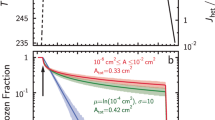
Abstract
Atmospheric ice nucleation is crucial for global precipitation and affects the structure, lifetime and reflectivity of clouds, thereby impacting climate. Ice nucleates in various ways from aerosol particles, termed ice-nucleating particles, over an extensive temperature and humidity range. Quantifying the kinetic and thermodynamic regimes of nucleation is necessary to relate fundamental physics to theoretically based predictions of ice formation for implementation in cloud and climate models. We review how the molecular picture of ice nucleation has advanced in recent years and consequential impacts on the interpretation and parameterization of ice nucleation. Advances include the role of interfacial free energy and pressure on ice nucleation rates, mobility regions of water that generate the critical ice nucleus, classical and non-classical pathways of nucleation, the type of ice polymorph that forms, the impact of solutes on freezing and the role of nanopores as surface features promoting ice nucleation. We also introduce currently debated and evaluated freezing parameterizations for application in model environments. Finally, we outline what we believe are the current needs for improving predictive understanding of ice nucleation.
Key points
-
Homogeneous ice nucleation is affected by pressure, temperature and solute strength and is predictable using water activity. However, research into the dynamics of water molecules and ice germ physicochemical properties is needed to better constrain nucleation parameters.
-
Heterogeneous ice nucleation from nano-sized confinements can lead to the formation of cubic ice or stacking disordered ice. Which ice phase forms impacts nucleation rate derivations owing to differences in the free energy of formation of the critical ice nucleus.
-
The water activity criterion is a thermodynamic predictor of kinetic ice nucleation for both homogeneous and heterogeneous ice nucleation. It can be applied to describe immersion freezing and reveals a direct relationship to the water structure at the ice-nucleating particle surface or the contact angle, allowing for interpretation of the interaction between the ice-nucleating particle and the critical ice nucleus.
-
Water in nanometre-sized confinements at the surface of aerosol particles can serve as the origin of ice formation. Pore condensation freezing in nanometre-sized pores can explain ice formation by particles in the absence of a coverage of liquid water.
-
Although nucleation is a stochastically driven process, an unambiguous evaluation of the underlying physical processes from experimental data is challenging. This is because it is difficult to obtain nanoscale control of the physicochemical properties of the nucleating substrate while observing a significant number of ice nucleation events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abbott, B. W. et al. Human domination of the global water cycle absent from depictions and perceptions. Nat. Geosci. 12, 533 (2019).
Boucher, O. et al. in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Storelvmo, T. in Annual Review of Earth and Planetary Sciences Vol. 45 (eds Jeanloz, R. & Freeman, K. H.) 199–222 (Annual Reviews, 2017).
Murray, B. J. & Liu, X. in Aerosols and Climate (ed. Carslaw, K. S.) Ch. 15, 619–649 (Elsevier, 2022).
Gasparini, B., Meyer, A., Neubauer, D., Munch, S. & Lohmann, U. Cirrus cloud properties as seen by the CALIPSO satellite and ECHAM-HAM global climate model. J. Clim. 31, 1983–2003 (2018).
Korolev, A. Limitations of the Wegener–Bergeron–Findeisen mechanism in the evolution of mixed-phase clouds. J. Atmos. Sci. 64, 3372–3375 (2007).
Mülmenstädt, J., Sourdeval, O., Delanoe, J. & Quaas, J. Frequency of occurrence of rain from liquid-, mixed-, and ice-phase clouds derived from A-train satellite retrievals. Geophys. Res. Lett. 42, 6502–6509 (2015).
Lau, K. M. & Wu, H. T. Warm rain processes over tropical oceans and climate implications. Geophys. Res. Lett. 30, 2290 (2003).
Lohmann, U. & Feichter, J. Global indirect aerosol effects: a review. Atmos. Chem. Phys. 5, 715–737 (2005).
Held, I. M. & Soden, B. J. Water vapor feedback and global warming. Annu. Rev. Energ. Env. 25, 441–475 (2000).
