Abstract
Requiem sharks (genus Carcharhinus) have previously been reported to form large aggregations around marine infrastructures in the eastern Mediterranean Sea. While this behaviour may offer fitness advantages at the individual level, the implications of extended residency at human-altered habitats for population persistence have yet to be assessed. In this work, we investigated the phylogeographic and demographic composition of sharks near a coal-fired power and desalination station in Israel. Our aim was to infer habitat use and the mechanisms underlying the aggregation behaviour, and to highlight potential conservation impacts. We sampled, measured, and released 70 individuals between 2016 and 2022 to assess genetic variability within the cytochrome C oxidase I (COI) region and to analyse the aggregation’s structure based on the sharks’ size and sex distribution. In addition, we performed meristic counts on a reference specimen collected dead at another power station in Israel to supplement species identification using the abovementioned techniques. Our findings indicate size-based sex segregation of adult female dusky and male sandbar sharks (Carcharhinus obscurus and Carcharhinus plumbeus, respectively), with each species comprising two COI haplotypes. In the dusky shark, one haplotype corresponded to an Indo-Pacific lineage, and the other matched an Atlantic lineage. In the sandbar shark, we observed a haplotype previously sampled in the Mediterranean Sea, the Red Sea, the Northwest Indian Ocean, and South Africa, and another haplotype that was unique to our study site and genetically closer to the former than to sequences sampled in other ocean basins. This study provides the first indication of sympatric aggregation amongst phylogeographically distinct dusky and sandbar shark lineages, suggesting that human-altered habitats in the eastern Mediterranean Sea may influence the distribution of these species. Based on the observed segregation pattern, we conclude that the site does not function as a nursery, parturition, or mating area, and discuss other plausible explanations that warrant further research. Finally, we highlight important directions for future research and the implications of our findings for management and conservation.
Similar content being viewed by others
Introduction
A shark aggregation is defined as the clustering of multiple individuals at the same time and location, influenced by environmental conditions or the availability of shared resources1. This behaviour is typically discussed within the context of foraging, refuging, or reproducing and has been documented across a wide range of species, from planktivores to high-level predators in offshore and coastal ecosystems2. Although forming large or dense groups may entail adverse impacts on individuals, such as elevated susceptibility to parasite infection, increased exposure to predator or prey species, and heightened competition for resources, it is generally assumed to provide a fitness advantage that outweighs those natural risks3. However, large aggregations have also been observed around marine infrastructures, and there is a growing recognition that human-altered habitats might function as ecological traps, i.e., sites that are more attractive for settlement than other available options, less suitable for reproduction and survival, or both4. Furthermore, in situations of a population size below a critical threshold, these factors might instigate a decline or even extinction5.
For example, Robinson et al.6 reported hundreds of whale sharks (Rhincodon typus) foraging seasonally around offshore gas platforms in Qatar, and various other studies have recorded requiem sharks (genus Carcharhinus) near fish farms and coastal power stations in Hawai’i and the eastern Mediterranean Sea (EMS)7,8,9,10. While not explicitly addressing the notion of ecological traps, some of these studies discuss potential impacts on population persistence (e.g., disruption of seasonal migration patterns7) and provide evidence of compromised habitat quality (e.g., presence of debilitated individuals8). Conceivably, the diversion from natural migration routes might further increase the sharks’ exposure to threats while occupying, approaching, or leaving these coastal areas. Finally, the cues evolutionarily used by sharks for settlement might not yield the expected reproductive or metabolic outcomes outside their usual environmental context. Thus, while aggregations in human-altered habitats may ostensibly manifest positive effects on individual fitness, considering potential risks at a population level and on a case-by-case basis is imperative, particularly in situations involving threatened species.
In the present study, we investigated the utility of phylogeographic and demographic composition to inform the drivers associated with aggregation behaviour of sharks at the Orot-Rabit power and desalination station in Israel (32° 27′ 52″ N, 034° 52′ 57″ E). This site has been reported to host female dusky- and male sandbar sharks (Carcharhinus obscurus and Carcharhinus plumbeus, respectively)11,12,13, yet their species identification and size assessment were based solely on visual estimates. Arguably, it is prudent to integrate molecular techniques to enhance the validity of those preliminary observations, as the two species are notoriously difficult to distinguish from one another and from other carcharhinids14. Moreover, C. obscurus and C. plumbeus are listed by the International Union for the Conservation of Nature (IUCN) as Data Deficient (DD) and Endangered (EN) in the Mediterranean Sea, respectively15,16; the striking contrast between their conservation status and seasonal occurrence in large numbers near marine infrastructures warrant a molecular investigation of population connectivity between that region and both the Atlantic and Indian Oceans.
Additionally, the demographic composition of sharks at human-altered habitats can be used as a proxy for the mechanisms underlying the aggregation behaviour1. For example, the occurrence of juvenile, sub-adult, or sexually mature individuals may provide insight into a site’s functionality as a parturition or nursery ground, mating area, or adult habitat17. In turn, establishing the proximate causes behind site occupancy may help evaluate its capacity to yield the expected outcomes in the context of potential or identified threats. By incorporating both phylogeographic and demographic data, this study aims to improve our understanding of shark population connectivity and structure in the EMS, and to provide insight into the factors influencing segregation in multi-species complexes, which is crucial for effective management and conservation efforts2,18.
