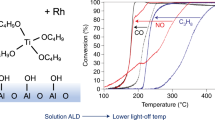
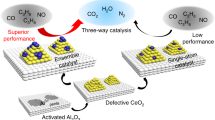
Abstract
CO oxidation is an important primary reaction in automotive catalysis, and has been studied extensively since the 1970s because of its fundamental nature and technological relevance to emission control regulations. In this Review, we investigate the development of state-of-the-art catalysts for CO oxidation and consider the important achievements in the design of good catalysts via a detailed scrutiny of CO oxidation pathways for single-atom and few-atom cluster catalysis, which constitute a subset of the emerging technology of atomically dispersed and nanostructured oxide-supported catalysts. We see a recent effort towards achieving high-performance catalysts via chemical potential tuning, in which the size, structure, shape and degree of alloys are controlled to alter the electronic structure, catalyst-oxide support interactions and resulting interactions between adsorbates and the catalyst. We present a missing link in modern catalysis research in terms of the future development of automotive catalysts and related issues that must be satisfactorily resolved for sustainable and environment-friendly solutions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gandhi, H. S., Graham, G. W. & McCabe, R. W. Automotive exhaust catalysis. J. Catal. 216, 433–442 (2003).
Kašpar, J., Fornasiero, P. & Hickey, N. Automotive catalytic converters: current status and some perspectives. Catal. Today 77, 419–449 (2003).
Miyoshi, N. et al. Development of new concept three-way catalyst for automotive lean-burn engines. SAE Trans. 104, 1361–1370 (1995).
Langmuir, I. The mechanism of the catalytic action of platinum in the reactions 2CO + O2= 2CO2 and 2H2+ O2= 2H2O. Trans. Faraday Soc. 17, 621–654 (1922).
Beniya, A., Ikuta, Y., Isomura, N., Hirata, H. & Watanabe, Y. Synergistic promotion of NO–CO reaction cycle by gold and nickel elucidated using a well-defined model bimetallic catalyst surface. ACS Catal. 7, 1369–1377 (2017).
2017 Outlook for Energy: A View to 2040 (Exxon Mobil Corporation, 2017).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Higashi, S., Lee, S. W., Lee, J. S., Takechi, K. & Cui, Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 7, 11801 (2016).
Zammit, M. et al. Future Automotive Aftertreatment Solutions: The 150°C Challenge Workshop Report (US Department of Energy, 2013). Describes the 150 °C challenge in the United States.
Golunski, S. E. Why use platinum in catalytic converters? Platin. Met. Rev. 51, 162–162 (2007).
Heiz, U. & Landman, U. Nanocatalysis (Springer-Verlag, Berlin, 2007).
Heiz, U., Sanchez, A., Abbet, S. & Schneider, W. D. Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: each atom counts. J. Am. Chem. Soc. 121, 3214–3217 (1999).
Vajda, S. et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 8, 213–216 (2009).
Kaden, W. E., Wu, T. P., Kunkel, W. A. & Anderson, S. L. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 326, 826–829 (2009).
Yoon, B. et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science 307, 403–407 (2005).
Neugebohren, J. et al. Velocity-resolved kinetics of site-specific carbon monoxide oxidation on platinum surfaces. Nature 558, 280–283 (2018).
Wintterlin, J., Völkening, S., Janssens, T. V. W., Zambelli, T. & Ertl, G. Atomic and macroscopic reaction rates of a surface-catalyzed reaction. Science 278, 1931–1934 (1997).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Salmeron, M. & Schlögl, R. Ambient pressure photoelectron spectroscopy: a new tool for surface science and nanotechnology. Surf. Sci. Rep. 63, 169–199 (2008).
Herzing, A. A., Kiely, C. J., Carley, A. F., Landon, P. & Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 321, 1331–1335 (2008).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water–gas shift catalysts. Science 301, 935–938 (2003).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Freund, H.-J., Meijer, G., Scheffler, M., Schlögl, R. & Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 50, 10064–10094 (2011).
Boudart, M. Heterogeneous catalysis by metals. J. Mol. Catal. 30, 27–38 (1985).
Masatake, H., Tetsuhiko, K., Hiroshi, S. & Nobumasa, Y. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 16, 405–408 (1987).
Li, L. et al. Investigation of catalytic finite-size-effects of platinum metal clusters. J. Phys. Chem. Lett. 4, 222–226 (2013).
Yang, C. & Garland, C. W. Infrared studies of carbon monoxide chemisorbed on Rhodium. J. Phys. Chem. 61, 1504–1512 (1957).
Yates, J. T., Duncan, T. M., Worley, S. D. & Vaughan, R. W. Infrared spectra of chemisorbed CO on Rh. J. Chem. Phys. 70, 1219–1224 (1979).
