Abstract
To evaluate the diagnostic accuracy of matrix-assisted laser desorption ionization time-of-flight mass spectrometry based on nucleotide (nucleotide MALDI-TOF MS) on bronchoalveolar lavage fluid (BALF) from suspected pulmonary tuberculosis (PTB) patients. A retrospective study was conducted on suspected PTB patients (total of 960) admitted to Chongqing Public Health Medical Center between May 2021 and January 2022. The sensitivity, specificity, positive predictive value, negative predictive value (NPV) and area under the curve values of nucleotide MALDI-TOF MS as well as smear microscopy, Mycobacterium Growth Indicator Tube 960 culture (MGIT culture), and Xpert MTB/RIF were calculated and compared. Total of 343 presumed PTB cases were enrolled. Overall, using the clinical diagnosis as reference, the sensitivity and NPV of nucleotide MALDI-TOF MS was 71.5% and 43.1%, respectively, significantly higher than smear microscopy (22.6%, 23.2%), MGIT culture (40.6%, 18.9%), Xpert MTB/RIF (40.8%, 27.9%). Furthermore, nucleotide MALDI-TOF MS also outperformed over Xpert MTB/RIF and MGIT culture on smear-negative BALFs. Approximately 50% and 30% of patients benefited from nucleotide MALDI-TOF MS compared with smear and MGIT culture or Xpert MTB/RIF, respectively. This study demonstrated that the analysis of BALF with nucleotide MALDI-TOF MS provided an accurate and promising tool for the early diagnosis of PTB.
Similar content being viewed by others
Introduction
Tuberculosis (TB), a communicable disease mainly due to infection with Mycobacterium tuberculosis (MTB), remains a major public health problem afflicts approximately 10 million people worldwide, of which, pulmonary tuberculosis (PTB) is the most common form1. Early PTB diagnosis and initial proper treatment are crucial for the prevention of disease progression2. Microbiological tests based on high-quality respiratory specimens are essential to enable accurate diagnosis of PTB. Sputum specimens are the most common type; however, the quality of them is difficult to ensure, especially in patients who unable to self-expectorate or have no sputum3,4. For example, a high proportion of children with susceptible TB are unable to provide sufficient sputum or have negative smear sputum5,6. Alternatively, bronchoalveolar lavage fluid (BALF) is another sample type to be considered and favoured by physicians7.
Currently, standard PTB microbiologically diagnostic methods in most hospitals usually include smear microscopy, culture, each with its own strengths and weaknesses8. Smear microscopy provides the advantage of speed, yet its sensitivity is insufficient nor can it differentiate MTB and nontuberculous mycobacteria (NTM). The culture method, including Mycobacterium Growth Indicator Tube 960 culture (MGIT 960 culture), remains the gold standard for the diagnosis of TB, but it demands high-level biosafety, high personnel expertise, poses risks of fragile contamination and most importantly has long turnaround time9, resulting the delayed diagnosis and potential drug resistance10. With the development of molecular technology, many methods for the rapid diagnosis of TB occur recent years, several of which have been recommended by the World Health Organization (WHO), like Xpert MTB/RIF assay, the first approved one11. However, the limit of detection (LOD) of many molecular detection techniques, including Xpert MTB/RIF, are relatively high and the sensitivity is inferior to that of culture method12.
Nucleotide MALDI-TOF MS assay, a relative novel technology, is matrix-assisted laser desorption ionization time-of-flight mass spectrometry based on nucleotide, possessing the advantages of rapidity, high throughput and high accuracy13. Su et al.14 reported that the LOD of nucleotide MALDI-TOF MS is 5 CFU/mL, exhibiting significantly lower than that of Xpert MTB/RIF (114 CFU/mL). Several studies have demonstrated the promising application of this technique in TB diagnosis and its drug resistance15,16; however, evaluation of this assay using BALF specimens in more real-world practices and different regions in China remains very limited. Here, we analysed the diagnostic performance of nucleotide MALDI-TOF MS assay on BALFs obtained from highly suspected PTB patients in southwestern region of China and compared the results to those of smear microscopy, MGIT 960 culture and Xpert MTB/RIF assay. Thus, we hope that the results of this study will contribute to the improvement of the clinic diagnosis of PTB.