Methods
Fieldwork
We captured, sampled, and released 70 sharks in the warm water effluents discharged from the cooling system of the Orot-Rabin plant between 17 January 2017 and 8 May 2022. Our capturing technique involved a handline operated from the vessel and autonomous passive drumlines distributed in the study area. The handline consisted of a 6 millimetre (mm)/150 metre (m) polypropylene rope attached to a 4 mm/3 m double-stranded monofilament leader and threaded through a protective vinyl tube. The leader terminated at a 16/0 5°-offset carbon steel circle hook (Lindgren-Pitman, FL, USA) baited with mackerel, squid, herring or bream. In addition, we attached a large, inflatable buoy float to the handline, approximately 2 m from the leader, to keep the captured shark near the water surface and prevent excessive tension while leading it towards the vessel. The drumlines consisted of a 6 mm/4–6 m polypropylene rope running vertically from a submerged weight on the seafloor to two surface floats approximately 3 m apart, i.e., one directly above the weight and another at the standing end. In addition, a 4 mm/10 m gangion line was attached to the vertical rope ~ 0.5 m below the first float and, at its other end, a leader of the abovementioned design. The snaps provided for the gangion line’s stepwise removal from the vertical and attachment at the end of the 150 m handline in case of a successful capture.
Following capture, we restrained the shark alongside the vessel with its anterior end upstream to facilitate irrigation of the gills. To identify individuals upon future recaptures, we marked each shark with a passive integrated transponder micro-tag (PIT; Biomark, ID, USA) at the dorsal surface, and inserted a stainless-steel dart tag (Hallprint Pty Ltd., Australia) labelled with a serial number on the opposite side of the fin. We then measured the precaudal length (PCL) to the nearest 0.5 centimetre (cm). In males, we also measured the inner clasper length (ICL; in cm) and noted whether they were calcified as a proxy of sexual maturity19 alongside previously published length-at-maturity estimates. Finally, we obtained a dorsal fin clip for deoxyribonucleic acid (DNA) extraction. We recorded our observations using the shark tagging feature on Delphis (version 0.350), a data collection mobile app, and utilised a custom script for automated post-survey data processing in the R statistical environment (version 4.3.1)20. The entire sampling procedure was approved by the Israel Nature and National Parks Protection Authority (INPA; permit numbers 2016/41315, 2017/41714, 2018/42027, 2019/42412, 2021/42699, 2022/42926), performed in compliance with its regulations, and reported in accordance with the ‘Animal Research: Reporting of In Vivo Experiments’ (ARRIVE) guidelines.
Molecular analysis
We extracted total genomic DNA from all available fin clip samples using the Wizard SV Genomic DNA Purification System (Promega, WI, USA). In addition, we included nine reference samples of C. obscurus from the Gulf of Mexico, provided by the Mote Marine Laboratory & Aquarium, USA. First, we amplified a partial sequence of 1355 base pairs (bp) from the mitochondrially encoded cytochrome C oxidase I (COI) gene, which is recognised as a standard marker for shark population studies21,23,23; we used a newly-designed Carcharhinus_COI57F forward primer (5’-AACCACAAAGATATTGGCACCCTTTAC-3’) and Carcharhinus_1550R reverse primer (3’-AGAGGGTCGTTGGACTTGAACAAATGC-5’). We performed the polymerase chain reaction (PCR) in 30 microlitre (µL) volumes with the PCRBIO HS Taq Mix Red enzyme and buffer system (PCR Biosystems Ltd, UK). Our thermal cycling profile included denaturation at 95 degrees Celcius (°C) for 1 minute (min), followed by 35 extension cycles at 94 °C for 15 seconds (sec), 50 °C for 20 s, 72 °C for 40 s, and a final extension cycle at 72 °C for 2 min. We submitted the PCR products to Macrogen (Seoul, South Korea) for purification and sequencing. Next, we assessed the homology of our sequences to reference deposits of high percent identity (> 99%) from the GenBank repository and performed a multiple sequence alignment using MAFFT (version 724). To find the most suitable phylogenetic model using likelihood-based criteria, we utilised the Smart Model Selection (SMS) function in PhyML25 with a Bayesian information criterion (BIC). Then, we used the MEGA11 software26 to construct a neighbour-joining (NJ) tree based on the maximum likelihood method, employing the TN93 + G model27 (100 bootstrap replications) and a sequence of the scalloped hammerhead shark (Sphyrna lewini; JX827259) as an outgroup. Finally, to assess the genetic variation amongst sharks at the site, we calculated haplotype diversity (h) and nucleotide diversity (π) within each identified species using the pegas package28 in R. Notably, we did not obtain complementary samples of C. plumbeus from other locations as we did for C. obscurus, and the number of previous GenBank submissions available for comparison based on our 1355 bp COI segment was limited. Therefore, we trimmed our sequences to 531 bp and produced an additional NJ tree for matching submissions on GenBank using the Hasegawa-Kishino-Yano model29 (100 bootstrap replications) and the aforementioned outgroup.
Morphometric analysis
First, we performed a Wilcoxon rank sum test with continuity correction to compare the PCL medians of intrasexual monophyletic subgroups identified in the molecular analysis. Additionally, we found a high genetic similarity between some of our C. plumbeus sequences and another carcharhinid species (Section "Results"), necessitating the use of a supplementary technique to validate species identification. Therefore, we opportunistically collected a dead specimen, presumably C. plumbeus, entrapped within the seawater intake pool of the Eshkol power station in Ashdod, Israel (31° 50′ 51″ N, 034° 39′ 43″ E) on 26 April 2022. We obtained a genetic sample which we included in the molecular analysis and transferred the animal to a nearby necropsy facility for apex marine predators, where it was stored at 16 °C until the following morning. We then performed meristic counts and morphometric measurements on that shark as a reference specimen for our molecular analysis. More specifically, we followed the guide by Voigt and Weber30 to assess its dental formula, precaudal- and caudal vertebral counts, and dimensions as a percentage of total length (TL), which we then compared to minimum and maximum values in other carcharhinids showing a high genetic similarity.