Cantrell, W. & Heymsfield, A. Production of ice in tropospheric clouds — a review. Bull. Am. Meteorol. Soc. 86, 795–807 (2005).
Chen, T., Rossow, W. B. & Zhang, Y. C. Radiative effects of cloud-type variations. J. Clim. 13, 264–286 (2000).
Pruppacher, H. R. & Klett, J. D. Microphysics of Clouds and Precipitation (Kluwer Academic Publishers, 1997).
Hegg, D. A. & Baker, M. B. Nucleation in the atmosphere. Rep. Prog. Phys. 72, 056801 (2009).
Seinfeld, J. H. & Pandis, S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change 2nd edn (Wiley-Interscience, 2006).
Pöschl, U. Atmospheric aerosols: composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 44, 7520–7540 (2005).
Fröhlich-Nowoisky, J. et al. Bioaerosols in the earth system: climate, health, and ecosystem interactions. Atmos. Res. 182, 346–376 (2016).
Knopf, D. A., Alpert, P. A. & Wang, B. The role of organic aerosol in atmospheric ice nucleation: a review. ACS Earth Space Chem. 2, 168–202 (2018).
Kanji, Z. A. et al. in Ice Formation and Evolution in Clouds and Precipitation: Measurement and Modeling Challenges Vol. 58 Meteorological Monographs Ch. 1, 1.1–1.33 (American Meteorological Society, 2017).
Cziczo, D. J. et al. in Ice Formation and Evolution in Clouds and Precipitation: Measurement and Modeling Challenges Vol. 58, Ch. 8, 8.1–8.13 (American Meteorological Society, 2017).
Yang, Y. & Liu, R. Anthropogenic aerosols effects on ice clouds: a review. Atmosphere 13, 9 (2022).
Vali, G., DeMott, P. J., Möhler, O. & Whale, T. F. Technical note: a proposal for ice nucleation terminology. Atmos. Chem. Phys. 15, 10263–10270 (2015).
Koop, T. Homogeneous ice nucleation in water and aqueous solutions. Z. Phys. Chem. Int. J. Res. Phys. Chem. Chem. Phys. 218, 1231–1258 (2004).
de Boer, G., Morrison, H., Shupe, M. D. & Hildner, R. Evidence of liquid dependent ice nucleation in high-latitude stratiform clouds from surface remote sensors. Geophys. Res. Lett. 38, L01803 (2011).
Ansmann, A. et al. Dust and smoke transport from Africa to South America: lidar profiling over Cape Verde and the Amazon rainforest. Geophys. Res. Lett. 36, L11802 (2009).
David, R. O. et al. Pore condensation and freezing is responsible for ice formation below water saturation for porous particles. Proc. Natl Acad. Sci. USA 116, 8184–8189 (2019).
Marcolli, C. Technical note: fundamental aspects of ice nucleation via pore condensation and freezing including Laplace pressure and growth into macroscopic ice. Atmos. Chem. Phys. 20, 3209–3230 (2020).
Marcolli, C. Deposition nucleation viewed as homogeneous or immersion freezing in pores and cavities. Atmos. Chem. Phys. 14, 2071–2104 (2014).
Ladino Moreno, L. A., Stetzer, O. & Lohmann, U. Contact freezing: a review of experimental studies. Atmos. Chem. Phys. 13, 9745–9769 (2013).
Hussain, S. & Haji-Akbari, A. Role of nanoscale interfacial proximity in contact freezing in water. J. Am. Chem. Soc. 143, 2272–2284 (2021).
Yang, F., Cantrell, W. H., Kostinski, A. B., Shaw, R. A. & Vogelmann, A. M. Is contact nucleation caused by pressure perturbation. Atmosphere 11, 16 (2020).
Niehaus, J. et al. A technique to measure ice nuclei in the contact mode. J. Atmos. Ocean. Technol. 31, 913–922 (2014).
Hoffmann, N., Duft, D., Kiselev, A. & Leisner, T. Contact freezing efficiency of mineral dust aerosols studied in an electrodynamic balance: quantitative size and temperature dependence for illite particles. Faraday Discuss. 165, 383–390 (2013).