Materials and methods
Participants and clinical diagnosis
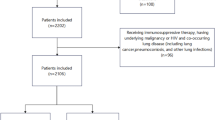
We retrospectively reviewed the clinical and laboratory test results of patients with suspected PTB who underwent nucleotide MALDI-TOF MS assay on BALFs from May 2021 to January 2022 at Chongqing Public Health Medical Center (Chongqing, China), a tertiary TB designated hospital located at in Southwest China. Medical data were collected, including age, sex, previous TB treatment, clinical diagnosis, and comorbidities. We had no access to information that could identify individual participants during or after data collection. The following conditions had to be simultaneously fulfilled for our inclusion criteria: (1) bronchoalveolar lavage was performed and a sufficient volume of BALF was obtained; (2) consent to send for nucleotide MALDI-TOF MS assay; and (3) a definitive clinical diagnosis was available. Those unable to fulfil any of the above conditions were excluded. During this time, a total of 343 patients agreed to conduct nucleotide MALDI-TOF assay on BALFs. These samples were subjected to acid-fast bacilli smear microscopy, culture or the Xpert MTB/RIF assay. However, not all of these BALF samples were performed using culture or Xpert MTB/RIF assay; that is, 280 for MGIT 960 culture and 220 for Xpert MTB/RIF.
In this study, we evaluated the diagnostic performance of various methods using clinical diagnosis as the gold standard. Clinically diagnosed PTB cases were according to the national diagnostic criteria (WS 288-2017 Diagnostic Criteria for Tuberculosis)17. A patient diagnosed with confirmed PTB had one or more of the following criteria: (1) positive microbiological results based on culture or molecular tests of sputum, BALF, or puncture fluid; (2) positive results for MTB based on histopathological examination of puncture, biopsy, or surgical samples; (3) radiological findings (chest X-ray or chest CT scan) suggestive of TB; and (4) improved symptoms after anti-TB treatment. Otherwise, those cases who did not fulfil the clinical diagnostic criteria for PTB were classified as non-PTB patients. A positive microbiological result was defined as any positive evidence of MTB detected in any sample provided by the patient admitted to our center, including BLAF, sputum, or puncture fluid samples, according to culture or Xpert MTB/RIF assay and regardless of the results of nucleotide MALDI-TOF MS assay.
BALF collection
Bronchoscopy was performed according to the Chinese Thoracic Society expert consensus. To collect BALF, 50–100 mL of isotonic saline was used for bronchial washing of the related sub-segment and 5–20 mL of the recovered BALFs were collected into the sterile containers and sealed. The containers were delivered immediately to the laboratory for smear microscopy, culture or Xpert MTB/RIF assay and nucleotide MALDI-TOF MS assay.
Conventional test methods for BALF specimens
The collected BALF samples were pre-incubated by decontamination with 4% (m/v) NaOH and centrifugated at 3000 ×g for 20 min. The sediment was resuspended and subjected for traditional test in our hospital as follows: (1) Acid-fast bacillus microscopy: The sediment was spread onto the front of a glass microscope slide, which was allowed to air dry and heat-fixed before staining with the Ziehl–Neelsen method. Smear results of 1 + or more acid-fast bacillus (AFB) were recorded as positive for statistical analysis. (2) MGIT 960 culture: The Mycobacteria Growth Indicator Tube (MGIT) 960 system (BD Diagnostics) was used for liquid culture. All samples were inoculated in the MGIT tubes were incubated in the corresponding instrument. (3) Xpert MTB/RIF assay: The testing was performed according to the manufacturer’s instructions (Cepheid, USA).
Nucleotide MALDI-TOF MS assay
The assay used Conlight TB&DR® assay, which was well-established and conducted by Shanghai Conlight Medical Co., Ltd. This assay integrates the sensitivity of PCR technology and the high throughput nature of mass spectrometry detection technology. One reaction can amplify up to 40 gene amplifications that can applied to single nucleotide polymorphism analysis, mutant detection, and other research areas. The specific procedures can be found in the literatures15,18. Briefly, the process includes the following six steps: extraction of nucleic acids from samples, PCR to obtain target fragments, shrimp alkaline phosphatase (SAP) reaction, extension reaction, detection on the MS instrument, and automatic analysis of results by MassARRAY analysis software.