Results
We captured 70 sharks from 17 January 2017 to 8 May 2022, and measured the PCL of 67 individuals and ICL of 21 males (Table 1; see Additional File 1 for detailed information on each individual). The molecular analysis included a subset of 57 sharks sampled between 17 January 2017 and 3 May 2021 and revealed a phylogenetic partition between two haplotypes of C. obscurus and two of C. plumbeus (Fig. 1), with an asymmetric sex ratio within each species (i.e., 33 genotyped females and 1 male in the former, corresponding to 1 and 22 in the latter). In C. obscurus, haplotypes 1 and 2 (henceforth, Cobs1 and Cobs2) differed at eight informative sites along the COI segment (Additional File 2). This divergence resulted in genetic diversity at both the haplotype (h = 0.7167) and nucleotide (π = 0.0034) levels. Additionally, Cobs1 (n = 22, females only; accession numbers ON428926-ON428947) displayed a single bp substitution relative to an individual sampled from eastern Australia, whereas Cobs2 (females, n = 11; males, n = 1; accession numbers ON428914-ON428925) showed seven bp substitutions relative to that sequence. In contrast, Cobs2 displayed two bp substitutions relative to our reference samples of C. obscurus from the Gulf of Mexico, USA (accession numbers ON428948-ON428956), excluding one specimen to which it was identical, whereas Cobs1 showed eight bp substitutions relative to those reference sequences.
Phylogenetic relationship among haplotypes of the mitochondrially encoded cytochrome C oxidase I (COI) gene (1355 base pairs) in sharks at the Orot-Rabin power and desalination station, Israel. Colours indicate species-level differentiation (warm—Carcharhinus obscurus; cold—Carcharhinus plumbeus), with shades representing distinct haplotypes (dark—Cobs1 and Cplu1; light—Cobs2 and Cplu2). Sequence labels denote the corresponding GenBank accession number, sampling location (HAD—Hadera; AUS—eastern Australia; GoM—Gulf of Mexico; ASH—Ashdod; CHN—China; NA—not available), sex (F—female; M—male), or, in reference samples, the species name. The numbers at the nodes represent corresponding bootstrap values equal to or greater than 50.
In C. plumbeus, the two identified haplotypes (henceforth, Cplu1 and Cplu2) diverged at two informative sites along the 1355 bp COI segment, resulting in lower genetic variation (h = 0.5688, π = 0.0008) than observed for C. obscurus. The first haplotype, Cplu1 (females, n = 1; males, n = 7; accession numbers ON880458-ON880465), exhibited seven bp substitutions relative to a C. plumbeus specimen sampled in Australia, to which Cplu2 (n = 15, males only; accession numbers ON880442-ON880455, ON880457) displayed nine bp substitutions (Additional File 3). Interestingly, Cplu1 exhibited eight bp substitutions when compared to previously submitted sequences of C. plumbeus and C. altimus from unspecified locations (accession numbers JQ65471 and JQ654712, respectively), both showing a percent identity of 99.41%. Similarly, Cplu2 showed ten and eight bp substitutions when compared to those sequences, respectively. Finally, Cplu2 had an identical sequence to the specimen collected dead at the Eshkol power station (accession number ON880456).
When assessing the similarity of our trimmed C. plumbeus sequences (531 bp) to GenBank submissions of equal length, the NJ analysis produced considerably low bootstrap values (Fig. 2). However, the tree’s topology corresponded with the geographical distribution of the sequence origins. Notably, one of the two informative sites differentiating Cplu1 and Cplu2 in the analysis of 1355 bp was retained (Additional File 4). We found that Cplu1 had an identical sequence to C. plumbeus sequences from Malta (n = 1), Saudi Arabia (n = 1), Oman (n = 6), the United Arab Emirates (n = 4), and South Africa (n = 1). Additionally, it exhibited a single bp substitution in comparison to specimens from the Western Indian Ocean and the Western Atlantic Ocean, specifically Madagascar (n = 1), Tanzania (n = 1), Virginia (n = 2), and South Carolina (n = 1). Furthermore, it differed at two sites from Indo-Pacific sequences obtained in eastern Australia (n = 1), western Australia (n = 2), Indonesia (n = 2), and Papua New Guinea (n = 1). Lastly, Cplu1 had three differences compared to the abovementioned C. plumbeus specimen sampled at an unknown location, which was situated on the same branch as the Indo-Pacific sequences. Concerning Cplu2, we found two substitutions relative to the sequences from the Western Indian Ocean and the Western Atlantic Ocean, and three substitutions relative to the sequences in the Indo-Pacific cluster. Additionally, it retained three of the eight bp substitutions differentiating it from the C. altimus sequence sampled at an unknown location, yet it was situated on the same phylogenetic branch.
Phylogenetic relationship among haplotypes of the mitochondrially encoded cytochrome C oxidase I (COI) gene (531 base pairs) in Carcharhinus plumbeus. Colour shades indicate the two lineages identified in the study (dark—Cplu1; light—Cplu2). Sequence labels denote the corresponding GenBank accession number, followed by the sampling location (HAD—Hadera; ASH—Ashdod; ZAF—South Africa; SAU—Saudi Arabia; UAE—United Arab Emirates; OMN—Oman; MLT—Malta; WAS—Western Australia; PNG—Papua New Guinea; AUS—Eastern Australia; MDG—Madagascar; TZA—Tanzania; VIR—Virginia; SCL—South Carolina; IDN—Indonesia; CHN—China; NA—not available), sex (F—female; M—male), or, in reference samples, the species name. The numbers at the nodes represent corresponding bootstrap values.