Shaw, R. A., Durant, A. J. & Mi, Y. Heterogeneous surface crystallization observed in undercooled water. J. Phys. Chem. B 109, 9865–9868 (2005).
Gurganus, C., Kostinski, A. B. & Shaw, R. A. Fast imaging of freezing drops: no preference for nucleation at the contact line. J. Phys. Chem. Lett. 2, 1449–1454 (2011).
Durant, A. J. & Shaw, R. A. Evaporation freezing by contact nucleation inside-out. Geophys. Res. Lett. 32, L20814 (2005).
Korolev, A. & Leisner, T. Review of experimental studies of secondary ice production. Atmos. Chem. Phys. 20, 11767–11797 (2020).
Field, P. R. et al. in Ice Formation and Evolution in Clouds and Precipitation: Measurement and Modeling Challenges Vol. 58, Ch. 7, 7.1–7.20 (American Meteorological Society, 2017).
Zipori, A. et al. The role of secondary ice processes in mid‐latitude continental clouds. J. Geophys. Res. 123, 12762–12777 (2018).
Phillips, V. T. J., Patade, S., Gutierrez, J. & Bansemer, A. Secondary ice production by fragmentation of freezing drops: formulation and theory. J. Atmos. Sci. 75, 3031–3070 (2018).
Korolev, A. et al. A new look at the environmental conditions favorable to secondary ice production. Atmos. Chem. Phys. 20, 1391–1429 (2020).
Murphy, D. M. & Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. Roy. Meteor. Soc. 131, 1539–1565 (2005).
DeMott, P. J. et al. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 107, 11217–11222 (2010).
Salzmann, C. G., Loveday, J. S., Rosu-Finsen, A. & Bull, C. L. Structure and nature of ice XIX. Nat. Commun. 12, 7 (2021).
Rosu-Finsen, A. et al. Medium-density amorphous ice. Science 379, 474–478 (2023).
Millot, M. et al. Nanosecond X-ray diffraction of shock-compressed superionic water ice. Nature 569, 251–255 (2019).
Yamane, R. et al. Experimental evidence for the existence of a second partially-ordered phase of ice VI. Nat. Commun. 12, 6 (2021).
Prakapenka, V. B., Holtgrewe, N., Lobanov, S. S. & Goncharov, A. F. Structure and properties of two superionic ice phases. Nat. Phys. 17, 1233 (2021).
Malkin, T. L., Murray, B. J., Brukhno, A. V., Anwar, J. & Salzmann, C. G. Structure of ice crystallized from supercooled water. Proc. Natl. Acad. Sci. USA 109, 1041–1045 (2012).
del Rosso, L. et al. Cubic ice Ic without stacking defects obtained from ice XVII. Nat. Mater. 19, 663–668 (2020).
Malkin, T. L. et al. Stacking disorder in ice I. Phys. Chem. Chem. Phys. 17, 60–76 (2015).
Koop, T., Luo, B. P., Tsias, A. & Peter, T. Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 406, 611–614 (2000).
Burrows, S. M. et al. Ice-nucleating particles that impact clouds and climate: observational and modeling research needs. Rev. Geophys. 60, 45 (2022).
Knopf, D. A. et al. Aerosol–ice formation closure: a southern great plains field campaign. Bull. Am. Meteorol. Soc. 102, E1952–E1971 (2021).
Amaya, A. J. & Wyslouzil, B. E. Ice nucleation rates near similar to 225 K. J. Chem. Phys. 148, 9 (2018).
Fitzner, M., Sosso, G. C., Cox, S. J. & Michaelides, A. Ice is born in low-mobility regions of supercooled liquid water. Proc. Natl. Acad. Sci. USA 116, 2009–2014 (2019).
Li, C., Liu, Z., Goonetilleke, E. C. & Huang, X. H. Temperature-dependent kinetic pathways of heterogeneous ice nucleation competing between classical and non-classical nucleation. Nat. Commun. 12, 9 (2021).
