Abstract
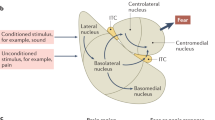
Post-traumatic stress disorder (PTSD) is a debilitating mental health condition for which current treatments have long-term efficacy in 50% of patients. There is a clear need for better understanding of the mechanisms underlying PTSD and the development of new treatment approaches. Analog trauma procedures in animals, such as the stress-enhanced fear learning (SEFL) procedure, can be used to produce behavioral and neurobiological changes that have validity in modeling PTSD. However, by necessity, the modeling of PTSD in animals requires them to potentially experience pain and suffering. Consistent with the ‘3Rs’ (reduction, refinement and replacement) of animal research, this study aimed to determine whether the SEFL procedure can be refined to reduce potential animal pain and suffering while retaining the same behavioral and neurobiological changes. Here we showed that PTSD-relevant changes could be produced in both behavior and the brain of rats that were group- rather than single-housed and that received lower-magnitude electric shocks in the ‘trauma analog’ session. We also varied the number of shock exposures in the trauma analog session, finding SEFL-susceptible and SEFL-resilient populations at all levels of shock exposure, but with greater levels of shock increasing the proportion of rats showing the SEFL-susceptible phenotype. These data demonstrate that the SEFL procedure can be used as an animal analog of PTSD with reduced potential pain and suffering to the animals and that variations in the procedure could be used to generate specific proportions of SEFL-susceptible and SEFL-resilient animals in future studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data accompanying this publication are available at the University of Cambridge data repository (https://doi.org/10.17863/CAM.75449).
References
Parsons, R. G. & Ressler, K. J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 16, 146–153 (2013).
Risbrough, V., Glenn, D. E. & Baker, D. G. in Translational Neuropsychopharmacology Current Topics in Behavioral Neurosciences (eds T. W. Robbins & B. J. Sahakian) 173–196 (Springer, 2016).
Bisson, J. I. et al. Psychological treatments for chronic post-traumatic stress disorder: systematic review and meta-analysis. Br. J. Psychiatry 190, 97–104 (2007).
Holmes, E. A., Craske, M. G. & Graybiel, A. M. A call for mental-health science. Nature 511, 287–289 (2014).
Siegmund, A. & Wotjak, C. T. Toward an animal model of posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 1071, 324–334 (2006).
Bowers, M. E. & Ressler, K. J. An overview of translationally informed treatments for posttraumatic stress disorder: animal models of pavlovian fear conditioning to human clinical trials. Biol. Psychiatry 78, e15–e27 (2015).
Flores, Á., Fullana, M. À., Soriano-Mas, C. & Andero, R. Lost in translation: how to upgrade fear memory research. Mol. Psychiatry 23, 2122–2132 (2018).
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Lopresto, D., Schipper, P. & Homberg, J. R. Neural circuits and mechanisms involved in fear generalization: implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 60, 31–42 (2016).
Rougemont-Bücking, A. et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther. 17, 227–236 (2011).
Milad, M. R., Rosenbaum, B. L. & Simon, N. M. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav. Res. Ther. 62, 17–23 (2014).
Richter-Levin, G., Stork, O. & Schmidt, M. V. Animal models of PTSD: a challenge to be met. Mol. Psychiatry 24, 1135–1156 (2019).
Yamamoto, S. et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress. Anxiety 26, 1110–1117 (2009).
Rau, V., DeCola, J. P. & Fanselow, M. S. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 29, 1207–1223 (2005).
Rau, V. & Fanselow, M. S. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12, 125–133 (2009).
Ponomarev, I., Rau, V., Eger, E. I., Harris, R. A. & Fanselow, M. S. Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology 35, 1402–1411 (2010).
Perusini, J. N. et al. Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacol. Rev. 41, 45–57 (2016).
Gonzalez, S. T., Marty, V., Spigelman, I., Reise, S. P. & Fanselow, M. S. Impact of stress resilience and susceptibility on fear learning, anxiety, and alcohol intake. Neurobiol. Stress 15, 100335 (2021).
Poulos, A. M., Zhuravka, I., Long, V., Gannam, C. & Fanselow, M. Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behav. Neurosci. 129, 62–67 (2015).
Quinn, J. J., Skipper, R. A. & Claflin, D. I. Infant stress exposure produces persistent enhancement of fear learning across development. Dev. Psychobiol. 56, 1008–1016 (2013).
Poulos, A. M. et al. Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol. Psychiatry 76, 306–314 (2014).
LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 10, 1062–1069 (1990).
Phillips, R. G. & LeDoux, J. E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 (1992).
Killcross, S., Robbins, T. W. & Everitt, B. J. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388, 377–380 (1997).
Humeau, Y. et al. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J. Neurosci. 27, 10947–10956 (2007).
Pizzimenti, C. L., Navis, T. M. & Lattal, K. M. Persistent effects of acute stress on fear and drug-seeking in a novel model of the comorbidity between post-traumatic stress disorder and addiction. Learn. Mem. 24, 422–431 (2017).
Russell, W. M. S. & Burch, R. L. The Principles of Humane Experimental Technique (Methuen, 1959).
Tribble, J. E. & Fanselow, M. S. Pair-housing rats does not protect from behavioral consequences of an acute traumatic experience. Behav. Neurosci. 133, 232–239 (2019).
Ruis, M. A. W. et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24, 285–300 (1999).
Sharp, J. L., Zammit, T. G., Azar, T. A. & Lawson, D. M. Stress-like responses to common procedures in male rats housed alone or with other rats. J. Am. Assoc. Lab. Anim. Sci. 41, 8–14 (2002).