In line with our molecular analysis, the morphological examination of the dead shark retrieved from the Eshkol power station (Fig. 3) and identified as Cplu2 confirmed that it was a C. plumbeus specimen, although not unequivocally. While most of the morphometric indices we examined for the shark aligned with the range of values reported for C. plumbeus by Voigt and Weber30 based on 23 individuals, other measurements corresponded with their sample of C. altimus (n = 18) only, both species, or neither (Additional File 5). The tooth count for that specimen was 14 in the left and right maxillary jaws, which were separated by one anterodorsal symphyseal tooth, 14 in the right mandibular jaw, one anteroventral symphyseal tooth, and 11 in the left mandibular jaw. Thus, the dental formula of that specimen was [14-1-14/14-1-11], compared to [13(15)-1(2)-13(15)/12(15)-1(2)-12(15)] in C. plumbeus and [14(16)-1(2)-14(16)/14(15)-1-14(15)] in C. altimus, with possible variations given in parentheses. However, the precaudal, caudal, and total vertebral counts, which are recognised as unambiguous species-level identifiers30, were 93, 86 and 179, showing complete concordance with C. plumbeus.
Reference specimen employed for validation of molecular species identification. (A) Individual following retrieval from the seawater intake pool of the Eshkol power station in Ashdod, Israel; (B) spinal cord utilised for vertebral counts; (C, D) maxillary and mandibular jaws used for dental counts, respectively. Photos: Ran Lustiger (A), Eyal Bigal (B–D).
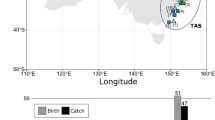
Our sample of 54 sharks identified to lineage level and included in the morphological analysis (Cobs1, n = 21; Cobs2, n = 11; Cplu1, n = 7; Cplu2, n = 15) was insufficient to reject the null hypothesis of equal PCL medians between the two haplotypes of C. obscurus females (W = 109.5, p-value = 0.8271; ES = 15.64286), nor the two haplotypes of C. plumbeus males (W = 64, p-value = 0.436, ES = 9.142857). The C. obscurus females belonging to Cobs1 had a PCL of 219.3 ± 12.6 cm (mean ± SD; n = 21) compared to 221.17 ± 8.21 in Cobs2 (mean ± SD; n = 11); the single C. obscurus male, which belonged to Cobs2, had a PCL of 205 cm and an ICL of 34 cm. In C. plumbeus, the males we identified as haplotype Cplu1 had a PCL of 138.57 ± 6.58 cm (mean ± SD; n = 7) and an ICL of 23.14 ± 4.02 cm (mean ± SD; n = 7) compared to 136.07 ± 5.47 cm and 22.69 ± 2.73 cm (mean ± SD; n = 15 and n = 13) in Cplu2, correspondently. The single C. plumbeus female included in the molecular analysis, which belonged to haplotype Cplu1, had a PCL of 149 cm. The ungenotyped females of that species, and the only measured ungenotyped male, had PCLs of 151.67 ± 8.14 cm (mean ± SD; n = 3) and 126 cm (ICL not measured), respectively. Although we did not establish distinctive phenotypic features for any of the above groups, the molecular analysis confirmed that we had correctly identified the species of all examined individuals based on their size differences. Figure 4 depicts the PCLs of each species and sex combination, including the morphologically identified individuals that we measured but did not genotype (C. obscurus females, n = 7; C. plumbeus females, n = 3; C. plumbeus males, n = 1) and those that we genotyped but excluded from the morphological analysis due to insufficient sample size in their corresponding sex group (C. obscurus male, n = 1; C. plumbeus females, n = 1).
Distribution of precaudal lengths (PCL) by species and sex at the Orot-Rabin coal-fired power and desalination station, Israel. Column widths represent the kernel density estimate (KDE) at the corresponding Y axis value. The number above each column denotes the overall sample size of sharks in the corresponding group. Colours indicate species-level differentiation (warm—Carcharhinus obscurus; cold—Carcharhinus plumbeus) with shades representing distinct haplotypes (dark circles—Cobs1 and Cplu1; light diamonds—Cobs2 and Cplu2; black triangles—ungenotyped). Groups comprising fewer than two observations were excluded. Photos: Hagai Nativ (left), Ran Golan (right).
Discussion
Phylogeography
In this study, we described the phylogeographic and demographic composition of requiem sharks aggregating at a power and desalination station in Israel. Our molecular analysis and morphological measurements confirmed the co-occurrence of C. obscurus and C. plumbeus sharks, with further differentiation of the examined COI segment within each species into two distinct haplotypes. In C. obscurus, we found a closer phylogenetic relationship between the first haplotype (Cobs1) and an Indo-Pacific lineage, and between the second haplotype (Cobs2) and an Atlantic lineage, than the similarity they exhibited amongst themselves. Notably, Barash et al.31 sampled C. obscurus specimens from various locations along Israel’s coastline and reported the occurrence of two haplotypes of the Nicotinamide Adenine Dinucleotide (NADH2) gene and two of the Displacement loop, that matched Indo-Pacific and Atlantic lineages. While we did not investigate the alignment of those haplotypes to ours, the presence of divergence across multiple markers, and the substantial size of our sample, suggest the possibility of two geographically isolated lineages. Notably, we also tested the phylogenetic relationship between the two C. obscurus haplotypes using the Internal Transcribed Spacer 2 (ITS2) marker but found no evidence of sequence divergence (unpublished observations).
