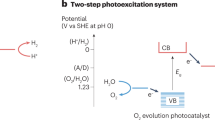
Abstract
Photocatalytic water splitting using particulate semiconductor materials has been studied as a simple means of hydrogen production. However, there are still many obstacles to the development of complete, practical and renewable solar hydrogen production processes. This review discusses particulate photocatalyst systems intended for large-scale solar hydrogen production via water splitting, focusing on their current status and potential impact. The cost and efficiency targets for solar-to-fuel conversion on a practical scale are also reviewed, based on the maximum allowable cost of solar hydrogen production systems, which has been estimated to be US$102 m–2, at most. Particulate photocatalyst material design principles are discussed, using efficient oxide photocatalysts as examples. Approaches to constructing photocatalytic reactors extensible to large areas are also introduced. Finally, challenges related to the development of efficient and inexpensive photocatalyst systems and potentially useful analytical methods are outlined.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
BP Statistical Review of World Energy (British Petroleum, 2018).
BP Energy Outlook 2018 Edition (British Petroleum, 2018).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Domen, K., Kondo, J. N., Hara, M. & Takata, T. Photo- and mechano-catalytic overall water splitting reactions to form hydrogen and oxygen on heterogeneous catalysts. Bull. Chem. Soc. Jpn. 73, 1307–1331 (2000).
Kudo, A., Kato, H. & Tsuji, I. Strategies for the development of visible-light-driven photocatalysts for water splitting. Chem. Lett. 33, 1534–1539 (2004).
Kudo, A., Niishiro, R., Iwase, A. & Kato, H. Effects of doping of metal cations on morphology, activity, and visible light response of photocatalysts. Chem. Phys. 339, 104–110 (2007).
Maeda, K. & Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 111, 7851–7861 (2007).
Osterloh, F. E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 20, 35–54 (2008).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009). Heterogeneous photocatalyst materials for water splitting are reviewed comprehensively along with the basis of photocatalytic water splitting.
Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C: Photochem. Rev. 11, 179–209 (2010).
Maeda, K. & Domen, K. Photocatalytic water splitting: recent progress and future challenges. J. Phys. Chem. Lett. 1, 2655–2661 (2010).
Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C 12, 237–268 (2011).
Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 3, 1486–1503 (2013).
Li, X., Yu, J., Low, J., Fang, Y., Xiao, Jing & Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 3, 2485–2534 (2015).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Zhang, X., Chen, Y. L., Liu, R. & Tsai, D. P. Plasmonic photocatalysis. Rep. Prog. Phys. 76, 046401 (2013).
Yang, J., Wang, D., Han, H. & Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 46, 1900–1909 (2013).
Hisatomi, T., Kubota, J. & Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535 (2014).
Zhou, P., Yu, J. & Jaroniec, M. All-solid-state z-scheme photocatalytic systems. Adv. Mater. 26, 4920–4935 (2014).
Fabian, D. M. et al. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 8, 2825–2850 (2015).
Hisatomi, T., Takanabe, K. & Domen, K. Photocatalytic water-splitting reaction from catalytic and kinetic perspectives. Catal. Lett. 145, 95–108 (2015).
Hisatomi, T. & Domen, K. Introductory lecture: sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis. Faraday Discuss. 198, 11–35 (2017).
Setoyama, T., Takewaki, T., Domen, K. & Tatsumi, T. The challenges of solar hydrogen in chemical industry: how to provide, and how to apply? Faraday Discuss. 198, 509–527 (2017).
Chen, S., Takata, T. & Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2, 17050 (2017). The basis and historical evolution of photocatalytic water splitting are reviewed along with emerging materials and technologies for overall water splitting.
Takanabe, K. Photocatalytic water splitting: quantitative approaches toward photocatalyst by design. ACS Catal. 7, 8006–8022 (2017). Physical and chemical processes in photocatalytic water splitting on particulate semiconductors are reviewed comprehensively.
Osterloh, F. E. Photocatalysis versus photosynthesis: a sensitivity analysis of devices for solar energy conversion and chemical transformations. ACS Energy Lett. 2, 445–453 (2017).
Wang, Y. et al. Mimicking natural photosynthesis: solar to renewable H2 fuel synthesis by z-scheme water splitting systems. Chem. Rev. 118, 5201–5241 (2018).