Statistical analysis
All information of patients enrolled in the study were recorded in EXCEL. R 4.2.0 was used for statistical analysis. Descriptive statistics were presented in terms of number and proportion of cases. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence intervals (95% CI) were calculated. For intergroup comparisons of categorical variables, chi-square test was carried out; for continuous variables that are not normally distributed, the Mann–Whitney U test was employed. Receiver operating characteristic (ROC) curves and their areas under the curve (AUC) were used to evaluate the diagnostic accuracy. A two-tailed value of p < 0.05 was considered as statistical significance.
Ethics statement
The study protocol was approved by the Ethics Committee of Chongqing Public Health Medical Center of China (2023-013). This study was conducted in accordance with the principles of the Declaration of Helsinki of the World Medical Association and with Good Clinical Practice Guidelines. The requirement of written informed consent was waived owing to the retrospective study design with approval from the Research Ethics Committee of the study hospital.
Results
Basic information of the enrolled participants
As shown in the flow chart (Fig. 1), we retrospectively screened suspected PTB cases who visited Chongqing Public Health Medical Center between May 2021 and January 2022 and found a total of 343 cases agreed to undergo nucleotide MALDI-TOF MS assay, which were enrolled in this study. BALF samples were obtained from all patients and subjected to nucleotide MALDI-TOF MS assay. At same time, routine laboratory tests were performed in our hospital as follows: all samples underwent smear microscopy, 280 of them were cultured on MGIT 960 system, and 220 were analyzed with Xpert MTB/RIF assay. Clinical diagnosis of PTB was finally made in 274 cases (79.9%) and the remaining (69, 20.1%) was classified as non-PTB patients.
Demographic and clinical features of the study population were presented in Table 1. Of the enrolled participants, 215 (62.7%) were males and 128 (37.3%) were females, with a median age of 46.0 years (interquartile range [IQR]: 31.0–58.5). Twenty-seven patients (7.9%) were diagnosed with diabetes mellitus (DM), 13 patients (3.8%) with hypertension, and 120 patients (35.0%) with a history of TB treatment.
We further compared the distribution of demographic and clinical characteristics between PTB and non-PTB patients in our study as shown in Table 2. The ratio of patients with previous TB treatment in PTB group (37.6%) was significantly higher than those in non-PTB group (24.6%). Additionally, PTB group had a greater percentage of male subjects, although this result was not statistically significant (65.3% vs. 52.2%, p = 0.051).
Clinical performance of nucleotide MALDI-TOF MS, smear microscopy, culture and Xpert MTB/RIF
In the present study, nucleotide MALDI-TOF MS and smear microscopy were performed for all BALF samples, whereas 280 and 220 samples were conducted using MGIT 960 culture and Xpert MTB/RIF, respectively. A total of 67/343 (19.5%) patients had a positive acid-fast bacilli smear microscopy, 105/343 (30.6%) had a positive MGIT 960 culture, 73/343 (21.3%) had a positive Xpert MTB/RIF and 206/343 (60.1%) had a positive nucleotide MALDI-TOF MS assay (Table 1). Out of the 189 cases with results using the three diagnostic tools, namely MGIT 960 culture, Xpert MTB/RIF and nucleotide MALDI-TOF MS assay, 124 cases were MTB positive for either method. For them, we used R software to create a venn diagram to analyse the distribution and overlap of MTB positivity by different methods. As Fig. 2 shown, 43 (34.7%, 43/124) cases were found positive for MTB by all the three methods, whereas 22 (17.7%, 22/124) were MTB positive by two methods. Additionally, 45 (36.3%, 45/124) patients were positive for MTB using nucleotide MALDI-TOF MS assay exclusively, with seven (5.6%, 7/124) cases positive by Xpert MTB/RIF or culture only (Fig. 2). These results suggested that nucleotide MALDI-TOF MS has more potential to diagnose TB.