The primary hypothesis that can be invoked to explain the founding of an Indo-Pacific population in the Mediterranean Sea is of Lessepsian migration, i.e., from the Red Sea via the Suez Canal, as previously suggested for other shark species32,34,35,35. In line with that postulation, other studies have noted that the Indo-Pacific distribution of C. obscurus indicates island hopping from Australia to southeast Asia and coastal movement along the shores of Africa36. Notwithstanding, Corrigan et al.37 found the abovementioned mitochondrial NADH2 haplotype of C. obscurus in Atlantic waters off Senegal and France which they clustered with sequences from Taiwan, northern Australia and South Africa, suggesting range expansion along the west coast of Africa and into the Mediterranean Sea via the Strait of Gibraltar. Finally, a third explanation for the close affinity between the first haplotype in our study and the Indo-Pacific lineage is entrapment in the Mediterranean Sea and sympatric differentiation following a historical anomalous migratory event across the Indian Ocean, Atlantic Ocean, and through the Strait of Gibraltar during extreme eustatic and climatic periods. This alternative route has been suggested as the cause of vicariance in populations of other pelagic predators, such as swordfish (Xiphias gladius)38 and white sharks (Carcharodon carcharias)39. Ultimately, the importance of our molecular analysis with respect to population persistence in C. obscurus depends on whether the single mutation differentiating Cobs1 and the reference specimen sampled in Australia indicates demographic links to the Indo-Pacific Ocean or an isolated Mediterranean subpopulation.
In C. plumbeus, we analysed two segments of the COI marker—one comprising 1355 bp and the other 531 bp. The longer segment indicated that the two haplotypes observed in the study site are distinct yet closer to each other and to a C. altimus sequence from an unknown location than they are to a C. plumbeus specimen sampled in Australia and another C. plumbeus specimen from an unknown location. In the shorter segment, the tree’s topology corresponded with the geographical distribution of the reference sequence origins, indicating allopatric divergence as expected. Arguably, this suggests that the low bootstrap values observed in that analysis primarily reflect relatively subtle differences between sequences within the same branch and that the overall clustering pattern was reliable. On that ground, the C. altimus sequence of an unknown origin corresponded with lineages from the Mediterranean Sea, the Red Sea, the Western Indian Ocean, and the Western Atlantic Ocean, whereas the unknown C. plumbeus sequence corresponded with Indo-Pacific lineages. These findings are in line with previous work which, based on other mitochondrial markers, indicated greater variability between Atlantic and Indo-Pacific C. plumbeus lineages compared to that between C. plumbeus and C. altimus within the Atlantic Ocean22,40,41. Moreover, the difficulty of differentiating between C. plumbeus and C. altimus based on the COI gene, specifically, has been reported in previous studies42,44,44. To establish unambiguous species-level identification, we implemented morphometric and meristic tools on a dead individual homologous with the Cplu2 haplotype, confirming it as C. plumbeus. Notably, Portnoy et al.45 investigated microsatellite and mitochondrial DNA control region variation within and between putative C. plumbeus populations worldwide and argued that nuclear markers might be more efficient to infer its phylogeography due to significant male-mediated gene flow. Moreover, they proposed several dispersal pathways for C. plumbeus between the Atlantic and Indian Oceans, including contemporary movement around the tip of Africa, but did not consider the possibility of migration through the Mediterranean Sea. Our results demonstrate sympatry between two distinct lineages of C. plumbeus in the study area—one connecting the western Mediterranean Sea, Red Sea, Western Indian Ocean, South Africa, and the Western Atlantic Ocean, and another which is potentially endemic to the Mediterranean Sea. The phylogeographic connectivity and relatively low haplotype and nucleotide diversity observed for C. plumbeus supports the possibility of Lessepsian migration. Future research should employ samples from multiple locations across the Mediterranean Sea and the Atlantic Ocean to provide a more comprehensive understanding of contemporary movement in that species. However, our observation of a dispersal pattern using a maternal marker, despite the presence of significant male-mediated gene flow, implies that the genetic structure of C. plumbeus may be influenced by various aspects of its biology and ecological dynamics.
Demography
Other biological traits that we investigated in the captured sharks were their sex, size, and maturity level. We recorded an uneven number of individuals between the sexes, with 41 females and one male in C. obscurus compared to four females and 24 males in C. plumbeus. Additionally, based on length measurements from previous studies46,48,48 and the presence of calcified claspers in the captured males, all individuals from both species were sexually mature.
The asymmetric sex ratio, alongside a narrow range of PCLs, indicates species-specific sex and size segregation—a common behaviour in coastal carcharhinids18. In line with this finding, previous studies have also reported sexually segregated adult aggregations of C. obscurus females and of C. plumbeus males, although not in mixed-species groups7,23,49. Arguably, the observed segregation pattern may provide insights into habitat use at our study site and the mechanisms underlying the aggregation behaviour.
For example, we did not observe neonate or juvenile sharks, nor are we aware of any evidence from site users during the aggregation season suggesting the area serves as a nursery or parturition ground. In addition, we did not record signs of recent copulation (e.g., bite marks or clasper abrasions) on any of the captured individuals which, alongside the presence of predominantly one sex from each species, indicates that the site does not function as a mating area, either. Interestingly, Wearmouth and Sims50 discussed mammal-focused hypotheses to explain sex segregation in ectotherms, which may help guide future research at our study site.
First, they propose that habitat divergence may occur as females and males respond to different resources (e.g., shelter and prey abundance) or environmental conditions (e.g., thermal optimums for egg and sperm development) to enhance reproductive success. Alternatively, they suggest that segregation might emerge as a direct outcome of sexual size dimorphism (SSD) rather than as a reproductive strategy. For example, individuals with analogous body size may exhibit comparable metabolic demands or foraging efficiencies and, thus, shared habitat preferences leading to separation from conspecifics. Another related explanation is that sharks of similar size may have comparable activity budgets and engage in behavioural synchronisation to avoid the costs of suboptimal grouping. Finally, the authors propose that segregation might occur as individuals exhibit intrasexual affinity by engaging in cooperation or information transfer, or social aversion to avoid potential aggressors.