Liu, X. et al. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 1502, 71–80 (2013).
Beery, A. K., Holmes, M. M., Lee, W. & Curley, J. P. Stress in groups: lessons from non-traditional rodent species and housing models. Neurosci. Biobehav. Rev. 113, 354–372 (2020).
Przybyl, K. J. et al. Genetic stress-reactivity, sex, and conditioning intensity affect stress-enhanced fear learning. Neurobiol. Learn. Mem. 185, 107523 (2021).
dos Santos Corrêa, M. et al. Relationship between footshock intensity, post-training corticosterone release and contextual fear memory specificity over time. Psychoneuroendocrinology 110, 104447 (2019).
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D. & Shansky, R. M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4, e11352 (2015).
Lebrón-Milad, K., Tsareva, A., Ahmed, N. & Milad, M. R. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav. Brain Res. 253, 217–222 (2013).
Olff, M., Langeland, W., Draijer, N. & Gersons, B. P. R. Gender differences in posttraumatic stress disorder. Psychol. Bull. 133, 183–204 (2007).
Shansky, R. M. Are hormones a “female problem” for animal research? Science 364, 825–826 (2019).
Fanselow, M. S. & Kim, J. J. Acquisition of contextual pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist d,l-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav. Neurosci. 108, 210–212 (1994).
Maren, S., Aharonov, G. & Fanselow, M. S. Retrograde abolition of conditioned fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav. Neurosci. 110, 718–726 (1996).
Wang, S.-H., de Oliveira Alvares, L. & Nader, K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat. Neurosci. 12, 905–912 (2009).
Ben Mamou, C., Gamache, K. & Nader, K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 9, 1237–1239 (2006).
Milton, A. L. et al. Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. J. Neurosci. 33, 1109–1115 (2013).
Clem, R. L. & Huganir, R. L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330, 1108–1112 (2010).
Espejo, P. J., Ortiz, V., Martijena, I. D. & Molina, V. A. Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology 109, 349–356 (2016).
Meyer, E. Behavioral, Biochemical, and Physiological Components of Stress-Enhanced Fear Learning in an Animal Model of Post-Traumatic Stress Disorder. PhD thesis, Univ. of California Los Angeles (2014).
Sotres-Bayon, F., Bush, D. E. A. & LeDoux, J. E. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology 32, 1929–1940 (2007).
Dalton, G. L., Wu, D. C., Wang, Y. T., Floresco, S. B. & Phillips, A. G. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology 62, 797–806 (2012).
Holehonnur, R. et al. Increasing the GluN2A/GluN2B ratio in neurons of the mouse basal and lateral amygdala inhibits the modification of an existing fear memory trace. J. Neurosci. 36, 9490–9504 (2016).
Rao-Ruiz, P. et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat. Neurosci. 14, 1302–1308 (2011).
Conoscenti, M. A., Smith, N. J. & Fanselow, M. S. Dissociable consequences of moderate and high volume stress are mediated by the differential energetic demands of stress. Soc. Sci. Res. Netw. https://doi.org/10.2139/ssrn.4029512 (2022).
Shimizu, K., Kurosawa, N. & Seki, K. The role of the AMPA receptor and 5-HT3 receptor on aggressive behavior and depressive-like symptoms in chronic social isolation-reared mice. Physiol. Behav. 153, 70–83 (2016).
Ellenbroek, B. & Youn, J. Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech. 9, 1079–1087 (2016).
Shi, Y.-W., Fan, B.-F., Xue, L., Wen, J.-L. & Zhao, H. Regulation of fear extinction in the basolateral amygdala by dopamine D2 receptors accompanied by altered GluR1, GluR1-Ser845 and NR2B levels. Front. Behav. Neurosci. 11, 116 (2017).
Jarome, T. J. et al. The timing of multiple retrieval events can alter GluR1 phosphorylation and the requirement for protein synthesis in fear memory reconsolidation. Learn. Mem. 19, 300–306 (2012).
Cardinal, R. N. & Aitken, M. R. F. Whisker: a client-server high-performance multimedia research control system. Behav. Res. Methods 42, 1059–1071 (2010).
Cardinal, R. N. & Aitken, M. R. F. ANOVA for the Behavioural Sciences Researcher (Lawrence Erlbaum Associates, Inc., 2006).
Glass, G. V., Peckham, P. D. & Sanders, J. R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Educ. Res. 42, 237–288 (1972).
Acknowledgements
This research was funded by the Department of Psychology, University of Cambridge. A.L.M. is the Ferreras-Willetts Fellow in Neuroscience at Downing College, University of Cambridge. The authors would like to thank G. Vousden for writing the MATLAB scripts that were used to quantify conditioned freezing.
Author information
Authors and Affiliations
Contributions
A.L.M. conceived and designed the experiments and analysis. I.A.V.A. and A.L.M. collected the data and performed the analysis for the behavioral experiments. M.S.P., O.S.R.P.S. and A.L.M. collected the data and performed the analysis for the western blotting experiments. A.L.M. wrote the manuscript, and all authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Julia Chester, Jennifer Quinn, Graziano Pinna and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Assche, I.A., Padilla, M.S., Stupart, O.S.R.P. et al. Refinement of the stress-enhanced fear learning model of post-traumatic stress disorder: a behavioral and molecular analysis. Lab Anim 51, 293–300 (2022). https://doi.org/10.1038/s41684-022-01054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-022-01054-4