Abstract
In this study, we conducted a comprehensive investigation on the influence of waste perlite powder (WPP) on the various properties of mortar and paste, including slurry performance, mechanical strength, hydration products, and microstructure. Additionally, we also aimed to uncover the underlying mechanism behind these effects. It was found that WPP reduced the workability and facilitated the setting process of cement-WPP pastes. WPP decreased the mechanical strength of mortar, but it exhibited significant strength enhancement during the subsequent stages. The incorporation of WPP worsened the water absorption behavior; however, this negative effect was mitigated as the curing age of the mortar was prolonged. The drying shrinkage cement-WPP binary system was significantly improved, with the prolongation of curing age. Moreover, WPP reduced the early heat release of the binary system, which may be beneficial for reducing the temperature gradient in mass concrete. It was confirmed that WPP accelerated the transformation from AFt to AFm, promoted the formation of C-A-S-H and optimized the pore structure of the system. It was confirmed that WPP was involved in the hydration reaction, which accelerated the transformation from AFt to AFm and promoted the formation of C-A-S-H. Such results suggested that the reuse of WPP in concrete production was technically feasible owing to its high pozzolanic reactivity.
Similar content being viewed by others
Introduction
In the year 2022, the cement production of China has achieved a significant milestone, reaching a staggering 2.13 billion tons. This remarkable figure accounts for approximately 51.20% of the global cement output, firmly establishing China as the leading producer in this industry. Meanwhile, other notable contributors to the worldwide cement production include India, Vietnam, and America, accounting for 9.24%, 2.95%, and 2.28% respectively. These countries have secured their positions at the top of the cement production list. In the realm of cement utilization, a significant proportion of 47% was allocated to the development of residential structures, while 32% was dedicated to the construction of commercial buildings. Moreover, an additional 21% was employed for civil works. The cement industry not only necessitated significant quantities of limestone, clay, and energy, but also discharged substantial quantities of dust and waste materials. The data illustrated that the manufacture of 1 ton of cement clinker requires around 1.5 tons of limestone, along with significant amounts of coal and petroleum for energy and power generation purposes. Besides, about 0.9 t CO2 and a large amount of nitrogen and sulfur oxides will be emitted in this process1. These emissions were identified as major contributors to the greenhouse effect and acid rain, thereby substantially elevating the environmental burden and presenting a significant hazard to both the natural ecosystem and human well-being. Therefore, it is of far-reaching significance for the sustainable development of construction industry and the harmonious coexistence of mankind and natural environment, and therefore it is necessary to take timely and effective measures to control CO2 emission of cement industry.
For several decades, the practitioners have been studied on the alternative materials to partially replace cement in concrete production, which was namely supplementary cementitious materials (SCMs), for instance, fly ash2,3, silica fume4,5 and slag6,7, thus decreasing the cement production and energy consumption, as well as the GHG (Greenhouse Gas) emissions. However, due to the uneven distribution of resources, there exist several problems in the utilization of SCMs for concrete production because of its scarce and costly in some countries and areas8. Therefore, the search for local resource-rich and low-cost SCMs, such as natural pozzolans, include natural rocks and volcanic sediments, is of great significance to the green and sustainable development of the concrete industry. Such natural pozzolans, for instance, kaolinite9, zeolite10 and volcanic ash11, etc., have been utilized successfully in construction projects. Perlite, which is considered as a typical natural pozzolan and rapidly cooled during volcanic eruptions and the interior of the particle presents a glassy structure with high SiO2 and Al2O3 content12 (Fig. 1), has also already attracted considerable attention of researchers.
Yu13 conducted a quantitative analysis on the pozzolanic impact of perlite powder on the mechanical characteristics of concrete by specific strength indices. The findings indicated a significant enhancement in the performance of the concrete due to its pozzolanic reactivity. Karein et al.8 conducted a comprehensive study employing perlite powder of varying specific surface areas as a substitute for cement, aiming to evaluate its impact on the workability, strength, and durability of self-compacting concrete (SCC). Research indicated that the mechanical grinding process significantly influenced the pozzolanic reactivity of perlite powder. Several studies have indicated that the incorporation of perlite as supplementary cementitious material could reduce the porosity and pore size in cement-based materials, altering the pore size and connectivity of the concrete matrix due to the reactivity of volcanic ash14. Erdem15 reported the utilization of natural perlite powder in blended cement production and found that blended cement incorporating perlite powder may decrease the early strength in comparison with Portland cement. Nevertheless, the mechanical properties gradually approached Portland cement with the increase in curing time because of its pozzolanic reaction. Notably, Turanli16 and Uzal17 have reported that the incorporation of a large amount of perlite powder in concrete could potentially exhibit lower strength in comparison with the control sample. Other research studies have delved into the issue of durability concerns. R. chihaoui et al.18 reported that the samples with 20% natural perlite powder showed similar mechanical strength compared to control, and the mortar sample modified by 20% natural perlite powder exhibited better resistance to sulfate attack and sulfuric acid attack. Fodil19 found that the incorporation of 10% pozzolan and 10% perlite significantly improved the resistance to Cl- ion ingress of concrete in corrosion environment within 1 year. However, the continuous increase of pozzolan and perlite content would have a detrimental effect on the durability properties.
Research showed that pozzolanic materials containing active SiO2 and Al2O3 could participate in the hydration process of cement and affect the generation of AFt, AFm, and C-S-H in the binary system, ultimately influencing the physical and mechanical properties of the system11,18,20,21,22. However, there was still limited research on the reaction mechanisms of dissolved silicates and aluminates, especially since the transformation of aluminate phases was often overlooked8,21,23,24. The purpose of this study was to evaluate the effect of natural waste perlite powder (WPP) on the fresh, mechanical properties of mortar, and the mechanism behind was revealed through the analysis of the hydration process, hydrates and microstructure. For this purpose, the workability, mechanical strength and hydration process were studied from the aspects of standard water requirement, setting time, compressive and flexural strength. The phase transformation in hydration process was characterized by XRD, SEM and NMR. Such results would give deep insight into hydration mechanism of cement-perlite powder system and also give useful experience for utilization of WPP in concrete industry.
