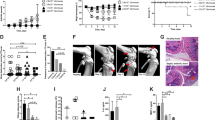
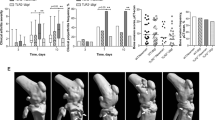
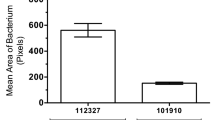
Abstract
Despite the creation of several experimental animal models for the study of septic arthritis, a protocol detailing the development of a reliable and easily reproducible animal model has not yet been reported. The experimental protocol described herein for the development of a clinically relevant mouse model of septic arthritis includes two main study stages: the first stage consisting of the preparation of the mice and of the methicillin-resistant Staphylococcus aureus (MRSA) cultures, followed by direct inoculation of MRSA into the knee joints of C57BL/6J mice (25–40 min); and a second study stage consisting of multiple sample collection and data analysis (1–3 days). This protocol may be carried out by researchers skilled in mouse care and trained to work with biosafety-level-2 agents such as MRSA. The model of septic arthritis described here has demonstrated clinical relevance in developing intra-articular inflammation and cartilage destruction akin to that of human patients. Moreover, we describe methods for serum, synovial fluid and knee joint tissue analysis that were used to confirm the development of septic arthritis in this model, and to test potential treatments. This protocol confers the advantages of enabling granular evaluation of the pathophysiology of MRSA infection and of the efficacy of therapeutic medications; it may also be employed to study a range of native joint diseases beyond inflammatory pathologies alone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Kaandorp, C. J., Krijnen, P., Moens, H. J., Habbema, J. D. & van Schaardenburg, D. The outcome of bacterial arthritis: a prospective community-based study. Arthritis Rheum. 40, 884–892 (1997).
Mathews, C. J., Weston, V. C., Jones, A., Field, M. & Coakley, G. Bacterial septic arthritis in adults. Lancet 375, 846–855 (2010).
Carpenter, C. R., Schuur, J. D., Everett, W. W. & Pines, J. M. Evidence-based diagnostics: adult septic arthritis. Acad. Emerg. Med. 18, 781–796 (2011).
Alder, K. D. et al. Intracellular Staphylococcus aureus in bone and joint infections: a mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone 141, 115568 (2020).
Herrmann, M. et al. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158, 693–701 (1988).
McGavin, M. H., Krajewska-Pietrasik, D., Ryden, C. & Hook, M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect. Immun. 61, 2479–2485 (1993).
Yacoub, A. et al. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur. J. Biochem. 222, 919–925 (1994).
McDevitt, D., Francois, P., Vaudaux, P. & Foster, T. J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11, 237–248 (1994).
Cheung, A. I., Projan, S. J., Edelstein, R. E. & Fischetti, V. A. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect. Immun. 63, 1914–1920 (1995).
Ryden, C., Tung, H. S., Nikolaev, V., Engstrom, A. & Oldberg, A. Staphylococcus aureus causing osteomyelitis binds to a nonapeptide sequence in bone sialoprotein. Biochem. J. 327, 825–829 (1997).
Shirtliff, M. E. & Mader, J. T. Acute septic arthritis. Clin. Microbiol. Rev. 15, 527–544 (2002).
Wang, J. & Wang, L. Novel therapeutic interventions towards improved management of septic arthritis. BMC Musculoskelet. Disord. 22, 530 (2021).
Mitchell, M., Howard, B., Haller, J., Sartoris, D. J. & Resnick, D. Septic arthritis. Radiol. Clin. North Am. 26, 1295–1313 (1988).
Smith, R. L., Schurman, D. J., Kajiyama, G., Mell, M. & Gilkerson, E. The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J. Bone Joint Surg. Am. 69, 1063–1068 (1987).
Oppegaard, O., Skodvin, B., Halse, A. K. & Langeland, N. CD64 as a potential biomarker in septic arthritis. BMC Infect. Dis. 13, 278 (2013).
Martinez-Aguilar, G. et al. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr. Infect. Dis. J. 23, 701–706 (2004).
Arnold, S. R. et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J. Pediatr. Orthop. 26, 703–708 (2006).
Al-Nammari, S. S., Bobak, P. & Venkatesh, R. Methicillin resistant Staphylococcus aureus versus methicillin sensitive Staphylococcus aureus adult haematogenous septic arthritis. Arch. Orthop. Trauma Surg. 127, 537–542 (2007).
Salgado, C. D., Dash, S., Cantey, J. R. & Marculescu, C. E. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin. Orthop. Relat. Res. 461, 48–53 (2007).
Vincent, G. M. & Amirault, J. D. Septic arthritis in the elderly. Clin. Orthop. Relat. Res. 251, 241–245 (1990).
Wang, C. L., Wang, S. M., Yang, Y. J., Tsai, C. H. & Liu, C. C. Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J. Microbiol. Immunol. Infect. 36, 41–46 (2003).
