejection in lung grafts. Am. J. Transplant. 13, 2540–2549 (2013).
Frayn, K. N., Coppack, S. W., Humphreys, S. M., Clark, M. L. & Evans, R. D. Periprandial regulation of lipid metabolism in insulin-treated diabetes mellitus. Metabolism 42, 504–510 (1993).
DeFronzo, R. A., Tobin, J. D. & Andres, R.Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. Endocrinol. Metab. 237, E214–E223 (1979).
Elahi, D. In praise of the hyperglycemic clamp. A method for assessment of β-cell sensitivity and insulin resistance. Diabetes Care 19, 278–286 (1996).
Kraegen, E. W., James, D. E., Jenkins, A. B. & Chisholm, D. J. Dose–response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. Endocrinol. Metab. 248, E353–E362 (1985).
Bonora, E. et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23, 57–63 (2000).
Miyazaki, Y. et al. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 89, 4312–4319 (2004).
Michael, M. D. et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Bali, D. et al. Animal model for maturity-onset diabetes of the young generated by disruption of the mouse glucokinase gene. J. Biol. Chem. 270, 21464–21467 (1995).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Hellerstein, M. K. & Neese, R. A. Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am. J. Physiol. Endocrinol. Metab. 263, E988–E1001 (1992).
Stanhope, K. L. et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 119, 1322–1334 (2009).
Bloch, K. & Rittenberg, D. On the utilization of acetic acid for cholesterol formation. J. Biol. Chem. 145, 625–636 (1942).
Shulman, G. I. et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322, 223–228 (1990).
Daurio, N. A. et al. Spatial and temporal studies of metabolic activity: contrasting biochemical kinetics in tissues and pathways during fasted and fed states. Am. J. Physiol. Endocrinol. Metab. 316, E1105–E1117 (2019).
Yuan, J., Bennett, B. D. & Rabinowitz, J. D. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat. Protoc. 3, 1328–1340 (2008).
Acknowledgements
We thank Yihui Shen, Wenyun Lu, Michael Neinast, Asael Roichman and other members of the Rabinowitz laboratory for helpful discussion. This work was supported by NIH Pioneer award 5DP1DK113643, Diabetes Research Center grant P30 DK019525 and Paul G. Allen Family Foundation grant 0034665 to J.D.R., NIH fellowship F32DK118856 to T.T. and Damon Runyon Cancer Research Foundation fellowship DRG-2373-19 to C.R.B.
Author information
Authors and Affiliations
Contributions
C.R.B., T.T. and J.D.R. conceived and wrote the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Julian Griffin and Elizabeth Want for their contribution to the peer review of this work. Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bartman, C.R., TeSlaa, T. & Rabinowitz, J.D. Quantitative flux analysis in mammals. Nat Metab 3, 896–908 (2021). https://doi.org/10.1038/s42255-021-00419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00419-2
This article is cited by
-
Metabolic pathway analysis using stable isotopes in patients with cancer
Nature Reviews Cancer (2023)
-
Metabolite signaling in the heart
Nature Cardiovascular Research (2023)
-
Endogenous renal adiponectin drives gluconeogenesis through enhancing pyruvate and fatty acid utilization
Nature Communications (2023)
-
Metabolic flux between organs measured by arteriovenous metabolite gradients
Experimental & Molecular Medicine (2022)
-
Tracing the lactate shuttle to the mitochondrial reticulum
Experimental & Molecular Medicine (2022)