Qureshi, M. & Takanabe, K. Insights on measuring and reporting heterogeneous photocatalysis: efficiency definitions and setup examples. Chem. Mater. 29, 158–167 (2017). Guiding principles for the correct measurement and reporting of photocatalytic efficiency are critically reviewed.
Kamat, P. V. Semiconductor photocatalysis: “Tell us the complete story!”. ACS Energy Lett. 3, 622–623 (2018).
Lan, R., Irvine, J. T. S. & Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 37, 1482–1494 (2012).
Kondratenko, E. V., Mul, G., Baltrusaitis, J., Larrazábalc, G. O. & Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 6, 3112–3135 (2013).
Gretz, J., Drolet, B., Kluyskens, D., Sandmann, F. & Ullmann, O. Status of the hydro-hydrogen pilot project (EQHHPP). Int. J. Hydrog. Energy 19, 169–174 (1994).
Okada, Y., Sasaki, E., Watanabe, E., Hyodo, S. & Nishijima, H. Development of dehydrogenation catalyst for hydrogen generation in organic chemical hydride method. Int. J. Hydrog. Energy 31, 1348–1356 (2006).
Alhumaidan, F., Cresswell, D. & Garforth, A. Hydrogen storage in liquid organic hydride: producing hydrogen catalytically from methylcyclohexane. Energy Fuels 25, 4217–4234 (2011).
Fujishima, A., Zhang, X. & Tryk, D. A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008).
Chong, M. N., Jin, B., Chow, C. W. K. & Saint, C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 44, 2997–3027 (2010).
Sayama, K. Significance of artificial photosynthesis and solar hydrogen technology: discussion using cost analysis. Optronics 34, 44–49 (2015).
Shaner, M. R., Atwater, H. A., Lewis, N. S. & McFarland, E. W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 9, 2354–2371 (2016).
Ager, J. W., Shaner, M. R., Walczak, K. A., Sharp, I. D. & Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 8, 2811–2824 (2015).
Nakamura, A. et al. A 24.4% solar to hydrogen energy conversion efficiency by combining concentrator photovoltaic modules and electrochemical cells. Appl. Phys. Express 8, 107101–107102 (2015).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 13237–13241 (2016).
Sathre, R. et al. Opportunities to improve the net energy performance of photoelectrochemical water-splitting technology. A. Energy Environ. Sci. 9, 803–819 (2016).
Fu, R. et al. U.S. solar Photovoltaic System Cost Benchmark: Q1 2016 (NREL, 2016).
Schmidt, O. et al. Future cost and performance of water electrolysis: an expert elicitation study. Int. J. Hydrog. Energy 42, 30470–30492 (2017).
Yoshida, M. et al. Role and function of noble-metal/Cr-layer core/shell structure cocatalysts for photocatalytic overall water splitting studied by model electrodes. J. Phys. Chem. C 113, 10151–10157 (2009). The mechanism of how a ultrathin Cr 2 O 3 layer prevents backward reactions on noble metals and preserves their hydrogen evolution activity is revealed via electrochemical approaches.
Takata, T., Pan, C., Nakabayashi, M., Shibata, N. & Domen, K. Fabrication of a Core−shell-type photocatalyst via photodeposition of group IV and V transition metal oxyhydroxides: an effective surface modification method for overall water splitting. J. Am. Chem. Soc. 137, 9627–9634 (2015).
Garcia-Esparza, A. T. et al. An oxygen-insensitive hydrogen evolution catalyst coated by molybdenum-based layer for overall water splitting. Angew. Chem. Int. Ed. 56, 5780–5784 (2017).
Muduli, S. K. et al. Evolution of hydrogen by few-layered black phosphorus under visible illumination. J. Mater. Chem. A 5, 24874–24879 (2017).
Tian, B. et al. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353 K. Nat. Commun. 9, 1397 (2018).
Zhu, M., Sun, Z., Fujitsuka, M. & Majima, T. Z-scheme photocatalytic water splitting on a 2D heterostructure of black phosphorus/bismuth vanadate using visible Light. Angew. Chem. Int. Ed. 57, 2160–2164 (2018).
Wang, X. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009).
Zhang, G., Lan, Z.-A., Lin, L., Lin, S. & Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 7, 3062–3066 (2016).
Lin, L. et al. Photocatalytic overall water splitting by conjugated semiconductors with crystalline poly(triazine imide) frameworks. Chem. Sci. 8, 5506–5511 (2017).
Zhang, G., Lan, Z.-A. & Wang, X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 8, 5261–5274 (2017).