Yu, K. et al. Recalcitrant methicillin-resistant Staphylococcus aureus infection of bone cells: Intracellular penetration and control strategies. Bone Joint Res. 9, 49–59 (2020).
Kwon, H. K. et al. Dual therapeutic targeting of intra-articular inflammation and intracellular bacteria enhances chondroprotection in septic arthritis. Sci. Adv. https://doi.org/10.1126/sciadv.abf2665 (2021).
Otto, G. Combination therapy for septic arthritis. Nat. Rev. Rheumatol. 17, 509 (2021).
Kwon, H. K. et al. Treating ‘septic’ with enhanced antibiotics and ‘arthritis’ by mitigation of excessive inflammation. Front. Cell Infect. Microbiol. 12, 897291 (2022).
Garcia-De La Torre, I. Advances in the management of septic arthritis. Rheum. Dis. Clin. North Am. 29, 61–75 (2003).
Long, B., Koyfman, A. & Gottlieb, M. Evaluation and management of septic arthritis and its mimics in the emergency department. West J. Emerg. Med. 20, 331–341 (2019).
Ross, K. et al. Outbreak of septic arthritis associated with intra-articular injections at an outpatient practice—New Jersey, 2017. MMWR Morb. Mortal. Wkly Rep. 66, 777–779 (2017).
Johnson, A. H., Campbell, W. G. Jr. & Callahan, B. C. Infection of rabbit knee joints after intra-articular injection of Staphylococcus aureus. Comparison with joints injected with Staphylococcus albus. Am. J. Pathol. 60, 165–202 (1970).
Wysenbeek, A. J. et al. Treatment of staphylococcal septic arthritis in rabbits by systemic antibiotics and intra-articular corticosteroids. Ann. Rheum. Dis. 57, 687–690 (1998).
Colavite, P. M. & Sartori, A. Septic arthritis: immunopathogenesis, experimental models and therapy. J. Venom Anim. Toxins Incl. Trop. Dis. 20, 19 (2014).
Hultgren, O., Kopf, M. & Tarkowski, A. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur. J. Immunol. 29, 2400–2405 (1999).
Shaw, L. N. et al. Identification and characterization of sigma, a novel component of the Staphylococcus aureus stress and virulence responses. PLoS ONE 3, e3844 (2008).
Narita, K. et al. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect. Immun. 78, 4234–4242 (2010).
Fatima, F. et al. Radiological features of experimental staphylococcal septic arthritis by micro computed tomography scan. PLoS ONE 12, e0171222 (2017).
Jin, T. et al. A novel mouse model for septic arthritis induced by Pseudomonas aeruginosa. Sci. Rep. 9, 16868 (2019).
Volzke, J. et al. Inflammatory joint disease is a risk factor for streptococcal sepsis and septic arthritis in mice. Front. Immunol. 11, 579475 (2020).
Morgan, D. S., Fisher, D., Merianos, A. & Currie, B. J. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol. Infect. 117, 423–428 (1996).
del Val del Amo, N. et al. Study of 112 patients with septic arthritis caused by pyogenic organisms and fungi: changes in the clinical spectrum during the last 2 decades. Rev. Clin. Esp. 197, 540–544 (1997).
Nissim, L. et al. The impact of gender on the clinical presentation, management, and surgical outcomes of patients with native-joint septic arthritis. J. Eval. Clin. Pract. 27, 371–376 (2021).
Wang, Y. et al. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc. Natl Acad. Sci. USA 114, E5094–E5102 (2017).
Mohammad, M. et al. The YIN and YANG of lipoproteins in developing and preventing infectious arthritis by Staphylococcus aureus. PLoS Pathog. 15, e1007877 (2019).
Liu, L. et al. High susceptibility to collagen-induced arthritis in mice with progesterone receptors selectively inhibited in osteoprogenitor cells. Arthritis Res. Ther. 22, 165 (2020).
Chia, W. T. et al. MMP-9 mRNA as a therapeutic marker in acute and chronic stages of arthritis induced by type II collagen antibody. J. Formos Med. Assoc. 107, 245–252 (2008).
Kung, L. H. W. et al. Comprehensive expression analysis of microRNAs and mRNAs in synovial tissue from a mouse model of early post-traumatic osteoarthritis. Sci. Rep. 7, 17701 (2017).
Smith, M. M. et al. Significant synovial pathology in a meniscectomy model of osteoarthritis: modification by intra-articular hyaluronan therapy. Rheumatology 47, 1172–1178 (2008).
Marty, I. et al. Amelioration of collagen-induced arthritis by thrombin inhibition. J. Clin. Invest. 107, 631–640 (2001).
Pritzker, K. P. et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 14, 13–29 (2006).
Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18, S17–S23 (2010).
Li, J. et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 79, 635–645 (2020).