Materials and methods
Materials
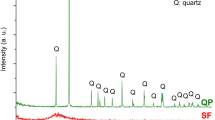
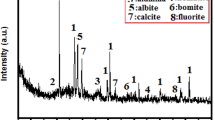
P·I 52.5 cement (PC) and WPP were used to prepare mortar and paste samples. The waste perlite powder was sourced from Henan, China. The chemical component of PC and WPP were examined by XRF, which is shown in Table 1. The (SiO2 + Al2O3 + Fe2O3) content of WPP reaches 86.76%. The mineral phases of PC and WPP were given in Fig. 2. The SEM image of WPP was shown in Fig. 3. The particle distributions of PC and WPP were shown in Fig. 4. The porosity and pore distribution of WPP particles were determined by nitrogen absorption and desorption method. The test results indicated that the total porosity of WPP particles was 0.01871 cm3/g, with an average pore size of 11.44 nm (Fig. 5).
Sample preparation
The mixture proportion of mortar is given in Table 2. The replacement level of WPP was 0% to 30%, and the corresponding number was recorded as M-C0, M-WP5, M-WP10, M-P20 and M-WP30, respectively. All the samples were prepared according to Chinese National Standard GB/T 17671-199925. Mortar samples for mechanical strength test were molded with a dimension of 40 × 40 × 160 mm3. Paste samples were molded with a dimension of 40 × 40 × 40 mm3, and the corresponding number was recorded as P-C0, P-WP5, P-WP10, P-WP20 and P-WP30, respectively. When the samples were cured to a specified time, broke the samples and terminated the hydration process with anhydrous ethanol. Then dried the samples at 40 °C for at least 24 h. The small pieces were used for microstructure analysis; and the remaining samples were ground to 200 mesh for hydration product analysis.
Testing methods
Macro tests
The standard water requirement and setting time was conducted based on Chinese National Standard GB/T 1346-201126. The workability of cement mortar was measured according to Chinese National Standard GB/T 2419-200527. The mechanical strength of mortar was tested at 3 days, 7 days, 28 days, 56 days and 91 days following Chinese National Standard GB/T 17671-199925.
The strength activity index (SAI) method could quantitatively analyze the pozzolanic reactivity of WPP according to Eq. (1)22.
where f0 and fp represent the compressive strength of M-C0 and WPP modified mortar, respectively.
In addition, the contribution of WPP to the compressive strength was calculated according to Eqs. (2)–(4)13,20.
where R is the specific strength that represents the contribution ratio of unit cement or WPP to the mortar compressive strength; f represents the compressive strength of mortar; q represents the ratio of cement or WPP to powder materials (cement + WPP); RC is the specific strength of control mortar; RM is the specific strength of WPP modified mortar; RP represents the contribution of pozzolanic reaction to mortar strength; P represents the contribution rate of pozzolanic activity to mortar strength.
According to the Chinese building materials industry standard JC/T603-2004, a drying shrinkage test was conducted. The mold dimension was set at 25 mm × 25 mm × 280 mm, with 3 samples retained per group. After demolding, the samples were placed in a drying shrinkage curing box for maintenance. The length of samples was recorded at different ages using a length comparator. The volume shrinkage rate (ε) of the mortar samples was calculated using the following Eq. (5):
where L0-the initial length; Lt-the length at time t; Lb-the standard length.
Mortar samples for the water absorption test were prepared with dimensions of Φ100 mm × 50 mm and were subsequently cured in a humidity chamber for a duration of 28 days. Following the curing process, the samples were immersed in water for a period of 2 days, with the resulting wet weight being denoted as M0. Finally, the samples were subjected to a vacuum oven at a temperature of 50 °C for a duration of 3 days in order to attain a constant weight, which was labeled as M1. The calculation of the total water absorption of the mortar was conducted using the provided Eq. (6):
Then, we applied a layer of paraffin on the surface of the sample, excluding the area in contact with water (Fig. 6). The weight of each sample was measured at various time intervals within a 7 days period. The water absorption per unit area was calculated according to Eq. (7), and then plotting it against the square root of time for linear fitting (Eq. (8)), the slope of the straight line represents the capillary water absorption coefficient S of the mortar.
where A—the area of specimen immersed in water, mm2; ΔM—the weight of absorbed water, g; S—the sorptivity coefficient of mortar, mm/min1/2; ρ—the water density, g/cm3; t—the absorption time, min.
Micro tests
The hydration heat of different cement pastes was characterized by an isothermal conduction calorimeter at 20 °C for 72 h.
The hydration products of different samples by a D8 Discovery diffractometer with a scanning angle of 5–60° and a scanning speed of 10°/min.
TG-DTG analysis was performed on NETZSCH STA 449C, Germany. The heating rate of the test was 10 °C/min, and the test temperature was from 35 °C to 1000 °C.
27Al and 29Si MAS-NMR analysis were conducted by a Bruker Avance III HD 400 MHz spectrometer. Cement hydration degree (αPC), waste perlite powder reaction degree (αWPP) and average chain length (ACL) of C-S-H gel was calculated according to Eqs. (9)–(12)28,29:
where I(Q0), I(Q1), I(Q2) and I(Q2 (Al)) represented the integrated intensities of signals Q0, Q1, Q2 and Q2 (Al) in hydrated cement sample, respectively.
A mercury intrusion porosimetry technique was employed to analyze the pore structure of the cement paste sample. The highest surface tension measured during testing was 480 MPa.
The analysis of the microstructure was performed by means of a scanning electron microscope. (JSM-5610LV, Japan).