Che et al. Fast photoelectron transfer in (Cring)−C3N4 plane heterostructural nanosheets for overall water splitting. J. Am. Chem. Soc. 139, 3021–3026 (2017).
Wang, L. et al. Conjugated microporous polymer nanosheets for overall water splitting using visible light. Adv. Mater. 29, 1702428 (2017).
Wang, L., Zheng, X., Chen, L., Xiong, Y. & Xu, H. Van der waals heterostructures comprised of ultrathin polymer nanosheets for efficient z-scheme overall water splitting. Angew. Chem. Int. Ed. 57, 3454–3458 (2018).
Tanaka, A., Teramura, K., Hosokawa, S., Kominami, H. & Tanaka, T. Visible light-induced water splitting in an aqueous suspension of a plasmonic Au/TiO2 photocatalyst with metal co-catalysts. Chem. Sci. 8, 2574–2580 (2017).
Wang, S. et al. Achieving overall water splitting on plasmon-based solid z-scheme photocatalysts free of redox mediators. J. Catal. 354, 250–257 (2017).
Naya, S., Kume, T., Akashi, R., Fujishima, M. & Tada, H. Red-light-driven water splitting by Au(Core)−CdS(Shell) half-cut nanoegg with heteroepitaxial junction. J. Am. Chem. Soc. 140, 1251–1254 (2018).
Sato, S. & White, J. M. Photodecomposition of water over Pt/TiO2 catalysts. Chem. Phys. Lett. 72, 83–86 (1980).
Domen, K., Naito, S., Soma, M., Onishi, T. & Tamaru, K. Photocatalytic decomposition of water vapour on an NiO–SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. 12, 543–544 (1980).
Lehn, J. M., Sauvage, J. P. & Ziessel, R. Photochemical water splitting. Continuous generation of hydrogen and oxygen by irradiation of aqueous suspensions of metal loaded strontium titanate. Nouv. J. Chim. 4, 623–627 (1980).
Kato, H., Asakura, K. & Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 125, 3082–3089 (2003). A particulate semiconductor is shown to split water into hydrogen and oxygen with an AQY ofgreater than 50%.
An, L. & Onishi, H. Electron−hole recombination controlled by metal doping sites in NaTaO3 photocatalysts. ACS Catal. 5, 3196–3206 (2015).
An, L. et al. Local environment of strontium cations activating NaTaO3 photocatalysts. ACS Catal. 8, 880–885 (2018).
Yamakata, A., Ishibashi, T., Kato, H., Kudo, A. & Onishi, H. Photodynamics of NaTaO3 catalysts for efficient water splitting. J. Phys. Chem. B 107, 14383–14387 (2003).
Maruyama, M., Iwase, A., Kato, H., Kudo, A. & Onishi, H. Time-resolved infrared absorption study of NaTaO3 photocatalysts doped with alkali earth metals. J. Phys. Chem. C 113, 13918–13923 (2009).
Sakata, Y., Hayashi, T., Yasunaga, R., Yanaga, N. & Imamura, H. Remarkably high apparent quantum yield of the overall photocatalytic H2O splitting achieved by utilizing Zn ion added Ga2O3 prepared using dilute CaCl2 solution. Chem. Commun. 51, 12935–12938 (2015).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018). Photocatalytic water-splitting panel reactors that can sustain a gas evolution rate envisioned at 10% STH and are scalable beyond the square-metre scale are demonstrated.
Ham, Y. et al. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 4, 3027–3033 (2016).
Chiang, T. H. et al. Efficient photocatalytic water splitting Using Al-Doped SrTiO3 Coloaded with Molybdenum Oxide and Rhodium–Chromium Oxide. ACS Catal. 8, 2782–2788 (2018).
Takata, T. & Domen, K. Defect engineering of photocatalysts by doping of aliovalent metal cations for efficient water splitting. J. Phys. Chem. C. 113, 19386–19388 (2009).
Mu et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting. Energy Environ. Sci. 9, 2463–2469 (2016).
Zhu, J. et al. Direct imaging of highly anisotropic photogenerated charge separations on different facets of a single BiVO4 photocatalyst. Angew. Chem. Int. Ed. 54, 9111–9114 (2015).
Chen, R., Zhu, J., An, H., Fan, F. & Can, Li Unravelling charge separation via surface built-in electric fields within single particulate photocatalysts. Faraday Discuss. 198, 473–479 (2017).