Indeed, previous studies conducted at the site have found supportive indications for some of the above hypotheses. For example, Barash et al.12 investigated the movement dynamics of three C. obscurus females tagged with acoustic transmitters incorporating a temperature sensor and found that, during their residency at the site, the sharks remained in the warm water plume for more than 90% of their time. Indeed, the locally elevated temperatures might attract sharks either directly, by supporting physiological functions, or indirectly, by increasing the local forage base.
Concerning SSD as the mechanism potentially underlying segregation at the site, supplementary drone footage we collected throughout the study period indicated occasional movement of C. obscurus females in shivers of 10 or fewer individuals, and underwater videos exhibited polarised swimming amongst large groups of C. plumbeus males (unpublished observations). Additionally, our morphological measurements indicate a higher variability of body size in C. obscurus which may explain the formation of relatively small subgroups in that species.
Finally, supporting the proposition that segregation might reflect social interactions amongst sharks at the aggregation, Zemah-Shamir et al.11 used acoustic telemetry to study 22 of the individuals included in our dataset to perform a social network analysis and identified strong assortment patterns in C. plumbeus (males, n = 10) relative to C. obscurus (females, n = 11; males, n = 1). Similarly, Barash et al.13 analysed depth records from acoustic detections in both species and found potential indication of spatiotemporal habitat partitioning that might reflect intraspecific affinity, interspecific aversion, or both.
Although the findings outlined above may help assess the observed demographic composition using the theoretical framework by Wearmouth and Sims, further research is warranted on resource availability at the study site, the sharks’ reproductive status, and social interactions among species, lineages, and sex groups to infer their habitat use.
Conservation implications
The phylogeographic composition we observed suggests a potential range expansion of both species into the EMS, which could have detrimental outcomes if individuals are more prone to increased mortality or fitness reduction in that region. Indeed, large numbers of C. obscurus specimens are occasionally encountered in the catch of fisheries throughout the EMS51. Furthermore, though all elasmobranch species are strictly protected by law within the marine jurisdiction of Israel (Wildlife Protection Law, 1955), anecdotal fatal entrapments of both species are known to occur within all human-altered habitats hosting large aggregations along the country’s coastline (unpublished observations), as demonstrated for C. plumbeus in our study. Further research employing various nuclear and mitochondrial markers is required to investigate contemporary dispersal patterns of C. obscurus and C. plumbeus in the EMS and adjacent ocean basins, and to verify our indication of Lessepsian migration in both species.
Additionally, it is imperative to assess the risks associated with residency at the Orot-Rabin plant or other infrastructures throughout the EMS on population persistence in comparison to their native habitats. Zemah Shamir et al.52 documented behavioural responses in individuals of both shark species to the presence of divers at our study site and proposed that such disturbances may have implications for their metabolic rates and, consequently, overall fitness. Similarly, Bregman et al.53 studied the microbiome community on sharks in the same aggregation and showed an increase of Streptococcus spp. in tissue samples from 27 of the sharks reported in our study, within and between the aggregation seasons of 2019–2021. This bacterial genus includes the highly pathogenic S. agalactiae, which was identified as the etiologic agent of an adult C. plumbeus female found moribund on a beach 15 km south of our study site54.
Arguably, the observed pattern of intraspecific sex and size-based segregation may exacerbate the potentially negative effects of long-term residency at human-altered habitats on population persistence. For example, the gestation period of C. obscurus females spans 22–24 months and is typically followed by one or more resting years55, potentially representing most of their reproductive years. Consequently, female-biased mortality would likely have a more pronounced effect on population fecundity compared to male-biased mortality, as male reproductive fitness generally depends on mating success and is, therefore, more variable across individuals50. Further research is needed on the risks of long-term residency at marine infrastructures throughout the EMS and the proportion of the population aggregating at these sites.
Conclusions
In this study, we investigated the phylogeographic and demographic composition of sharks aggregating at the Orot-Rabin coal-fired power and desalination station in the eastern Mediterranean Sea (EMS). We observed sympatric occurrence of two geographically isolated lineages of the dusky shark (Carcharhinus obscurus) along with two distinct lineages of the sandbar shark (Carcharhinus plumbeus). Additionally, all individuals were mature adults segregated by sex and size, with predominantly females of the former species and males of the latter. Our molecular analysis indicates complex biogeographical patterns, potentially involving Lessepsian migration in one lineage of each species and distinct genetic differentiation from the other. However, further research is warranted to investigate contemporary dispersal patterns of coastal carcharhinids in the region. Finally, the observed segregation pattern suggests that the Orot-Rabin plant does not function as a nursery, parturition, or mating area. To investigate habitat use at our study site and the implications of extended residency at marine infrastructures throughout the EMS, it is necessary to test existing frameworks that explain sex- or size-based segregation, particularly in the context of mixed species aggregations.
Data availability
The datasets generated and/or analysed during the current study are available in the GenBank repository [accession numbers ON428914-ON428956, ON880442-ON880465].
References
McInturf, A. G. et al. A unified paradigm for defining elasmobranch aggregations. ICES J. Mar. Sci. 2, 1–16 (2023).
Speed, C. W. et al. Spatial and temporal movement patterns of a multi-species coastal reef shark aggregation. Mar. Ecol. Prog. Ser. 429, 261–275 (2011).
Heupel, M. R. & Simpfendorfer, C. A. Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar. Biol. 147, 1239–1249 (2005).
Robertson, B. A. & Hutto, R. L. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87, 1075–1085 (2006).