Results and analysis
Setting time
From the results of the setting test in Fig. 7, the incorporation of WPP has little effect on the initial setting time, but greatly reduce the final setting time of cement pastes. For instance, the final setting of samples P-WP5, P-WP10, P-WP20 and P-WP30 were 46 min, 44 min, 45 min and 39 min earlier than that of control, which did not seem to be linear with WPP content. The shortening of the final setting time of pastes was mainly ascribed to the filler effect of fine WPP particle, which accelerated the hydration of C3A, C4AF and C3S30. As reported by Li31 and Jiang32, alkaline oxides, such as sodium oxide and potassium oxide, which were abundantly existed in natural perlite powder, can also promote the hydration of cement when dissolved in solution.
Standard water requirement and fluidity
Standard water requirement reflected the water demand for the pastes to achieve the standard consistency, which depended on water that needed to cover the surface and fill the voids between cement and WPP particles as well as the inner pores20. As seen in Fig. 8a, the incorporation of WPP significantly raised the standard water requirement. The water demand for WPP modified cement pastes increased by 4.76%, 10.50%, 17.15% and 29.53%, respectively.
Accordingly, the fluidity of mortar samples was also measured to investigate the influence of WPP on the workability of cement-based materials. A tendency similar to the results of the water demand test was observed from Fig. 8b. The fluidity value of samples M-WP5, M-WP10, M-WP20 and M-WP30 decreased by 4.76%, 9.85%, 22.17% and 31.53% in comparison with the control (M-C0), respectively. The test results of fluidity further revealed the adverse effect of WPP on the workability of cement. This may be due to the irregular morphology of WPP particles. It increased the friction between particles and hindered the mutual displacement of particles, leading to an increase in the water demand of the slurry and a decrease in flowability.
Mechanical strength
As seen in Fig. 9, at the early stages, the compressive strength of WPP samples was obviously reduced in comparison with M-C0. For example, the compressive strength of the control sample M-C0 at 7 days was 47.6 MPa. However, the compressive strength of samples M-WP5, M-WP10, M-WP20, and M-WP30 decreased by 7.9%, 11.3%, 27.9%, and 35.3% respectively when compared to M-C0. The results implied that the incorporation of WPP was not conducive to the early strength development of the cement-WPP binary system, which was due to the reduction of cement and the dilution of hydrates. Nevertheless, the margin between the compressive strength of WPP modified mortar and control sample gradually become smaller at later ages. For instance, the compressive strength of sample M-WP5 at 84 d was shown 2.1% higher, and that for samples M-WP10, M-WP20 and M-WP30 were 4.0%, 8.5% and 13.4% lower in comparison with sample M-C0. Based on the above analysis, it is obviously observed that the pozzolanic reaction activity of WPP was carried out slowly in the cement-WPP system until it began to accelerate in the later stage. For the cement-WPP binary system, the optimum content of WPP may be between 10 and 20%, thus the waste perlite powder can be fully utilized without excessive compromise in compressive strength.
The flexural strength of WPP modified mortar exhibits a similar tendency with its compressive strength in the early stage and the results were shown in Fig. 10. The 3 d, 7 d, and 28 d flexural strength of cement-WPP mortar was lower than that of the control, with the exception of sample M-WP5. However, after curing the WPP modified mortars for 56 days, the flexural strength significantly increased compared to the control. The flexural strength of M-WP5, M-WP10, M-WP20 and M-WP30 at 84 d was increased by 1.1%, 2.3%, 9.2% and 4.1%, respectively, which indicated that the incorporation of WPP would be beneficial to the later flexural strength of mortar. Furthermore, the progression of flexural strength for M-WP10, M-WP20 and M-WP30 between the 28 d and 84 d exhibited growth rates of 16.3%, 28.9%, and 13.3% respectively. In comparison, M-C0 displayed a rate of 6.9%. The result also demonstrated the high pozzolanic reaction activity of natural perlite powder in the later stage in the cement-WPP system.
Pozzolanic reaction activity of WPP
As shown in Table 3, the 28 d SAI value of 30% WPP modified mortar was 74.4%, which meets the Chinese National Standard GB/T 2847-200533. It can be inferred that the effect of WPP on the mechanical strength of the binary system was similar to that of the other SCMs. The increased SAI value at 28 d was primarily because of that the active silica and alumina took part in the hydration to form C-(A)-S-H which was beneficial to the mechanical strength34.
The strength contribution of WPP exhibited a sluggish progress during the early stage but demonstrated a steady rise over time, particularly as the curing period advanced. Notably, the strength contribution percentage attributed to the pozzolanic reaction in the M-WP30 sample surged to 18.5% after 56 days and further increased to 19.2% by 84 days (Fig. 11). The findings suggested that the pozzolanic effect of WPP cannot be brought into full play at early stage, however, it may gradually develop in the subsequent secondary reaction. These experimental outcomes offered valuable insights for the widespread incorporation of WPP in cementitious materials on a larger scale.
Water absorption
Saturated water absorption
The saturation water absorption rate of mortar is one of the characterization methods reflecting the size of its porosity, which can to a certain extent reflect the open porosity and structural compactness of cementitious materials. Harmful solutes and impurity ions in the external environment will enter the concrete through these connected pores together with water, thereby reducing the durability of the concrete. The better the impermeability, the more difficult it is for external solutions and harmful ions to enter the interior of concrete, resulting in better erosion resistance and freeze–thaw resistance.
Based on Fig. 12, it can be observed that the saturated water absorption of cement-WPP samples increased gradually with WPP content at 28 days. The saturated water absorption of M-WP5, M-WP10, M-WP20 and M-WP30 increased by 12.59%, 9.91% and 12.41%, respectively, compared with M-C0. However, after 180 days of curing, there was no significant difference in the saturated water absorption rate between the cement-WPP samples and M-C0. In fact, the saturated water absorption rate of M-WP30 even decreased by 4.58%. In essence, the increase in water absorption of mortar was attributed to the interconnected pore structure within the mixture34,35,36. It can be deduced that the pore structure of mortar was not deteriorated in the long-term due to the development of the pozzolanic reaction, which over time, also increased the compressive strength24.