Scaife, D. E. Oxide semiconductors in photoelectrochemical conversion of solar energy. Sol. Energy 25, 41–45 (1980).
Jo, W. et al. Phase transition-induced band edge engineering of BiVO4 to split pure water under visible light. Proc. Natl Acad. Sci. USA 112, 13774–13778 (2015).
Zhang, J., Zhang, M., Lin, S., Fu, X. & Wang, X. Molecular doping of carbon nitride photocatalysts with tunable bandgap and enhanced activity. J. Catal. 310, 24–30 (2014).
Sayama, K., Mukasa, K., Abe, R., Abe, Y. & Arakawa, H. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3 –/I– shuttle redox mediator under visible light irradiation. Chem. Commun. 23, 2416–2417 (2001).
Miyoshi, A. et al. Nitrogen/fluorine-codoped rutile titania as a stable oxygen-evolution photocatalyst for solar-driven z-scheme water splitting. Sustain. Energ. Fuels 2, 2025–2035 (2018).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016). Particulate photocatalyst sheets that split water into hydrogen and oxygen by two-step excitation at high STH values and maintain the intrinsic high water-splitting activity regardless of the size are demonstrated.
Wang, Q. et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 139, 1675–1683 (2017).
Wang, Q., Hisatomi, T., Ma, S. S. K., Li, Y. & Domen, K. Core/shell structured La- and Rh-codoped SrTiO3 as a hydrogen evolution photocatalyst in z-scheme overall water splitting under visible light irradiation. Chem. Mater. 26, 4144–4150 (2014).
Asai, R., Nemono, H., Jia, Q., Saito, K., Iwase, A. & Kudo, A. A visible light responsive rhodium and antimony codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting. Chem. Commun. 50, 2543–2546 (2014).
Xing, Z., Zong, X., Pan, J. & Wang, Li On the engineering part of solar hydrogen production from water splitting: photoreactor design. Chem. Eng. Sci. 104, 125–146 (2013).
Jing, D., Liu, H., Zhang, X., Zhao, L. & Guo, L. Photocatalytic hydrogen production under direct solar light in a CPC based solar reactor: reactor design and preliminary results. Energy Convers. Manag. 50, 2919–2926 (2009).
Xiong, A. et al. Fabrication of photocatalyst panels and the factors determining their activity for water splitting. Catal. Sci. Technol. 4, 325–328 (2014).
Wang, Q. et al. Z-scheme water splitting using particulate semiconductors immobilized onto metal layers for efficient electron relay. J. Catal. 328, 308–315 (2015).
Schröder, M. et al. Hydrogen evolution reaction in a large-scale reactor using a carbon nitride photocatalyst under natural sunlight irradiation. Energy Technol. 3, 1014–1017 (2015).
Hisatomi, T. & Domen, K. in Advances in Photoelectrochemical Water Splitting: Theory, Experiment and Systems Analysis (eds Tilley, S. D., Lany, S. & van de Krol, R.) Ch. 7 (Royal Society of Chemistry, 2018).
Sun, S. et al. Efficient redox-mediator-free z-scheme water splitting employing oxysulfide photocatalysts under visisble light. ACS Catal. 8, 1690–1696 (2018).
Wang, Q. et al. Printable photocatalyst sheets incorporating a transparent conductive mediator for z-scheme water splitting. Joule 2, 2667–2680 (2018). Efficient and scalable photocatalyst sheets for z-scheme water splitting are fabricated and operated in ambient-pressure processes.
Hisatomi, T. et al. Particulate photocatalyst sheets based on non-oxide semiconductor materials for water splitting under visible light irradiation. Catal. Sci. Technol. 8, 3918–3925 (2018).
Pan, C. et al. A complex perovskite-type oxynitride: the first photocatalyst for water splitting operable at up to 600 nm. Angew. Chem. Int. Ed. 54, 2955–2959 (2015). The applicability of narrow band gap oxynitrides to one-step excitation overall water splitting is demonstrated via surface modifications with oxide thin layers.
Pan, C., Takata, T. & Domen, K. Overall water splitting on the transition-metal oxynitride photocatalyst LaMg1/3Ta2/3O2N over a large portion of the visible-light spectrum. Chem. Eur. J. 22, 1854–1862 (2016).
