Abstract
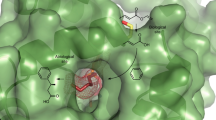
Artificial enzymes, which are hybrids of proteins with abiological catalytic groups, have emerged as a powerful approach towards the creation of enzymes for new-to-nature reactions. Typically, only a single abiological catalytic moiety is incorporated. Here we introduce a design of an artificial enzyme that comprises two different abiological catalytic moieties and show that these can act synergistically to achieve high activity and enantioselectivity (up to >99% e.e.) in the catalysed Michael addition reaction. The design is based on the lactococcal multidrug resistance regulator as the protein scaffold and combines a genetically encoded unnatural p-aminophenylalanine residue (which activates an enal through iminium ion formation) and a supramolecularly bound Lewis acidic Cu(ii) complex (which activates the Michael donor by enolization and delivers it to one preferred prochiral face of the activated enal). This study demonstrates that synergistic combination of abiological catalytic groups is a robust way to achieve catalysis that is normally outside of the realm of artificial enzymes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All data that support the findings of this study are available in the Article and its Supplementary Information, or from the corresponding author on reasonable request.
References
Hilvert, D. Design of protein catalysts. Annu. Rev. Biochem. 82, 447–470 (2013).
Nanda, V. & Koder, R. L. Designing artificial enzymes by intuition and computation. Nat. Chem. 2, 15–24 (2010).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Drienovská, I., Mayer, C., Dulson, C. & Roelfes, G. A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue. Nat. Chem. 10, 946–952 (2018).
Mayer, C., Dulson, C., Reddem, E., Thunnissen, A. M. W. H. & Roelfes, G. Directed evolution of a designer enzyme featuring an unnatural catalytic amino acid. Angew. Chem. Int. Ed. 58, 2083–2087 (2019).
Nödling, A. R. et al. Reactivity and selectivity of iminium organocatalysis improved by a protein host. Angew. Chem. Int. Ed. 57, 12478–12482 (2018).
Burke, A. J. et al. Design and evolution of an enzyme with a non-canonical organocatalytic mechanism. Nature 570, 219–223 (2019).
Markel, U., Sauer, D. F., Schiffels, J., Okuda, J. & Schwaneberg, U. Towards the evolution of artificial metalloenzymes—a protein engineer’s perspective. Angew. Chem. Int. Ed. 58, 4454–4464 (2019).
Hyster, T. K. & Ward, T. R. Genetic optimization of metalloenzymes: enhancing enzymes for non-natural reactions. Angew. Chem. Int. Ed. 55, 7344–7357 (2016).
Sträter, N., Lipscomb, W. N., Klabunde, T. & Krebs, B. Two-metal ion catalysis in enzymatic acyl- and phosphoryl-transfer reactions. Angew. Chem. Int. Ed. 35, 2024–2055 (1996).
Allen, A. E. & MacMillan, D. W. C. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 3, 633–658 (2012).
Patil, N. T., Shinde, V. S. & Gajula, B. A one-pot catalysis: the strategic classification with some recent examples. Org. Biomol. Chem. 10, 211–224 (2012).
Afewerki, S. & Córdova, A. Combinations of aminocatalysts and metal catalysts: a powerful cooperative approach in selective organic synthesis. Chem. Rev. 116, 13512–13570 (2016).
Deng, Y., Kumar, S. & Wang, H. Synergistic-cooperative combination of enamine catalysis with transition metal catalysis. Chem. Commun. 50, 4272–4284 (2014).
Krautwald, S., Schafroth, M. A., Sarlah, D. & Carreira, E. M. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 136, 3020–3023 (2014).
Zhang, M. et al. Synergetic iridium and amine catalysis enables asymmetric [4+2] cycloadditions of vinyl aminoalcohols with carbonyls. Nat. Commun. 10, 2716 (2019).
Du, Z. & Shao, Z. Combining transition metal catalysis and organocatalysis—an update. Chem. Soc. Rev. 42, 1337–1378 (2012).
Agustiandari, H., Lubelski, J., Van Den Berg Van Saparoea, H. B., Kuipers, O. P. & Driessen, A. J. M. LmrR is a transcriptional repressor of expression of the multidrug ABC transporter LmrCD in lactococcus lactis. J. Bacteriol. 190, 759–763 (2008).