Capillary water absorption
It is generally believed that the capillary water behavior of concrete can be divided into two stages: the first stage is from the initial time to 6 h, during which the capillary adsorption coefficient is called the initial sorptivity coefficient; the second stage is from 1 to 7 days, during which the capillary adsorption coefficient is called the secondary sorptivity coefficient. As shown in Fig. 13, the initial adsorption stage of the mortar is characterized by a higher slope of the fitted straight line, indicating a faster capillary absorption rate during this stage. However, in the secondary adsorption stage, the slope of the fitted straight line is noticeably smaller.
The fitting results of the capillary adsorption coefficients for cement-WPP mortar are shown in Table 4, and the variations of the initial and secondary adsorption coefficients are presented in Fig. 14. From Fig. 14a, it can be observed that the initial sorption coefficient of the mortar increased gradually with the increase of WPP content in the samples cured for 28 days and 180 days. Due to the continuous hydration of cement and ongoing secondary reactions, the initial sorptivity coefficient of the samples cured for 180 days was significantly lower compared to the samples cured for 56 days. Among them, samples with 5%, 10%, 20%, and 30% WPP dosage reduced the initial sorption coefficient by 77.16%, 64.72%, 60.89%, and 69.10%, respectively. In Fig. 14b, it can also be observed that the secondary sorption coefficient of the mortar containing WPP increases to varying degrees. Moreover, the secondary sorptivity coefficient of the samples cured for 28 days was lower than that of the specimens cured for 180 days. This was mainly due to the insufficient early hydration reaction of the specimens, the existence of large numbers of larger connected pores in the samples, resulting in higher initial sorptivity. The mortar samples reaching water saturation at this stage, leading to a lower secondary sorptivity coefficient. Overall, a large amount of WPP deteriorated the capillary water absorption performance of the mortar, but it would be improved with the prolongation of the curing age.
Drying shrinkage
In this section, the effect of WPP on the volume stability of cement-based cementable materials was investigated. Figure 15 showed the variation of drying shrinkage of mortar in cement-perlite powder system. As can be seen from the figure, cement-WPP mortar had a faster drying shrinkage growth rate in the early stage and poor volume stability. Among them, the 3d drying shrinkage of M-WP10, M-WP20 and M-WP30 increased by 0.83%, 33.33% and 35.83%, respectively, compared with the control sample. This was mainly due to the physical and chemical characteristics of WPP particles themselves: WPP was a product formed during volcanic eruption, with large numbers of pores inside, which might absorb the free water in the early curing stage, resulting in shrinkage of the system volume. With the extension of curing age, the filling effect of WPP particles took a dominant position, and the fine WPP particles optimized the pore structure inside the mortar. In addition, the pozzolanic effect of WPP began to gradually play out, and reacted with Ca(OH)2 to form C-S-H gel and filled the pores, which reduced the connectivity of the internal pore structure and increased the density of the structure, thus improving the drying shrinkage of the system. After curing to 28 days, the drying shrinkage of 10%, 20% and 30%WPP mortar decreased by 3.80%, 6.96% and 8.26%, respectively. At 90 days, drying shrinkage decreased by 6.02%, 12.21% and 13.83%, respectively. On the whole, cement-WPP mortar shows fast early-age drying shrinkage growth and poor volume stability. However, with the prolongation of curing age, the cement-WPP binary system has been significantly improved, as evidenced by the reference specimens.
Analysis of the hydration process
Hydration heat evolution
Figure 16 presents the hydration flow and cumulative hydration heat curve of blended pastes. The heat flow shown in Fig. 16a indicated that cement hydration in the early age was slightly facilitated by incorporating WPP. The main peak of P-WP30 paste appeared 54.5 min in advance compared to P-C0. Moreover, an additional peak appeared at around 25–30 h in cement-WPP binary pastes, which was likely associate with the formation of AFm. It is speculated that WPP promoted the transformation from AFt to AFm because of its high aluminum content.
The induction period was slightly delayed in the pastes with WPP addition (Fig. 16b). However, the hydration heat flow per mass of cement in the acceleration period was facilitated in the paste samples containing WPP compared to P-C0. Despite this, the cumulative heat of cement-WPP samples was still much lower than pure cement paste (Fig. 16c and Table 5). For instance, 5%, 10%, 20% and 30% reduced the 72 h cumulative heat by 5.3%, 9.6%, 17.1% and 25.6%, respectively. From the point of view of cement hydration, the hydration heat release per mass of cement in cement-WPP paste samples was obviously higher than P-C0, especially the early age cumulative heat (Fig. 16d). Combined the statistics in Table 5, the 24 h cumulative heat per mass of cement in pastes containing 5–30% WPP increased by 1.6%, 3.7%, 9.4% and 15.5%, which meant the promotion in cement hydration degree. The main reason was ascribed to the dilution and nucleation effect of fine WPP particles, which facilitated the hydration process and the formation of the C-(A)-S-H phase. Celik11 found that 30–50% natural pozzolan increased the heat release in the acceleration period slightly. Kunal37 reported that fine natural pozzolan could participate in the hydration. As a result, the formation of AFm and C-(A)-S-H was facilitated. The results further proved the benefits of WPP utilization in concrete production, especially in reducing heat release of mass concrete in the early stage.
XRD and thermal analysis
The XRD traces for different paste samples versus curing time are presented in Fig. 17. No obvious mineralogical changes are observed in the hydrated samples with or without WPP addition. The main mineralogical phases of the hydrated paste are tricalcium silicate (C3S), dicalcium silicate (C2S), portlandite (CH), and ettringite (AFt). Additionally, C-S-H gel cannot be detected by XRD since its poor crystallinity.