Barahona, D. On the thermodynamic and kinetic aspects of immersion ice nucleation. Atmos. Chem. Phys. 18, 17119–17141 (2018).
Schwidetzky, R. et al. Specific ion-protein interactions influence bacterial ice nucleation. Chem. Eur. J. 27, 7402–7407 (2021).
David, R. O. et al. The role of contact angle and pore width on pore condensation and freezing. Atmos. Chem. Phys. 20, 9419–9440 (2020).
Mohammed, S., Asgar, H., Benmore, C. J. & Gadikota, G. Structure of ice confined in silica nanopores. Phys. Chem. Chem. Phys. 23, 12706–12717 (2021).
Knopf, D. A., Alpert, P. A., Zipori, A., Reicher, N. & Rudich, Y. Stochastic nucleation processes and substrate abundance explain time-dependent freezing in supercooled droplets. npj. Clim. Atmos. Sci. 3, 2 (2020).
Turnbull, D. & Fisher, J. C. Rate of nucleation in condensed systems. J. Chem. Phys. 17, 71–73 (1949).
Laidler, K. J. & King, M. C. The development of transition-state theory. J. Phys. Chem. 87, 2657–2664 (1983).
Espinosa, J. R. et al. Interfacial free energy as the key to the pressure-induced deceleration of ice nucleation. Phys. Rev. Lett. 117, 6 (2016).
Barahona, D. Analysis of the effect of water activity on ice formation using a new thermodynamic framework. Atmos. Chem. Phys. 14, 7665–7680 (2014).
Knopf, D. A. & Rigg, Y. J. Homogeneous ice nucleation from aqueous inorganic/organic particles representative of biomass burning: water activity, freezing temperatures, nucleation rates. J. Phys. Chem. A 115, 762–773 (2011).
Alpert, P. A., Aller, J. Y. & Knopf, D. A. Initiation of the ice phase by marine biogenic surfaces in supersaturated gas and supercooled aqueous phases. Phys. Chem. Chem. Phys. 13, 19882–19894 (2011).
Bullock, G. & Molinero, V. Low-density liquid water is the mother of ice: on the relation between mesostructure, thermodynamics and ice crystallization in solutions. Faraday Discuss. 167, 371–388 (2013).
Murata, K. & Tanaka, H. General nature of liquid–liquid transition in aqueous organic solutions. Nat. Commun. 4, 2844 (2013).
Knopf, D. A. & Alpert, P. A. A water activity based model of heterogeneous ice nucleation kinetics for freezing of water and aqueous solution droplets. Faraday Discuss. 165, 513–534 (2013).
Baker, M. B. & Baker, M. A new look at homogeneous freezing of water. Geophys. Res. Lett. 31, L19102 (2004).
Berkemeier, T., Shiraiwa, M., Pöschl, U. & Koop, T. Competition between water uptake and ice nucleation by glassy organic aerosol particles. Atmos. Chem. Phys. 14, 12513–12531 (2014).
Koop, T., Bookhold, J., Shiraiwa, M. & Pöschl, U. Glass transition and phase state of organic compounds: dependency on molecular properties and implications for secondary organic aerosols in the atmosphere. Phys. Chem. Chem. Phys. 13, 19238–19255 (2011).
Shiraiwa, M. et al. Global distribution of particle phase state in atmospheric secondary organic aerosols. Nat. Commun. 8, 15002 (2017).
Barahona, D. Thermodynamic derivation of the activation energy for ice nucleation. Atmos. Chem. Phys. 15, 13819–13831 (2015).
Mishima, O. Relationship between melting and amorphization of ice. Nature 384, 546–549 (1996).
Kanno, H. & Angell, C. A. Homogeneous nucleation and glass formation in aqueous alkali-halide solutions at high-pressures. J. Phys. Chem. 81, 2639–2643 (1977).
Kanno, H., Speedy, R. J. & Angell, C. A. Supercooling of water to −92 degrees C under pressure. Science 189, 880–881 (1975).
Cai, Y. Q. et al. Ordering of hydrogen bonds in high-pressure low-temperature H2O. Phys. Rev. Lett. 94, 4 (2005).