Madoori, P. K., Agustiandari, H., Driessen, A. J. M. & Thunnissen, A. M. W. H. Structure of the transcriptional regulator LmrR and its mechanism of multidrug recognition. EMBO J. 28, 156–166 (2009).
Roelfes, G. LmrR: a privileged scaffold for artificial metalloenzymes. Acc. Chem. Res. 52, 545–556 (2019).
Bos, J., Browne, W. R., Driessen, A. J. M. & Roelfes, G. Supramolecular assembly of artificial metalloenzymes based on the dimeric protein LmrR as promiscuous scaffold. J. Am. Chem. Soc. 137, 9796–9799 (2015).
Drienovská, I., Rioz-Martínez, A., Draksharapu, A. & Roelfes, G. Novel artificial metalloenzymes by in vivo incorporation of metal-binding unnatural amino acids. Chem. Sci. 6, 770–776 (2015).
Liu, C. C. & Schultz, P. G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Dumas, A., Lercher, L., Spicer, C. D. & Davis, B. G. Designing logical codon reassignment—expanding the chemistry in biology. Chem. Sci. 6, 50–69 (2015).
Erkkilä, A., Majander, I. & Pihko, P. M. Iminium catalysis. Chem. Rev. 107, 5416–5470 (2007).
Mehl, R. A. et al. Generation of a bacterium with a 21 amino acid genetic code. J. Am. Chem. Soc. 125, 935–939 (2003).
Guo, C., Saifuddin, M., Saravanan, T., Sharifi, M. & Poelarends, G. J. Biocatalytic asymmetric Michael additions of nitromethane to α,β-unsaturated aldehydes via enzyme-bound iminium ion intermediates. ACS Catal. 9, 4369–4373 (2019).
Garrabou, X., Verez, R. & Hilvert, D. Enantiocomplementary synthesis of γ-nitroketones using designed and evolved carboligases. J. Am. Chem. Soc. 139, 103–106 (2017).
Garrabou, X., Macdonald, D. S., Wicky, B. I. M. & Hilvert, D. Stereodivergent evolution of artificial enzymes for the michael reaction. Angew. Chem. Int. Ed. 57, 5288–5291 (2018).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Huang, X., Webster, R. D., Harms, K. & Meggers, E. Asymmetric catalysis with organic azides and diazo compounds initiated by photoinduced electron transfer. J. Am. Chem. Soc. 138, 12636–12642 (2016).
Zhang, D. H., Knelles, J. & Plietker, B. Iron-catalyzed michael addition of ketones to polar olefins. Adv. Synth. Catal. 358, 2469–2479 (2016).
Villarino, L. et al. An artificial heme enzyme for cyclopropanation reactions. Angew. Chem. Int. Ed. 57, 7785–7789 (2018).
Chin, J. W. et al. Addition of p-azido-l-phenylalanine to the genetic code of Escherichia coli. J. Am. Chem. Soc. 124, 9026–9027 (2002).
Bos, J., García-Herraiz, A. & Roelfes, G. An enantioselective artificial metallo-hydratase. Chem. Sci. 4, 3578–3582 (2013).
Acknowledgements
We thank R. B. Leveson-Gower for assistance in preparation of the figures and K. E. Splan for useful discussions. Support from the Netherlands Organisation for Scientific Research (NWO) (Vici grant no. 724.013.003) and the Ministry of Education, Culture and Science (Gravitation programme no. 024.001.035) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
G.R. conceived and directed the project. Z.Z. performed the experimental work and analysed the data. The authors discussed the results and wrote the manuscript together.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Figs. 1–7, methods and references.
Rights and permissions
About this article
Cite this article
Zhou, Z., Roelfes, G. Synergistic catalysis in an artificial enzyme by simultaneous action of two abiological catalytic sites. Nat Catal 3, 289–294 (2020). https://doi.org/10.1038/s41929-019-0420-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0420-6
This article is cited by
-
Rationally introducing non-canonical amino acids to enhance catalytic activity of LmrR for Henry reaction
Bioresources and Bioprocessing (2024)
-
Hydrolase mimic via second coordination sphere engineering in metal-organic frameworks for environmental remediation
Nature Communications (2023)
-
Data-informed discovery of hydrolytic nanozymes
Nature Communications (2022)
-
Single-atom nanozymes catalytically surpassing naturally occurring enzymes as sustained stitching for brain trauma
Nature Communications (2022)
-
Engineering and emerging applications of artificial metalloenzymes with whole cells
Nature Catalysis (2021)