Furthermore, the intensity of diffraction peaks corresponding to C3S and C2S in samples with WPP addition was weaker than that of control sample P-C0 because of the reduction of cement amount. The intensity of diffraction peaks corresponding to AFm phase of sample P-C0 was slightly increased when WPP was incorporated, which may be ascribed to the increase of aluminum available in the cement-WPP binary system11. Nevertheless, the intensity of diffraction peaks of CH in the samples with WPP addition was less intense in comparison with the reference at all periods up to 56 d. To analyze the changes of the amount of hydration products further quantitatively, thermal analysis was employed in this study.
As seen in Fig. 18, the thermogravimetric curve of different paste samples cured for 28 d and 56 d were analyzed. The mass loss of CH and calcite was calculated according to the TG curve and the calculation results were shown in Table 6. The CH crystal decomposes in the temperature range of 400–550 °C; and the calcite, which was carbonized from CH, decomposes in the temperature range of 650–800 °C. The total CH content of paste samples was obtained by the following Eq. (13).
Figure 19 presents the CH contents in different paste samples cured for 28 d and 56 d. The value of CH content per unit mass binder was observed to decline with the increasing WPP substitution level (Fig. 19a): the CH content in samples with 5%, 10%, 20% and 30% WPP at 28 d were 1.75%, 5.24%, 13.54% and 20.09% lower than that of reference sample P-C0, respectively; and the reduced value at 56 d was 4.31%, 7.76%, 15.52% and 22.8%, respectively. The declining level was lower than the replacement ratio of cement, and the possible reason was that the dilution effect of WPP promoted the cement hydration. Furthermore, the CH content of P-C0 at 56 d was 1.31% higher than that at 28 d; however, the value decreased by 1.33%, 1.38%, 1.01% and 2.19% in the samples with 5%, 10%, 20% and 30% WPP, respectively. The decreased CH content with the curing time further demonstrated the pozzolanic reactivity of WPP at later ages, which was related to the C-(A)-S-H formation. However, the CH content per unit mass cement of blended pastes in Fig. 19b was observed to increase with WPP addition at both 28 d and 56 d.
Solid-state 27Al and 29Si MAS NMR spectroscopy analysis
27Al NMR was used to trace the changes of aluminum phase in cement and cement-WPP system and the spectrum was shown in Fig. 20. The 27Al NMR spectra had a higher resolution than that of XRD patterns due to the smaller proportion of aluminum phase in cement hydration products. As seen in Fig. 20, the characteristic peaks of WPP were located at around 2 ppm and 52 ppm. According to the previous studies, the characteristic peak between 12 and 14 ppm belongs to ettringite (AFt)38,39,40; the characteristic peak between 9 and 12 ppm belongs to monosulfate (AFm)39,40; the characteristic peak between 4 and 5 ppm belongs to the third aluminate hydrate (TAH)39,41; and the characteristic peak at 40–90 ppm was associated with the substitution of Si4+ ions by Al3+ ions in C-S-H gel42,43,44,45. In comparison with P-C0 at 56d, the AFt characteristic peak of P-WP30 shifted to the characteristic peak of AFm, implying that incorporation of WPP promoted the transformation from AFt to AFm. Celik11 also reported similar results in 70% cement and 30% natural pozzolan blend paste. He found that in the binary system of cement and natural pozzolan, AFt became unstable after 7 d hydration, and it would react with the unreacted aluminates and ferrites to form AFm. In this research, it was further confirmed that the formation of C-A-S-H, TAH and AFm was facilitated because of the incorporation of WPP. The transformation process of AFt to AFm was carried out according to Eq. (14)45,46.
The raw materials and hydrated samples were tested by 29Si NMR analysis (Figs. 21, 22). In Fig. 21, the Q0 singal in cement and Q3, Q4 singal in perlite were clearly observed. The appearance of Q1, Q2 and Q2(1Al) indicated the formation of different hydrates.
Combined with the calculation results in Table 7, the Q0 sin gal accounts for 40.03% and the (Q3 + Q4) singal accounts for 59.97% in the unhydrated P-WP30 sample. In P-C0 sample cured for 56 d, the cement hydration degree was 62.17%. Nevertheless, the cement hydration degree of P-WP30 was 78.12%, which was 25.90% higher than that of control. This was mainly due to: (i) the nucleation effect caused by fine WPP particles facilitated the hydration process; (ii) the cement particles disperse better because of the dilution effect, which was beneficial to the cement hydration. Furthermore, the ACL of P-WP30 was 4.53, which was increased by 58.4% compared to that of P-C0, suggesting the increase of polymerization degree. The appearance of Q2(1Al) indicated the substitution of Si4+ ions by Al3+ ions in silicon tetrahedron and the Al/Si ratio was 0.046. The results further confirmed that the aluminum phase of WPP dissolved in solution could be involved in the hydration reaction. Based on the analysis above, it was inferred that WPP modified the chemical phase of the hydrates and the pozzolanic reactivity developed rapidly in the later period. As a result, the reaction degree of WPP at 56 d reached 19.53%.
Pore structure analysis
The pore size distribution and cumulative porosity of P-C0 and P-WP30 samples at 28 d was shown in Fig. 23. From the perspective of cumulative pore volume, the total porosity of P-C0 sample was 0.1327 mL g−1, while that of P-WP30 sample was 0.1327 mL g−1, which was almost consistent. However, there were obvious differences in the distribution of pore structure between P-C0 and P-WP30. In the P-WP30 sample, the total volume of pores with dimensions above 50 nm was 0.0146 mL g−1, which was reduced by 73.51% compared to the P-C0 sample (Table 8). Moreover, the most probable aperture of sample P-WP30 was 30.09 nm, indicating a reduction of 55.24% compared to sample P-C0. This was primarily due to the participation of WPP in the late-stage pozzolanic reaction in the binary system, leading to the formation of a large amount of C-(A)-S-H gel. The additional C-S-H gels filled the voids between cement and WPP particles, effectively optimizing the pore structure and significantly reducing the pore size. According to Ghrici et al.47, it was also noted that the pozzolanic reaction played a significant role in refining the pore structures of the hardened cement paste.