Battin, J. When animals love bad habitats: Ecological traps and the conservation of animal populations. Conserv. Biol. 18, 1482–1491 (2004).
Robinson, D. P. et al. Whale sharks, Rhincodon typus, aggregate around offshore platforms in Qatari waters of the Arabian gulf to feed on fish spawn. PLoS One 8, e58255 (2013).
Papastamatiou, Y. P., Itano, D. G., Dale, J. J., Meyer, C. G. & Holland, K. N. Site fidelity and movements of sharks associated with ocean-farming cages in Hawaii. Mar. Freshw. Res. 61, 1366 (2010).
Barash, A. et al. Seasonal arrival and feeding of injured coastal sharks at fish farms in the Eastern Mediterranean. J. Black Sea/Mediterr. Environ. 24, 86–90 (2018).
Barash, A., Pickholtz, R., Pickholtz, E., Blaustein, L. & Rilov, G. Seasonal aggregations of sharks near coastal power plants in Israel: An emerging phenomenon. Mar. Ecol. Prog. Ser. 590, 145–154 (2018).
Ergüden, D., Ayas, D. & Kabasakal, H. Provoked non-fatal attacks to divers by sandbar shark, Carcharhinus plumbeus (Carcharhiniformes: Carcharhinidae), off taŞucu coast (Ne Mediterranean sea, Turkey). Ann. Ser. Hist. Nat. 30, 1–13 (2020).
Zemah-Shamir, Z. et al. Preliminary insights of a mixed-species shark aggregation: A case study of two carcharhinids from the Mediterranean sea. Environ. Biol. Fish. 105, 623–634 (2022).
Barash, A. et al. Some like it hot: Investigating thermoregulatory behavior of carcharhinid sharks in a natural environment with artificially elevated temperatures. Fishes 8, 428 (2023).
Barash, A. et al. Depth partitioning and diel movement of two large carcharhinid sharks in extremely shallow waters. Fishes 8, 85 (2023).
Pank, M., Stanhope, M., Natanson, L., Kohler, N. & Shivji, M. Rapid and simultaneous identification of body parts from the morphologically similar sharks Carcharhinus obscurus and Carcharhinus plumbeus (Carcharhinidae) using multiplex PCR. Mar. Biotechnol. 3, 231–240 (2001).
Musick, J., Grubbs, D., Baum, J. K. & Cortés, E. Carcharhinus obscurus (Mediterranean assessment). The IUCN red list of threatened species https://www.iucnredlist.org/species/3852/16527849 (2016).
Ferretti, F. et al. Carcharhinus plumbeus (Mediterranean assessment). The IUCN red list of threatened species https://www.iucnredlist.org/species/3853/16527809 (2016).
Chapman, D. D., Feldheim, K. A., Papastamatiou, Y. P. & Hueter, R. E. There and back again: A review of residency and return migrations in sharks, with implications for population structure and management. Ann. Rev. Mar. Sci. 7, 547–570 (2015).
Speed, C. W., Field, I. C., Meekan, M. G. & Bradshaw, C. J. A. Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 408, 275–293 (2010).
Carlson, J. K. & Goldman, K. J. Special issue: Age and growth of chondrichthyan fishes: new methods, techniques and analysis. Environ. Biol. Fish. 77 (2006).
Team, R. C. R: A language and environment for statistical computing. (2023).
Toha, A. H. et al. The genetic relationships and Indo-Pacific connectivity of whale sharks (Rhincodon typus) with particular reference to mitochondrial COI gene sequences from Cenderawasih Bay, Papua, Indonesia. (2020).
Naylor, G. J. P. et al. A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Mus. Nat. Hist. https://doi.org/10.1206/754.1 (2012).
Hoffmayer, E. R. et al. Habitat, movements and environmental preferences of dusky sharks, Carcharhinus obscurus, in the northern Gulf of Mexico. Mar. Biol. 161, 911–924 (2014).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Lefort, V., Longueville, J.-E. & Gascuel, O. SMS: Smart model selection in PhyML. Mol. Biol. Evol. 34, 2422–2424 (2017).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Paradis, E. pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 26, 419–420 (2010).
Hasegawa, M., Kishino, H. & Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 22, 160–174 (1985).
Voigt, M. & Weber, D. Field guide for sharks of the genus Carcharhinus. (F. Pfeil, 2011).
Barash, A. Species identification, phylogeography and spatio-temporal distribution of requiem sharks (genus Carcharhinus) along the Israeli Mediterranean coast. (University of Haifa, 2014).
Turan, C., Gürlek, M., Ergüden, D. & Kabasakal, H. A new record for the shark fauna of the Mediterranean sea: Whale shark, Rhincodon typus (Orectolobiformes: Rhincodontidae). Ann. Ser. Hist. Nat. 31, 167–172 (2021).
Ben Amor, M. M., Diatta, Y., Diop, M., Ben Salem, M. & Capape, C. Confirmed occurrence in the Mediterranean Sea of milk shark Rhizoprionodon acutus (Chondrichthyes: Carcharhinidae) and first record off the Tunisian coast. Cah. Biol. Mar. 57, 145–149 (2016).
De Maddalena, A. & Rovere, G. First record of the pigeye shark, Carcharhinus amboinensis (Müller & Henle, 1839), in the Mediterranean sea. Ann. (Ann. Istran Mediterr. Stud.) Ser. Hist. Nat. 16, 209–212 (2005).
Spaet, J. L. Y., Cochran, J. E. M. & Berumen, M. L. First record of the pigeye shark, Carcharhinus amboinensis (Müller & Henle, 1839) (Carcharhiniformes: Carcharhinidae), in the Red Sea. Zool. Middle East 52, 118–121 (2011).