Laksmono, H. et al. Anomalous behavior of the homogeneous ice nucleation rate in ‘No-Man’s Land’. J. Phys. Chem. Lett. 6, 2826–2832 (2015).
Prielmeier, F. X., Lang, E. W., Speedy, R. J. & Ludemann, H. D. The pressure-dependence of self-diffusion in supercooled light and heavy water. Ber. Bunsen-Ges. Phys. Chem. Chem. Phys. 92, 1111–1117 (1988).
Koop, T. & Murray, B. J. A physically constrained classical description of the homogeneous nucleation of ice in water. J. Chem. Phys. 145, 211915 (2016).
Espinosa, J. R., Navarro, C., Sanz, E., Valeriani, C. & Vega, C. On the time required to freeze water. J. Chem. Phys. 145, 14 (2016).
Espinosa, J. R., Vega, C. & Sanz, E. Ice-water interfacial free energy for the TIP4P, TIP4P/2005, TIP4P/Ice, and mW models as obtained from the mold integration technique. J. Phys. Chem. C 120, 8068–8075 (2016).
Xu, L. M. et al. Relation between the Widom line and the dynamic crossover in systems with a liquid–liquid phase transition. Proc. Natl. Acad. Sci. USA 102, 16558–16562 (2005).
Kim, K. H. et al. Maxima in the thermodynamic response and correlation functions of deeply supercooled water. Science 358, 1589–1593 (2017).
Lupi, L. et al. Role of stacking disorder in ice nucleation. Nature 551, 218–222 (2017).
Zobrist, B., Koop, T., Luo, B. P., Marcolli, C. & Peter, T. Heterogeneous ice nucleation rate coefficient of water droplets coated by a nonadecanol monolayer. J. Phys. Chem. C. 111, 2149–2155 (2007).
Khvorostyanov, V. I. & Curry, J. A. Thermodynamic theory of freezing and melting of water and aqueous solutions. J. Phys. Chem. A 108, 11073–11085 (2004).
Barahona, D. On the ice nucleation spectrum. Atmos. Chem. Phys. 12, 3733–3752 (2012).
Maattanen, A. et al. Nucleation studies in the Martian atmosphere. J. Geophys. Res. Planets 110, 12 (2005).
Wang, B. & Knopf, D. A. Heterogeneous ice nucleation on particles composed of humic-like substances impacted by O3. J. Geophys. Res. 116, D03205 (2011).
Metya, A. K. & Molinero, V. Is ice nucleation by organic crystals nonclassical? An assessment of the monolayer hypothesis of ice nucleation. J. Am. Chem. Soc. 143, 4607–4624 (2021).
Davies, M. B., Fitzner, M. & Michaelides, A. Routes to cubic ice through heterogeneous nucleation. Proc. Natl Acad. Sci. USA 118, 8 (2021).
China, S. et al. Ice cloud formation potential by free tropospheric particles from long-range transport over the Northern Atlantic Ocean. J. Geophys. Res. 122, 3065–3079 (2017).
Alpert, P. A. et al. Ice nucleation imaged with X-ray spectro-microscopy. Environ. Sci. Atmos. 2, 335–351 (2022).
Knopf, D. A. et al. Micro-spectroscopic and freezing characterization of ice-nucleating particles collected in the marine boundary layer in the eastern North Atlantic. Atmos. Chem. Phys. 22, 5377–5398 (2022).
Alpert, P. A. et al. Ice-nucleating agents in sea spray aerosol identified and quantified with a holistic multimodal freezing model. Sci. Adv. 8, eabq6842 (2022).
Gavish, M., Popovitzbiro, R., Lahav, M. & Leiserowitz, L. Ice nucleation by alcohols arranged in monolayers at the surface of water drops. Science 250, 973–975 (1990).
Cantrell, W. & Robinson, C. Heterogeneous freezing of ammonium sulfate and sodium chloride solutions by long chain alcohols. Geophys. Res. Lett. 33, L07802 (2006).