Microstructure analysis
Figure 24 shows the micrographs of the hardened pastes. A large number of C-S-H gels formed a dense microstructure in P-C0 3d sample. In addition, a small number of hexagonal plate-shaped CH crystals and needle-shaped AFt crystals were embedded in hydrates. In P-WP30 3d sample, plenty of C-S-H crystals grew outwardly along the WPP particle surface. Moreover, some flocculent hydrates were observed to form on WPP particle surface, which further confirmed the pozzolanic activity of WPP. As previous literature reported20, in alkaline environment, the Si–O and Al-O bonds of WPP were broken and reacted with calcium ions to produce C-(A)-S-H that adsorbed on the surface of WPP particles.
In P-C0 28d sample, a much denser microstructure of the matrix was observed, and a small amount of whisker-like C-S-H existed as well. Nevertheless, a large number of unreacted WPP particles were observed in P-WP30 28d sample, indicating the low pozzolanic activity of WPP in the early stage. The connection between the WPP particles and hydration products was relatively tight, and no obvious pores were found in the interface transition zone.
Overall discussion
Several test methods include standard water requirement, fluidity, setting time, mechanical strength, drying shrinkage, hydration heat, pore structure, XRD, TG, NMR and SEM was employed to assess the impact of WPP on fresh properties, mechanical characteristics, hydration products and microstructure of cement-based materials. The findings revealed that WPP facilitated the process of setting and raised the standard water demand of the binary system. Erdem et al.15 observed similar findings in their study where perlite was employed as a pozzolanic admixture for producing blended cements. WPP contributed little to the early-stage strength; however, notable advancements were observed during the later stages due to its pozzolanic reactivity, particularly evident in the enhancement of flexural strength. R. Chihaouia18 has also documented a comparable outcome. The incorporation of WPP worsened the water absorption behavior; nonetheless, this negative effect was alleviated with the extension of the curing period. Furthermore, the drying shrinkage cement-WPP binary system was significantly improved as the curing duration was prolonged.
Microscopically speaking, it was inferred that the incorporation of WPP would alter the phase of hydration product. Due to high content of aluminum phase, the formation of AFm in cement-WPP binary system was facilitated in the later stage. Meanwhile, a more stable C-(A)-S-H phase was also formed. The early-stage hydration exothermic analysis demonstrated that the incorporation of WPP reduced the hydration exothermic rate of the binary system, exhibiting a trend similar to that of natural pozzolan48 and fly ash49. Nevertheless, the early hydration of cement was accelerated due to the dilution effect and nucleation effect of WPP. In general, the appropriate amount of WPP reduced the hydration heat of the system, improved the late strength and optimized the microstructure, which was of great significance for its wide application in cement-based materials.
From the perspective of waste resource utilization, the WPP involved in this study was considered a by-product of the mining industry, suitable for application for the locally concrete production. Therefore, WPP presented itself as a promising substitute material for cement due to its abundant availability. The incorporation of WPP into concrete production not only enables the effective utilization of WPP resources but also carries potential benefits. It is of great significance to the resource utilization of waste and the sustainable development of concrete industry.
Conclusions
Several conclusions could be summarized based on the discussions above.
-
1.
The incorporation of WPP raised the standard water requirement compared with pure cement paste. The water demand for paste increased by 29.53% at 30% substitution ratio.
-
2.
The incorporation of WPP shortened the setting process of pastes, especially the final setting. The final setting of pastes with 5%, 10%, 20% and 30% WPP were 46 min, 44 min, 45 min and 39 min earlier than that of cement paste. Despite that, the setting process of cement-WPP binary pastes still complied with the Chinese National Standard GB1346-201126.
-
3.
WPP contributed little to the early strength but exhibited significant strength development in the later stages due to its pozzolanic reactivity, particularly in terms of flexural strength.
-
4.
The incorporation of WPP deteriorated the capillary water absorption performance of the mortar, but it would be improved with the prolongation of the curing age.
-
5.
Cement-WPP mortar shows fast early-age drying shrinkage growth and poor volume stability. However, with the prolongation of curing age, the cement-WPP binary system has been significantly improved, as evidenced by the reference specimens.
-
6.
The incorporation of WPP significantly decreased the 72 h cumulative heat of the binary system. However, the released heat per mass of cement was increased with WPP content, which could be ascribed to its dilution effect and nucleation effect in cement-WPP system.
-
7.
The incorporation of WPP induced the changes of chemical phase in hydrates since a large amount of aluminum phase existed in WPP, which accelerated the transformation of AFt to AFm, and promoted the formation of C-(A)-S-H phase with higher stability. Accordingly, the reaction degree of WPP at 56 d reached 19.53%.
The findings of this study have shown that the incorporation of waste perlite powder in the concrete mix design, in order to generate concrete with enhanced economic advantages, is indeed feasible from a technical standpoint. The utilization of WPP not only helps to reduce its accumulation, thus leading to a decrease in potential health hazards associated with airborne particles, but also serves as a valuable contribution towards the sustainable development of the ecological environment.
Data availability
All data generated or analysed during this study are included in this published article.
References
Novak, R., Schneider, W. & Lang, E. New knowledge regarding the supersulphated cement Slagstar. ZKG Int. 58, 70–78 (2005).
Khan, M. & Ali, M. Improvement in concrete behavior with fly ash, silica-fume and coconut fibres. Constr. Build. Mater. 203, 174–187. https://doi.org/10.1016/j.conbuildmat.2019.01.103 (2019).