Rice, C. A., Worley, S. D., Curtis, C. W., Guin, J. A. & Tarrer, A. R. The oxidation state of dispersed Rh on Al2O3. J. Chem. Phys. 74, 6487–6497 (1981).
Wovchko, E. A. & Yates, J. T. Activation of O2 on a photochemically generated RhI site on an Al2O3 surface: low-temperature O2 dissociation and CO oxidation. J. Am. Chem. Soc. 120, 10523–10527 (1998).
Asakura, K., Nagahiro, H., Ichikuni, N. & Iwasawa, Y. Structure and catalytic combustion activity of atomically dispersed Pt species at MgO surface. Appl. Catal. A. 188, 313–324 (1999).
Abbet, S. et al. Acetylene cyclotrimerization on supported size-selected Pdn clusters (1 ≤ n ≤ 30): one atom is enough! J. Am. Chem. Soc. 122, 3453–3457 (2000).
Zhang, X., Shi, H. & Xu, B.-Q. Catalysis by gold: isolated surface Au3+ ions are active sites for selective hydrogenation of 1,3-butadiene over Au/ZrO2 catalysts. Angew. Chem. Int. Ed. 44, 7132–7135 (2005).
Hackett, S. F. J. et al. High-activity, single-site mesoporous Pd/Al2O3 catalysts for selective aerobic oxidation of allylic alcohols. Angew. Chem. Int. Ed. 46, 8593–8596 (2007).
Nagai, Y. et al. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 242, 103–109 (2006).
Farmer, J. A. & Campbell, C. T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 329, 933–936 (2010).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Wang, C. et al. Water-mediated Mars–Van Krevelen mechanism for CO oxidation on ceria-supported single-atom Pt1 catalyst. ACS Catal. 7, 887–891 (2017).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Chen, J. et al. Surface engineering protocol to obtain an atomically dispersed Pt/CeO2 catalyst with high activity and stability for CO oxidation. ACS Sustain. Chem. Eng. 6, 14054–14062 (2018).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017). Demonstrates a relationship between CO IR and single atom/few-atom clusters.
Liu, S. et al. Stabilizing single-atom and small-domain platinum via combining organometallic chemisorption and atomic layer deposition. Organometallics 36, 818–828 (2017).
Moses-DeBusk, M. et al. CO oxidation on supported single Pt atoms: experimental and ab initio density functional studies of CO interaction with Pt atom on θ-Al2O3(010) surface. J. Am. Chem. Soc. 135, 12634–12645 (2013).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Neitzel, A. et al. Atomically dispersed Pd, Ni, and Pt species in ceria-based catalysts: principal differences in stability and reactivity. J. Phys. Chem. C. 120, 9852–9862 (2016).
Spezzati, G. et al. Atomically dispersed Pd–O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Qiao, B. et al. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 8, 2913–2924 (2015).
Guo, L.-W. et al. Contributions of distinct gold species to catalytic reactivity for carbon monoxide oxidation. Nat. Commun. 7, 13481 (2016).
Jeong, H. et al. Fully dispersed Rh ensemble catalyst to enhance low-temperature activity. J. Am. Chem. Soc. 140, 9558–9565 (2018).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Mars, P. & van Krevelen, D. W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 3, 41–59 (1954).
Wang, J., Tan, H., Yu, S. & Zhou, K. Morphological effects of gold clusters on the reactivity of ceria surface oxygen. ACS Catal. 5, 2873–2881 (2015).
Bliem, R. et al. An atomic-scale view of CO and H2 oxidation on a Pt/Fe3O4 model catalyst. Angew. Chem. Int. Ed. 54, 13999–14002 (2015).
Daté, M. & Haruta, M. Moisture effect on CO oxidation over Au/TiO2 catalyst. J. Catal. 201, 221–224 (2001).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Ghosh, T. K. & Nair, N. N. Rh1/γ-Al2O3 single-atom catalysis of O2 activation and CO oxidation: mechanism, effects of hydration, oxidation state, and cluster size. ChemCatChem 5, 1811–1821 (2013).
Zhou, X. et al. Stable Pt single atoms and nanoclusters on ultrathin CuO film and their performances in CO oxidation. J. Phys. Chem. C. 120, 1709–1715 (2016).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Bamwenda, G. R., Tsubota, S., Nakamura, T. & Haruta, M. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO2 for CO oxidation. Catal. Lett. 44, 83–87 (1997).
Mavrikakis, M., Stoltze, P. & Nørskov, J. K. Making gold less noble. Catal. Lett. 64, 101–106 (2000).
Sanchez, A. et al. When gold is not noble: nanoscale gold catalysts. J. Phys. Chem. A 103, 9573–9578 (1999).