Little, C. B. et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733 (2009).
Hayer, S. et al. ‘SMASH’ recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2020-219247 (2021).
Fitzgerald, J., Endicott, J., Hansen, U. & Janowitz, C. Articular cartilage and sternal fibrocartilage respond differently to extended microgravity. NPJ Microgravity 5, 3 (2019).
Liphardt, A. M. et al. Changes in mechanical loading affect arthritis-induced bone loss in mice. Bone 131, 115149 (2020).
Dubost, J. J. et al. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis 61, 267–269 (2002).
Li, H. K. et al. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 380, 425–436 (2019).
Bernard, L. et al. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N. Engl. J. Med. 384, 1991–2001 (2021).
Argyriou, A. et al. Single cell sequencing identifies clonally expanded synovial CD4+ TPH cells expressing GPR56 in rheumatoid arthritis. Nat. Commun. 13, 4046 (2022).
Penkava, F. et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 11, 4767 (2020).
Chou, C. H. et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci Rep. 10, 10868 (2020).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
McGonagle, D., Baboolal, T. G. & Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 13, 719–730 (2017).
Cheng, L. et al. New insights from single-cell sequencing data: synovial fibroblasts and synovial macrophages in rheumatoid arthritis. Front. Immunol. 12, 709178 (2021).
Evans, C. H., Kraus, V. B. & Setton, L. A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 10, 11–22 (2014).
Uson, J. et al. EULAR recommendations for intra-articular therapies. Ann. Rheum. Dis. 80, 1299–1305 (2021).
Lin, X., Tsao, C. T., Kyomoto, M. & Zhang, M. Injectable natural polymer hydrogels for treatment of knee osteoarthritis. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202101479 (2021).
Liu, M. et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 5, 17014 (2017).
Achaerandio-de Nova, A. et al. Development of an experimental model of septic knee arthritis in rats through intra-articular inoculation of Staphylococcus aureus. Lab Anim. 55, 270–280 (2021).
Olney, B. W., Papasian, C. J. & Jacobs, R. R. Risk of iatrogenic septic arthritis in the presence of bacteremia: a rabbit study. J. Pediatr. Orthop. 7, 524–526 (1987).
Smith, R. L., Kajiyama, G. & Schurman, D. J. Staphylococcal septic arthritis: antibiotic and nonsteroidal anti-inflammatory drug treatment in a rabbit model. J. Orthop. Res. 15, 919–926 (1997).
Linhart, W. E., Spendel, S., Weber, G. & Zadravec, S. Septic arthritis—an experimental animal model useful in free oxygen radical research. Z. Versuchstierkd 33, 65–71 (1990).
Huang, D. B., Noviello, S. & Gemmell, C. G. Iclaprim reduces the incidence and severity of Staphylococcus aureus-induced septic arthritis in a murine model. Access Microbiol. 1, e000052 (2019).
Ali, A. et al. IL-1 receptor antagonist treatment aggravates staphylococcal septic arthritis and sepsis in mice. PLoS ONE 10, e0131645 (2015).
Bremell, T., Lange, S., Yacoub, A., Ryden, C. & Tarkowski, A. Experimental Staphylococcus aureus arthritis in mice. Infect. Immun. 59, 2615–2623 (1991).
Daum, R. S. et al. A model of Staphylococcus aureus bacteremia, septic arthritis, and osteomyelitis in chickens. J. Orthop. Res. 8, 804–813 (1990).
Calander, A. M. et al. Matrix metalloproteinase-9 (gelatinase B) deficiency leads to increased severity of Staphylococcus aureus-triggered septic arthritis. Microbes Infect. 8, 1434–1439 (2006).
Staurengo-Ferrari, L. et al. Interleukin-33 receptor (ST2) deficiency improves the outcome of Staphylococcus aureus-induced septic arthritis. Front. Immunol. 9, 962 (2018).
Boff, D. et al. CXCR2 is critical for bacterial control and development of joint damage and pain in Staphylococcus aureus-induced septic arthritis in mouse. Eur. J. Immunol. 48, 454–463 (2018).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
This research was supported by National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants AR056246 and AR068353. We appreciate the histological assistance of N. Troiano and J. Fretz (Department of Orthopaedics and Rehabilitation, Yale School of Medicine).
Author information
Authors and Affiliations
Contributions
H.-K.K. conceptualized and performed all experiments. K.E.Y. analyzed the data. H.-K.K., K.E.Y. and F.Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Tao Jin, Johann Volzke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Video 1
Visual instructions for Steps 7–9.
Rights and permissions
About this article
Cite this article
Kwon, HK., Yu, K.E. & Lee, F.Y. Construction and evaluation of a clinically relevant model of septic arthritis. Lab Anim 52, 11–26 (2023). https://doi.org/10.1038/s41684-022-01089-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-022-01089-7