Cho, Y. K., Jung, S. H. & Choi, Y. C. Effects of chemical composition of fly ash on compressive strength of fly ash cement mortar. Constr. Build. Mater. 204, 255–264. https://doi.org/10.1016/j.conbuildmat.2019.01.208 (2019).
Chu, S. H. & Kwan, A. K. H. Co-addition of metakaolin and silica fume in mortar: effects and advantages. Constr. Build. Mater. 197, 716–724. https://doi.org/10.1016/j.conbuildmat.2018.11.244 (2019).
Gökçe, H. S., Hatungimana, D. & Ramyar, K. Effect of fly ash and silica fume on hardened properties of foam concrete. Constr. Build. Mater. 194, 1–11. https://doi.org/10.1016/j.conbuildmat.2018.11.036 (2019).
Xie, J., Wang, J., Zhang, B., Fang, C. & Li, L. Physicochemical properties of alkali activated GGBS and fly ash geopolymeric recycled concrete. Constr. Build. Mater. 204, 384–398. https://doi.org/10.1016/j.conbuildmat.2019.01.191 (2019).
Xie, J., Wang, J., Rao, R., Wang, C. & Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. B Eng. 164, 179–190. https://doi.org/10.1016/j.compositesb.2018.11.067 (2019).
Karein, S. M. M., Joshaghani, A., Ramezanianpour, A. A., Isapour, S. & Karakouzian, M. Effects of the mechanical milling method on transport properties of self-compacting concrete containing perlite powder as a supplementary cementitious material. Constr. Build. Mater. 172, 677–684. https://doi.org/10.1016/j.conbuildmat.2018.03.205 (2018).
Fan, Y., Zhang, S., Kawashima, S. & Shah, S. P. Influence of kaolinite clay on the chloride diffusion property of cement-based materials. Cement Concr. Compos. 45, 117–124. https://doi.org/10.1016/j.cemconcomp.2013.09.021 (2014).
Chen, J. J., Li, L. G., Ng, P. L. & Kwan, A. K. H. Effects of superfine zeolite on strength, flowability and cohesiveness of cementitious paste. Cement Concr. Compos. 83, 101–110. https://doi.org/10.1016/j.cemconcomp.2017.06.010 (2017).
Celik, K., Hay, R., Hargis, C. W. & Moon, J. Effect of volcanic ash pozzolan or limestone replacement on hydration of Portland cement. Constr. Build. Mater. 197, 803–812. https://doi.org/10.1016/j.conbuildmat.2018.11.193 (2019).
Rashad, A. M. A synopsis about perlite as building material—A best practice guide for Civil Engineer. Constr. Build. Mater. 121, 338–353. https://doi.org/10.1016/j.conbuildmat.2016.06.001 (2016).
Yu, L. H., Ou, H. & Lee, L. L. Investigation on pozzolanic effect of perlite powder in concrete. Cem. Concr. Res. 33, 73–76 (2003).
Yu, L. H., Ou, H. & Zhou, S. X. Influence of perlite admixture on pore structure of cement paste. Adv. Mater. Res. 97–101, 552–555 (2010).
Erdem, T. K., Meral, Ç., Tokyay, M. & Erdoğan, T. Y. Use of perlite as a pozzolanic addition in producing blended cements. Cement Concr. Compos. 29, 13–21. https://doi.org/10.1016/j.cemconcomp.2006.07.018 (2007).
Turanli, L., Uzal, B. & Bektas, F. Effect of large amounts of natural pozzolan addition on properties of blended cements. Cem. Concr. Res. 35, 1106–1111. https://doi.org/10.1016/j.cemconres.2004.07.022 (2005).
Uzal, B., Turanli, L. & Mehta, P. K. High-volume natural pozzolan concrete for structural applications. ACI Mater. J. 104, 535–538 (2007).
Chihaoui, R., Khelafi, H., Senhadji, Y. & Mouli, M. Potential use of natural perlite powder as a pozzolanic mineral admixture in Portland cement. J. Adhes. Sci. Technol. 30, 1930–1944. https://doi.org/10.1080/01694243.2016.1171568 (2016).
Fodil, D. & Mohamed, M. Compressive strength and corrosion evaluation of concretes containing pozzolana and perlite immersed in aggressive environments. Constr. Build. Mater. 179, 25–34. https://doi.org/10.1016/j.conbuildmat.2018.05.190 (2018).
Medina, G., Sáez del Bosque, I. F., Frías, M., Sánchez de Rojas, M. I. & Medina, C. Granite quarry waste as a future eco-efficient supplementary cementitious material (SCM): Scientific and technical considerations. J. Clean. Prod. 148, 467–476. https://doi.org/10.1016/j.jclepro.2017.02.048 (2017).
Motahari Karein, S. M., Vosoughi, P., Isapour, S. & Karakouzian, M. Pretreatment of natural perlite powder by further milling to use as a supplementary cementitious material. Constr. Build. Mater. 186, 782–789. https://doi.org/10.1016/j.conbuildmat.2018.08.012 (2018).
Zeyad, A. M., Tayeh, B. A. & Yusuf, M. O. Strength and transport characteristics of volcanic pumice powder based high strength concrete. Constr. Build. Mater. 216, 314–324. https://doi.org/10.1016/j.conbuildmat.2019.05.026 (2019).
Sayadi, A., Neitzert, T. R. & Charles Clifton, G. Influence of poly-lactic acid on the properties of perlite concrete. Constr. Build. Mater. 189, 660–675. https://doi.org/10.1016/j.conbuildmat.2018.09.029 (2018).
Guenanou, F., Khelafi, H. & Aattache, A. Behavior of perlite-based mortars on physicochemical characteristics, mechanical and carbonation: Case of perlite of Hammam Boughrara. J. Build. Eng. 24, 100734 (2019).
Method of testing cements-Determination of strength, GB/T 17671-1999, China (1999).