Knopf, D. A. & Forrester, S. M. Freezing of water and aqueous NaCl droplets coated by organic monolayers as a function of surfactant properties and water activity. J. Phys. Chem. A 115, 5579–5591 (2011).
Yang, H. Y. et al. Ordered hydrogen bonding structure of water molecules adsorbed on silver iodide particles under subsaturated conditions. J. Phys. Chem. C 125, 11628–11635 (2021).
Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 24, 247–260 (1888).
Cacace, M. G., Landau, E. M. & Ramsden, J. J. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 30, 241–277 (1997).
Kumar, A., Marcolli, C. & Peter, T. Ice nucleation activity of silicates and aluminosilicates in pure water and aqueous solutions — part 2: quartz and amorphous silica. Atmos. Chem. Phys. 19, 6035–6058 (2019).
Kumar, A., Marcolli, C. & Peter, T. Ice nucleation activity of silicates and aluminosilicates in pure water and aqueous solutions — part 3: aluminosilicates. Atmos. Chem. Phys. 19, 6059–6084 (2019).
Kumar, A., Marcolli, C., Luo, B. P. & Peter, T. Ice nucleation activity of silicates and aluminosilicates in pure water and aqueous solutions — part 1: the K-feldspar microcline. Atmos. Chem. Phys. 18, 7057–7079 (2018).
Whale, T. F., Holden, M. A., Wilson, T. W., O’Sullivan, D. & Murray, B. J. The enhancement and suppression of immersion mode heterogeneous ice-nucleation by solutes. Chem. Sci. 9, 6313 (2018).
Kumar, A., Bertram, A. K. & Patey, G. N. Molecular simulations of feldspar surfaces interacting with aqueous inorganic solutions: interfacial water/ion structure and implications for ice nucleation. ACS Earth Space Chem. 5, 2169–2183 (2021).
Whale, T. F. Disordering effect of the ammonium cation accounts for anomalous enhancement of heterogeneous ice nucleation. J. Chem. Phys. 156, 14 (2022).
Wang, B. et al. Direct observation of ice nucleation events on individual atmospheric particles. Phys. Chem. Chem. Phys. 18, 29721–29731 (2016).
Zimmermann, F., Ebert, M., Worringen, A., Schutz, L. & Weinbruch, S. Environmental scanning electron microscopy (ESEM) as a new technique to determine the ice nucleation capability of individual atmospheric aerosol particles. Atmos. Environ. 41, 8219–8227 (2007).
Kiselev, A. et al. Active sites in heterogeneous ice nucleation — the example of K-rich feldspars. Science 355, 367–371 (2017).
Friddle, R. W. & Thürmer, K. Mapping ice formation to mineral-surface topography using a micro mixing chamber with video and atomic-force microscopy. Atmos. Meas. Tech. 13, 2209–2218 (2020).
Holden, M. A. et al. High-speed imaging of ice nucleation in water proves the existence of active sites. Sci. Adv. 5, eaav4316 (2019).
Mahrt, F. et al. Aging induced changes in ice nucleation activity of combustion aerosol as determined by near edge X-ray absorption fine structure (NEXAFS) spectroscopy. Environ. Sci. Process. Impacts 22, 895–907 (2020).
Holden, M. A., Campbell, J. M., Meldrum, F. C., Murray, B. J. & Christenson, H. K. Active sites for ice nucleation differ depending on nucleation mode. Proc. Natl. Acad. Sci. USA 118, 9 (2021).
Friddle, R. W. & Thürmer, K. How nanoscale surface steps promote ice growth on feldspar: microscopy observation of morphology-enhanced condensation and freezing. Nanoscale 11, 21147–21154 (2019).
Knopf, D. A. et al. Microspectroscopic imaging and characterization of individually identified ice nucleating particles from a case field study. J. Geophys. Res. 119, 10365–10381 (2014).
Fukuta, N. Activation of atmospheric particles as ice nuclei in cold and dry air. J. Atmos. Sci. 23, 741–750 (1966).