Benavides, M. T. et al. Global phylogeography of the dusky shark Carcharhinus obscurus: Implications for fisheries management and monitoring the shark fin trade. Endang. Species Res. 14, 13–22 (2011).
Corrigan, S. et al. Historical introgression drives pervasive mitochondrial admixture between two species of pelagic sharks. Mol. Phylogenet. Evol. 110, 122–126 (2017).
Alvarado Bremer, J. R., Viñas, J., Mejuto, J., Ely, B. & Pla, C. Comparative phylogeography of Atlantic bluefin tuna and swordfish: The combined effects of vicariance, secondary contact, introgression, and population expansion on the regional phylogenies of two highly migratory pelagic fishes. Mol. Phylogenet. Evol. 36, 169–187 (2005).
Gubili, C. et al. Antipodean white sharks on a Mediterranean walkabout? Historical dispersal leads to genetic discontinuity and an endangered anomalous population. Proc. R. Soc. B: Biol. Sci. 278, 1679–1686 (2011).
Heist, E. J. & Gold, J. R. Genetic identification of sharks in the U.S. Atlantic large coastal shark fishery. Fish. Bull. 97, 53–61 (1999).
Greig, T. W., Moore, M. K., Woodley, C. M. & Quattro, J. M. Mitochondrial gene sequences useful for species identification of western North Atlantic Ocean sharks. (2005).
Ward, R. D., Hanner, R. & Hebert, P. D. N. The campaign to DNA barcode all fishes. FISH-BOL J. Fish. Biol. 74, 329–356 (2009).
Moftah, M., Aziz, S. H. A., El Ramah, S. & Favereaux, A. Classification of sharks in the Egyptian Mediterranean waters using morphological and DNA barcoding approaches. PLoS One 6, e27001 (2011).
Spaet, J. L. Y. & Berumen, M. L. Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish. Res. 161, 356–364 (2015).
Portnoy, D. S., McDowell, J. R., Heist, E. J., Musick, J. A. & Graves, J. E. World phylogeography and male-mediated gene flow in the sandbar shark, Carcharhinus plumbeus. Mol. Ecol. 19, 1994–2010 (2010).
Shoou-Jeng, J. & Che-Tsung, C. Reproduction in the sandbar shark, Carcharhinus plumbeus, in the waters off northeastern Taiwan. Copeia 1995, 659–665 (1995).
Saïumldi, B., Bradaï, M. N., Bouaïumln, A., Guéacutelorget, O. & Capapé, C. The reproductive biology of the sandbar shark, Carcharhinus plumbeus (Chondrichthyes: Carcharhinidae), from the Gulf of Gabès (southern Tunisia, central Mediterranean). Acta Adriat.: Int. J. Mar. Sci. 46, 47–62 (2005).
Joung, S. J., Chen, J. H., Chin, C. P. & Liu, K. M. Age and growth of the dusky shark, Carcharhinus obscurus, in the Western North Pacific Ocean. Terr., Atmos. Oceanic Sci. 26, 153–160 (2015).
McAuley, R. B., Lenanton, R., Chidlow, J., Allison, R. & Heist, E. J. Biology and stock assessment of the thickskin (sandbar) shark, Carcharhinus plumbeus, in Western Australia and further refinement of the dusky shark, Carcharhinus obscurus, stock assessment. Final FRDC Report - Project 2000/134. Fisheries Western Australia Fisheries Research Report Fisheries, 132 (2005).
Wearmouth, V. J. & Sims, D. W. Sexual segregation in marine fish, reptiles, birds and mammals. Behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol. 54, 107–170 (2008).
Bradai, M. N., Saidi, B. & Enajjar, S. Elasmobranchs of the Mediterranean and black sea: Status, ecology and biology, biographic analysis. (FAO, 2012).
Zemah Shamir, Z., Zemah Shamir, S., Becker, N., Scheinin, A. & Tchernov, D. Evidence of the impacts of emerging shark tourism in the Mediterranean. Ocean Coast. Manag. 178, 104847 (2019).
Bregman, G. et al. Preliminary study of shark microbiota at a unique mix-species shark aggregation site, in the Eastern Mediterranean Sea. Front. Microbiol. 14, 1027804 (2023).
Morick, D., Davidovich, N., Bigal, E., Rosenbluth, E. & Bouznach, A. Fatal infection in a wild sandbar shark (Carcharhinus plumbeus), caused by streptococcus agalactiae, type Ia-ST7. Animals https://doi.org/10.3390/ani10020284 (2020).
Romine, J. G., Musick, J. A. & Burgess, G. H. Demographic analyses of the dusky shark, Carcharhinus obscurus, in the Northwest Atlantic incorporating hooking mortality estimates and revised reproductive parameters. Environ. Biol. Fish. 84, 277–289 (2009).
Acknowledgements
We are grateful to Dr Demian Chapman of the Sharks and Rays Conservation Program, Mote Marine Laboratory & Aquarium, USA, for providing tissue samples and valuable comments on the manuscript. Special thanks go to the volunteers, boat operators, and students supporting our fieldwork.
Funding
This research was funded by the Kahn Foundation.
Author information
Authors and Affiliations
Contributions
E.B., D.T. and A.S. conceived the ideas and designed methodology; E.B., L.L., Z.Z.S. and A.S. collected the data; E.B., L.L., T.L. and E.S. analysed the data; E.B. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bigal, E., Livne, L., Zemah-Shamir, Z. et al. Shark shuffle: segregated co-occurrence of multiple dusky and sandbar lineages at a human-altered habitat in the eastern Mediterranean Sea. Sci Rep 14, 19924 (2024). https://doi.org/10.1038/s41598-024-69460-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69460-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.