Lee, S., Fan, C., Wu, T. & Anderson, S. L. CO oxidation on Aun/TiO2 catalysts produced by size-selected cluster deposition. J. Am. Chem. Soc. 126, 5682–5683 (2004).
Watanabe, Y., Wu, X., Hirata, H. & Isomura, N. Size-dependent catalytic activity and geometries of size-selected Pt clusters on TiO2(110) surfaces. Catal. Sci. Technol. 1, 1490–1495 (2011).
Bonanni, S., Aït-Mansour, K., Harbich, W. & Brune, H. Reaction-induced cluster ripening and initial size-dependent reaction rates for CO oxidation on Ptn/TiO2(110)-(1×1). J. Am. Chem. Soc. 136, 8702–8707 (2014).
Lou, Y. & Liu, J. CO oxidation on metal oxide supported single Pt atoms: the role of the support. Ind. Eng. Chem. Res. 56, 6916–6925 (2017).
Li, J. et al. In situ formation of isolated bimetallic PtCe sites of single-dispersed Pt on CeO2 for low-temperature CO oxidation. ACS Appl. Mater. Interfaces 10, 38134–38140 (2018).
Liang, J.-X. et al. Theoretical and experimental investigations on single-atom catalysis: Ir1/FeOx for CO oxidation. J. Phys. Chem. C. 118, 21945–21951 (2014).
Li, S. et al. Low-temperature CO oxidation over supported Pt catalysts prepared by colloid-deposition method. Catal. Commun. 9, 1045–1049 (2008).
Han, Y.-F., Zhong, Z., Ramesh, K., Chen, F. & Chen, L. Effects of different types of γ-Al2O3 on the activity of gold nanoparticles for CO oxidation at low-temperatures. J. Phys. Chem. C. 111, 3163–3170 (2007).
Lin, S. D., Bollinger, M. & Vannice, M. A. Low temperature CO oxidation over Au/TiO2 and Au/SiO2 catalysts. Catal. Lett. 17, 245–262 (1993).
Ayastuy, J. L., González-Marcos, M. P., Gil-Rodríguez, A., González-Velasco, J. R. & Gutiérrez-Ortiz, M. A. Selective CO oxidation over CeXZr1−XO2-supported Pt catalysts. Catal. Today 116, 391–399 (2006).
Lee, J., Ryou, Y., Kim, J., Chan, X., Kim, T. J. & Kim, D. H. Influence of the defect concentration of ceria on the Pt dispersion and the CO oxidation activity of Pt/CeO2. J. Phys. Chem. C. 122, 4972–4983 (2018).
Jia, C.-J., Liu, Y., Bongard, H. & Schüth, F. Very low temperature CO oxidation over colloidally deposited gold nanoparticles on Mg(OH)2 and MgO. J. Am. Chem. Soc. 132, 1520–1522 (2010).
Aguilar-Guerrero, V. & Gates, B. C. Kinetics of CO oxidation catalyzed by highly dispersed CeO2-supported gold. J. Catal. 260, 351–357 (2008).
Qiao, B. et al. Highly active Au1/Co3O4 single-atom catalyst for CO oxidation at room temperature. Chin. J. Catal. 36, 1505–1511 (2015).
Kunwar, D. et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 9, 3978–3990 (2019). This study demonstrates that cerium oxide can support Pt single atoms at high metal loading (3 wt% Pt).
Campbell, C. T. & Sellers, J. R. V. Anchored metal nanoparticles: effects of support and size on their energy, sintering resistance and reactivity. Faraday Discuss. 162, 9–30 (2013).
Yudanov, I. V., Genest, A., Schauermann, S., Freund, H.-J. & Rösch, N. Size dependence of the adsorption energy of CO on metal nanoparticles: a DFT search for the minimum value. Nano Lett. 12, 2134–2139 (2012).
Hammer, B., Morikawa, Y. & Nørskov, J. K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 76, 2141–2144 (1996).
Falsig, H. et al. Trends in the catalytic CO oxidation activity of nanoparticles. Angew. Chem. Int. Ed. 47, 4835–4839 (2008).
Fischer-Wolfarth, J.-H. et al. Particle-size dependent heats of adsorption of CO on supported Pd nanoparticles as measured with a single-crystal microcalorimeter. Phys. Rev. B 81, 241416 (2010).
Ruiz Puigdollers, A., Schlexer, P., Tosoni, S. & Pacchioni, G. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 7, 6493–6513 (2017).
Su, Y.-Q., Filot, I. A. W., Liu, J.-X., Tranca, I. & Hensen, E. J. M. Charge transport over the defective CeO2(111) surface. Chem. Mater. 28, 5652–5658 (2016).