Marcolli, C. Pre-activation of aerosol particles by ice preserved in pores. Atmos. Chem. Phys. 17, 1596–1623 (2017).
Gao, K. F., Zhou, C. W., Meier, E. J. B. & Kanji, Z. A. Laboratory studies of ice nucleation onto bare and internally mixed soot-sulfuric acid particles. Atmos. Chem. Phys. 22, 5331–5364 (2022).
Gao, K. F., Friebel, F., Zhou, C. W. & Kanji, Z. A. Enhanced soot particle ice nucleation ability induced by aggregate compaction and densification. Atmos. Chem. Phys. 22, 4985–5016 (2022).
Marcolli, C., Mahrt, F. & Karcher, B. Soot PCF: pore condensation and freezing framework for soot aggregates. Atmos. Chem. Phys. 21, 7791–7843 (2021).
Campbell, J. M. & Christenson, H. K. Nucleation- and emergence-limited growth of ice from pores. Phys. Rev. Lett. 120, 5 (2018).
Campbell, J. M., Meldrum, F. C. & Christenson, H. K. Observing the formation of ice and organic crystals in active sites. Proc. Natl. Acad. Sci. USA 114, 810–815 (2017).
Roudsari, G., Pakarinen, O. H., Reischl, B. & Vehkamaki, H. Atomistic and coarse-grained simulations reveal increased ice nucleation activity on silver iodide surfaces in slit and wedge geometries. Atmos. Chem. Phys. 22, 10099–10114 (2022).
Vali, G. Quantitative evaluation of experimental results on heterogeneous freezing nucleation of supercooled liquids. J. Atmos. Sci. 28, 402–409 (1971).
Connolly, P. J. et al. Studies of heterogeneous freezing by three different desert dust samples. Atmos. Chem. Phys. 9, 2805–2824 (2009).
Alpert, P. A. & Knopf, D. A. Analysis of isothermal and cooling-rate-dependent immersion freezing by a unifying stochastic ice nucleation model. Atmos. Chem. Phys. 16, 2083–2107 (2016).
Coluzza, I. et al. Perspectives on the future of ice nucleation research: research needs and unanswered questions identified from two International Workshops. Atmosphere https://doi.org/10.3390/atmos8080138 (2017).
Bauer, S. E. et al. MATRIX (multiconfiguration aerosol tracker of mixing state): an aerosol microphysical module for global atmospheric models. Atmos. Chem. Phys. 8, 6003–6035 (2008).
Liu, X. et al. Description and evaluation of a new four-mode version of the Modal Aerosol Module (MAM4) within version 5.3 of the Community Atmosphere Model. Geosci. Model. Dev. 9, 505–522 (2016).
Fan, J. W., Wang, Y., Rosenfeld, D. & Liu, X. H. Review of aerosol–cloud interactions: mechanisms, significance, and challenges. J. Atmos. Sci. 73, 4221–4252 (2016).
Ke, Z. M., Liu, X. H., Wu, M. X., Shan, Y. P. & Shi, Y. Improved dust representation and impacts on dust transport and radiative effect in CAM5. J. Adv. Model. Earth Syst. 14, 24 (2022).
Zhao, X., Liu, X. H., Burrows, S. M. & Shi, Y. Effects of marine organic aerosols as sources of immersion-mode ice-nucleating particles on high-latitude mixed-phase clouds. Atmos. Chem. Phys. 21, 2305–2327 (2021).
Fridlind, A. M. et al. Ice properties of single-layer stratocumulus during the mixed-phase arctic cloud experiment: 2. Model results. J. Geophys. Res. 112, D05208 (2007).
Morrison, H. et al. Intercomparison of model simulations of mixed-phase clouds observed during the ARM mixed-phase arctic cloud experiment. II: multilayer cloud. Q. J. R. Meteorol. Soc. 135, 1003–1019 (2009).
Yang, F., Ovchinnikov, M. & Shaw, R. A. Minimalist model of ice microphysics in mixed-phase stratiform clouds. Geophys. Res. Lett. 40, 3756–3760 (2013).