Iwashina, K., Iwase, A., Ng, Y., Amal, R. & Kudo, A. Z-schematic water splitting into H2 and O2 using metal sulfide as a hydrogen-evolving photocatalyst and reduced graphene oxide as a solid-state electron mediator. J. Am. Chem. Soc. 137, 604–607 (2015).
Ma, G. et al. Visible light-driven z-scheme water splitting using oxysulfide H2 evolution photocatalysts. J. Phys. Chem. Lett. 7, 3892–3896 (2016).
Kobayashi, R. et al. A heterojunction photocatalyst composed of zinc rhodium oxide, single crystal derived bismuth vanadium oxide, and silver for overall pure-water splitting under visible light up to 740 nm. Phys. Chem. Chem. Phys. 18, 27754–27760 (2016).
Hara, Y. et al. Silver-inserted heterojunction photocatalyst consisting of zinc rhodium oxide and silver antimony oxide for overall pure-water splitting under visible light. Appl. Catal. B. 209, 663–668 (2017).
Ohno, T., Bai, L., Hisatomi, T., Maeda, K. & Domen, K. Photocatalytic water splitting using modified GaN:ZnO solid solution under visible light: long-time operation and regeneration of activity. J. Am. Chem. Soc. 134, 8254–8259 (2012).
Wang, Z. et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 1, 756–763 (2018). Single crystal semiconductor nitride nanorods free from inside grain boundaries. and active in the overall water splitting reaction are fabricated by unique short nitridation.
Zhang, F. Cobalt-modified porous single-crystalline LaTiO2N for highly efficient water oxidation under visible light. J. Am. Chem. Soc. 134, 8348–8351 (2012).
Kim, T. W. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Godin, R., Kafizas, A. & Durrant, J. R. Electron transfer dynamics in fuel producing photosystems. Curr. Opin. Electrochem. 2, 136–143 (2017).
Mei, B., Han, K. & Mul, G. Driving surface redox reactions in heterogeneous photocatalysis: the active state of illuminated semiconductor-supported nanoparticles during overall water-splitting. ACS Catal. 8, 9154–9164 (2018).
Sambur, J. B. et al. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified photoanodes. Nature 530, 77–80 (2016).
Yabuta, M. et al. Particle size dependence of carrier dynamics and reactivity of photocatalyst BiVO4 probed with single-particle transient absorption microscopy. J. Phys. Chem. C 121, 22060–22066 (2017).
Sakai, E. et al. Investigation of the enhanced photocathodic activity of La5Ti2CuS5O7 photocathodes in H2 evolution by synchrotron radiation nanospectroscopy. Nanoscale 8, 18893–18896 (2016).
Fuku, K. & Sayama, K. Efficient oxidative hydrogen peroxide production and accumulation in photoelectrochemical water splitting using a tungsten trioxide/bismuth vanadate photoanode. Chem. Commun. 52, 5406–5409 (2016).
Fuku, K., Miyase, Y., Miseki, Y., Gunji, T. & Sayama, K. WO3/BiVO4 photoanode coated with mesoporous Al2O3 layer for oxidative production of hydrogen peroxide from water with high selectivity. RSC Adv. 7, 47619–47623 (2017).
Miyase, Y. et al. Modification of BiVO4/WO3 composite photoelectrodes with Al2O3 via chemical vapor deposition for highly efficient oxidative H2O2 production from H2O. Sustain. Energ. Fuels 2, 1621–1629 (2018).
Acknowledgements
This work was financially supported by the Artificial Photosynthesis Project of the New Energy and Industrial Technology Development Organization (NEDO), by a Grant-in-Aid for Scientific Research (A) (no. 16H02417), a Grant-in-Aid for Young Scientists (A) (no. 15H05494), and a Grand-in-Aid for Scientific Research on Innovative Areas (no. 18H05156) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hisatomi, T., Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat Catal 2, 387–399 (2019). https://doi.org/10.1038/s41929-019-0242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0242-6
This article is cited by
-
Light alters reaction pathways
Nature Energy (2024)
-
Induced dipole moments in amorphous ZnCdS catalysts facilitate photocatalytic H2 evolution
Nature Communications (2024)
-
Efficient and stable visible-light-driven Z-scheme overall water splitting using an oxysulfide H2 evolution photocatalyst
Nature Communications (2024)
-
Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting
Nature Catalysis (2024)
-
Bubble-water/catalyst triphase interface microenvironment accelerates photocatalytic OER via optimizing semi-hydrophobic OH radical
Nature Communications (2024)