Migani, A., Vayssilov, G. N., Bromley, S. T., Illas, F. & Neyman, K. M. Dramatic reduction of the oxygen vacancy formation energy in ceria particles: a possible key to their remarkable reactivity at the nanoscale. J. Mater. Chem. 20, 10535–10546 (2010).
Tsunekawa, S., Wang, J. T. & Kawazoe, Y. Lattice constants and electron gap energies of nano- and subnano-sized cerium oxides from the experiments and first-principles calculations. J. Alloy. Compd. 408–412, 1145–1148 (2006).
Trovarelli, A. & Llorca, J. Ceria catalysts at nanoscale: how do crystal shapes shape catalysis? ACS Catal. 7, 4716–4735 (2017).
Bruix, A. & Neyman, K. M. Modelling ceria-based nanomaterials for catalysis and related applications. Catal. Lett. 146, 2053–2080 (2016).
Mavrikakis, M., Hammer, B. & Nørskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 81, 2819–2822 (1998).
Schlapka, A., Lischka, M., Groβ, A., Käsberger, U. & Jakob, P. Surface strain versus substrate interaction in heteroepitaxial metal layers: Pt on Ru(0001). Phys. Rev. Lett. 91, 016101 (2003).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2, 454–460 (2010).
De Clercq, A., Margeat, O., Sitja, G., Henry, C. R. & Giorgio, S. Core–shell Pd–Pt nanocubes for the CO oxidation. J. Catal. 336, 33–40 (2016).
Nilsson Pingel, T., Jørgensen, M., Yankovich, A. B., Grönbeck, H. & Olsson, E. Influence of atomic site-specific strain on catalytic activity of supported nanoparticles. Nat. Commun. 9, 2722 (2018).
Park, J. Y., Zhang, Y., Grass, M., Zhang, T. & Somorjai, G. A. Tuning of catalytic CO oxidation by changing composition of Rh−Pt bimetallic nanoparticles. Nano Lett. 8, 673–677 (2008).
Jin, M. et al. Synthesis of Pd nanocrystals enclosed by {100} facets and with sizes <10 nm for application in CO oxidation. Nano Res. 4, 83–91 (2011).
Wang, R., He, H., Liu, L.-C., Dai, H.-X. & Zhao, Z. Shape-dependent catalytic activity of palladium nanocrystals for the oxidation of carbon monoxide. Catal. Sci. Technol. 2, 575–580 (2012).
Wang, R., He, H., Wang, J., Liu, L. & Dai, H. Shape-regulation: an effective way to control CO oxidation activity over noble metal catalysts. Catal. Today 201, 68–78 (2013).
Wilde, M. & Fukutani, K. Hydrogen detection near surfaces and shallow interfaces with resonant nuclear reaction analysis. Surf. Sci. Rep. 69, 196–295 (2014).
Wang, Y.-G. et al. CO oxidation on Au/TiO2: condition-dependent active sites and mechanistic pathways. J. Am. Chem. Soc. 138, 10467–10476 (2016).
Nilius, N. Properties of oxide thin films and their adsorption behaviour studied by scanning tunnelling microscopy and conductance spectroscopy. Surf. Sci. Rep. 64–67, 595–659 (2009).
Sugimoto, Y. et al. Chemical identification of individual surface atoms by atomic force microscopy. Nature 446, 64 (2007). Identifies single Ge atoms substituted with Si on the Si(111) surface by analysing the AFM frequency.
Yurtsever, A. et al. The local electronic properties of individual Pt atoms adsorbed on TiO2(110) studied by Kelvin probe force microscopy and first-principles simulations. Nanoscale 9, 5812–5821 (2017). Single Pt atoms on TiO 2 (110) are identified by this technique.
Dai, Y. et al. Inherent size effects on XANES of nanometer metal clusters: size-selected platinum clusters on silica. J. Phys. Chem. C. 121, 361–374 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–3, Supplementary Notes 1–2 and Supplementary References.
Rights and permissions
About this article
Cite this article
Beniya, A., Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat Catal 2, 590–602 (2019). https://doi.org/10.1038/s41929-019-0282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0282-y
This article is cited by
-
Sequential co-reduction of nitrate and carbon dioxide enables selective urea electrosynthesis
Nature Communications (2024)
-
Highly active copper-intercalated weakly crystallized δ-MnO2 for low-temperature oxidation of CO in dry and humid air
Frontiers of Environmental Science & Engineering (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
The practically renewable and highly efficient electrocatalysts derived from a newly-designed Mo8Pt polyoxometalate compound
Science China Chemistry (2023)
-
Study on persulfate activated by Ce-modified tea waste biochar to degrade tetracycline
Environmental Science and Pollution Research (2023)