Fridlind, A. M. & Ackerman, A. S. in Mixed-Phase Clouds: Observation and Modeling (ed. Andronache, C.) Ch. 7, 153–182 (Elsevier, 2018).
Fridlind, A. M. et al. A FIRE-ACE/SHEBA case study of mixed-phase arctic boundary layer clouds: entrainment rate limitations on rapid primary ice nucleation processes. J. Atmos. Sci. 69, 365–389 (2012).
Savre, J. & Ekman, A. M. L. Large-eddy simulation of three mixed-phase cloud events during ISDAC: conditions for persistent heterogeneous ice formation. J. Geophys. Res. 120, 7699–7725 (2015).
Solch, I. & Karcher, B. A large-eddy model for cirrus clouds with explicit aerosol and ice microphysics and Lagrangian ice particle tracking. Q. J. R. Meteorol. Soc. 136, 2074–2093 (2010).
Harrington, J. Y. & Olsson, P. Q. On the potential influence of ice nuclei on surface-forced marine stratocumulus cloud dynamics. J. Geophys. Res. 106, 27473–27484 (2001).
Solomon, A. et al. The relative impact of cloud condensation nuclei and ice nucleating particle concentrations on phase partitioning in Arctic mixed-phase stratocumulus clouds. Atmos. Chem. Phys. 18, 17047–17059 (2018).
Ovchinnikov, M. et al. Intercomparison of large-eddy simulations of Arctic mixed-phase clouds: importance of ice size distribution assumptions. J. Adv. Model. Earth Syst. 6, 223–248 (2014).
Westbrook, C. D. & Illingworth, A. J. The formation of ice in a long-lived supercooled layer cloud. Q. J. Roy. Meteor. Soc. 139, 2209–2221 (2013).
Murray, B. J. et al. Kinetics of the homogeneous freezing of water. Phys. Chem. Chem. Phys. 12, 10380–10387 (2010).
Riechers, B., Wittbracht, F., Hutten, A. & Koop, T. The homogeneous ice nucleation rate of water droplets produced in a microfluidic device and the role of temperature uncertainty. Phys. Chem. Chem. Phys. 15, 5873–5887 (2013).
Stöckel, P., Weidinger, I. M., Baumgärtel, H. & Leisner, T. Rates of homogeneous ice nucleation in levitated H2O and D2O droplets. J. Phys. Chem. A 109, 2540–2546 (2005).
Stan, C. A. et al. A microfluidic apparatus for the study of ice nucleation in supercooled water drops. Lab Chip 9, 2293–2305 (2009).
Hagen, D. E., Anderson, R. J. & Kassner, J. L. Homogeneous condensation-freezing nucleation rate measurements for small water droplets in an expansion cloud chamber. J. Atmos. Sci. 38, 1236–1243 (1981).
Bhabhe, A., Pathak, H. & Wyslouzil, B. E. Freezing of heavy water (D2O) nanodroplets. J. Phys. Chem. A 117, 5472–5482 (2013).
Bartell, L. S. & Chushak, Y. G. in Water in Confining Geometries Springer Series in Cluster Physics (eds Buch, V. & Devlin, J. P.) Ch. 16 (Springer, 2003).
Koop, T., Luo, B. P., Biermann, U. M., Crutzen, P. J. & Peter, T. Freezing of HNO3/H2SO4/H2O solutions at stratospheric temperatures: nucleation statistics and experiments. J. Phys. Chem. A 101, 1117–1133 (1997).
Acknowledgements
This study was supported by the Atmospheric System Research Program and Atmospheric Radiation Measurement Program sponsored by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Climate and Environmental Sciences Division, grant DE-SC0021034. The authors are grateful to J. Aller for careful proofreading of the initial manuscript.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Physics thanks Xiaohong Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knopf, D.A., Alpert, P.A. Atmospheric ice nucleation. Nat Rev Phys 5, 203–217 (2023). https://doi.org/10.1038/s42254-023-00570-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42254-023-00570-7
This article is cited by
-
Observing growth and interfacial dynamics of nanocrystalline ice in thin amorphous ice films
Nature Communications (2024)