Test methods for water requirement of normal consistency, setting time and soundness of the portland cement, GB/T 1346-2011, China (2011).
Test method for fluidity of cement mortar, GB/T 2419-2005, China (2005).
Andersen, M. D., Jakobsen, H. J. & Skibsted, J. Characterization of white Portland cement hydration and the CSH structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 34, 857–868 (2004).
Richardson, I. & Groves, G. The structure of the calcium silicate hydrate phases present in hardened pastes of white Portland cement/blast-furnace slag blends. J. Mater. Sci. 32, 4793–4802 (1997).
Frías, M. et al. Scientific and technical aspects of blended cement matrices containing activated slate wastes. Cem. Concrete Compos. 48, 19–25. https://doi.org/10.1016/j.cemconcomp.2014.01.002 (2014).
Li, Z., Afshinnia, K. & Rangaraju, P. R. Effect of alkali content of cement on properties of high performance cementitious mortar. Constr. Build. Mater. 102, 631–639. https://doi.org/10.1016/j.conbuildmat.2015.10.110 (2016).
Jiang, S., Kim, B. G. & Aïtcin, P. Importance of adequate soluble alkali content to ensure cement/superplasticizer compatibility. Cem. Concrete Res. 29, 71–78 (1999).
Pozzolanic materials used for cement production, GB/T 2847-2005 China (2005).
Hossain, K. M. A. Volcanic ash and pumice as cement additives: pozzolanic, alkali-silica reaction and autoclave expansion characteristics. Cem. Concr. Res. 35, 1141–1144. https://doi.org/10.1016/j.cemconres.2004.09.025 (2005).
Awoyera, P. O., Dawson, A. R., Thom, N. H. & Akinmusuru, J. O. Suitability of mortars produced using laterite and ceramic wastes: Mechanical and microscale analysis. Constr. Build. Mater. 148, 195–203. https://doi.org/10.1016/j.conbuildmat.2017.05.031 (2017).
Bisht, K. & Ramana, P. Sustainable production of concrete containing discarded beverage glass as fine aggregate. Constr. Build. Mater. 177, 116–124 (2018).
Kupwade-Patil, K., Palkovic, S. D., Bumajdad, A., Soriano, C. & Büyüköztürk, O. Use of silica fume and natural volcanic ash as a replacement to Portland cement: Micro and pore structural investigation using NMR, XRD, FTIR and X-ray microtomography. Constr. Build. Mater. 158, 574–590. https://doi.org/10.1016/j.conbuildmat.2017.09.165 (2018).
Qu, B., Martin, A., Pastor, J. Y., Palomo, A. & Fernández-Jiménez, A. Characterisation of pre-industrial hybrid cement and effect of pre-curing temperature. Cem. Concr. Compos. 73, 281–288. https://doi.org/10.1016/j.cemconcomp.2016.07.019 (2016).
Rottstegge, J., Wilhelm, M. & Spiess, H. W. Solid state NMR investigations on the role of organic admixtures on the hydration of cement pastes. Cem. Concr. Compos. 28, 417–426. https://doi.org/10.1016/j.cemconcomp.2005.12.002 (2006).
Brunet, F., Charpentier, T., Chao, C. N., Peycelon, H. & Nonat, A. Characterization by solid-state NMR and selective dissolution techniques of anhydrous and hydrated CEM V cement pastes. Cem. Concr. Res. 40, 208–219. https://doi.org/10.1016/j.cemconres.2009.10.005 (2010).
Murgier, S., Zanni, H. & Gouvenot, D. Blast furnace slag cement: a 29Si and 27Al NMR study. Comptes Rendus Chimie 7, 389–394. https://doi.org/10.1016/j.crci.2004.02.004 (2004).
Le Saout, G., Lécolier, E., Rivereau, A. & Zanni, H. Chemical structure of cement aged at normal and elevated temperatures and pressures. Cem. Concr. Res. 36, 71–78. https://doi.org/10.1016/j.cemconres.2004.09.018 (2006).
Coleman, N. J. & Li, Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white Portland cement. Mater. Sci. Eng. C Mater. Biol. Appl. 33, 427–433. https://doi.org/10.1016/j.msec.2012.09.009 (2013).
Andersen, M. D., Jakobsen, H. J. & Skibsted, J. Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 34, 857–868. https://doi.org/10.1016/j.cemconres.2003.10.009 (2004).
De Weerdt, K. et al. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 41, 279–291. https://doi.org/10.1016/j.cemconres.2010.11.014 (2011).
Schöler, A., Lothenbach, B., Winnefeld, F. & Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 55, 374–382. https://doi.org/10.1016/j.cemconcomp.2014.10.001 (2015).
Ghrici, M., Kenai, S. & Said-Mansour, M. Mechanical properties and durability of mortar and concrete containing natural pozzolana and limestone blended cements. Cem. Concr. Compos. 29, 542–549 (2007).
Senhadji, Y., Escadeillas, G., Khelafi, H., Mouli, M. & Benosman, A. S. Evaluation of natural pozzolan for use as supplementary cementitious material. Eur. J. Environ. Civil Eng. 16 (2012).
Rojas, M. I. S. d., Luxan, M. P., Frias, M. & Garcia, N. The influence of different additions on portland cement hydration heat. Cem. Concr. Res. 23, 46–54 (1993).
Acknowledgements
This research was financially supported by Huanggang Normal University (202212004) and Huanggang Ecological Architecture and Renewable Resources Research Center (202315904).
Author information
Authors and Affiliations
Contributions
J.W., X.L., and H.T. wrote the main manuscript text. J.W., C.H., B.M. and, B.L. conducted experiments and prepared figures. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Lu, X., He, C. et al. Mechanical properties and microscopic characterization of waste perlite powder as supplementary cementitious material. Sci Rep 14, 21963 (2024). https://doi.org/10.1038/s41598-024-71968-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71968-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.