Analyses for diagnostic performance of nucleotide MALDI-TOF MS on BALF specimens
Using finally clinical diagnosis as reference standard, the overall sensitivity of nucleotide MALDI-TOF MS assay was 71.5% (95%CI 65.8–76.8%), significantly higher than that of smear microscopy (22.6%, 17.8–28.0%), MGIT 960 culture (40.6%, 34.3–47.1%) and Xpert MTB/RIF assay (40.8%, 33.5–48.4%). Meanwhile, the specificity of nucleotide MALDI-TOF MS assay was 85.5% (75, 0–92.8%), slightly lower than that of smear microscopy (92.8%, 83.9–97.6%) and Xpert MTB/RIF assay (100.00%, 91.4–100.0%), a little higher than that of MGIT 960 culture (80.5%, 65.1–91.2%). Nevertheless, no significant difference existed among those detection methods. The respective accuracy for smear microscopy, MGIT 960 culture, Xpert MTB/RIF and nucleotide MALDI-TOF MS assay were 36.7% (31.6–42.1%), 46.4% (40.5–52.5%), 51.8% (45.0–58.6%), and 74.3% (69.4–78.9%) (Table 3). The area under the curve (AUC) value for nucleotide MALDI-TOF MS assay was 0.800, larger than those obtained from smear microscopy (0.558), MGIT culture (0.614) and Xpert MTB/RIF (0.593) (Fig. 3a).
The diagnostic accuracy of different detection techniques on BALF samples. (a) The ROCs of nucleotide MALDI-TOF MS assay, Xpert MTB/RIF, Smear microscopy, and MGIT 960 culture on all of BALF samples with AUC to be 0.800, 0.687, 0.558, and 0.614, respectively. (b) The ROCs of nucleotide MALDI-TOF MS assay, Xpert MTB/RIF, and MGIT 960 culture on smear-negative BALF samples with AUC to be 0.776, 0.652 and 0.586, respectively.
Notably, 78 (28.5%, 78/274) and 10 (14.5%, 10/69) cases were false negative and false positive according to nucleotide MALDI-TOF MS, respectively. Among the false negatives, 18 cases (23.1%, 18/78) were incorrectly identified as NTM. We made a comparison between the true positive group and false negative group, which showed no difference in age and gender compsition, comorbidity with hypertension, whereas a significant sifference existed in history of TB treatment and comorbidity with diabetes mellitus in the two groups (Supplemental Table @1). Among the true negatives of nucleotide MALDI-TOF MS, NTM was identified in 17 cases (28.8%, 17/59). We also compared the true negative group with the false positive group and no differences were found in terms of gender, age composition, history of TB treatment, and the two comorbidities (Supplemental Table @2), which may be due to the small number of cases in the sample.
To investigate the diagnostic performance of nucleotide MALDI-TOF MS assay for the detection of MTB in BALFs from smear-negative patients, we have separately analyzed 276 samples with negative results using smear microscopy, with total of 212 cases diagnosed to be PTB. Using finally clinical diagnosis as reference standard, the sensitivity of nucleotide MALDI-TOF MS assay was 65.1% (95%CI 58.3–71.5%), significantly higher than that of MGIT 960 culture (25.4%, 19.2–32.4%) and Xpert MTB/RIF assay (30.7%, 23.2–39.1%). The corresponding specificity of these methods was 84.4% (73.1–92.2%), 88.9% (73.9–96.9%) and 100.0%, (90.7–100.0%), respectively, without significantly differences (Table 4). The AUC values for nucleotide MALDI-TOF MS, MGIT 960 culture and Xpert MTB/RIF were 0.692, 0.525 and 0.562, respectively (Fig. 3b). Collectively, our results indicated nucleotide MALDI-TOF MS assay exhibited better diagnostic performance on the smear-negative BALFs from suspected PTB patients.
Analyses for rifampicin resistance detection of nucleotide MALDI-TOF MS on BALF specimens
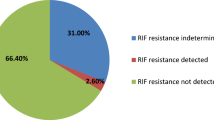
In order to evaluate the concordance of nucleotide MALDI-TOF MS assay and Xpert MTB/RIF for the rifampicin (RIF) resistance detection, those cases with positive results for MTB using both methods were included in the analyses. Of the 63 cases, six showed discordant results (Table 5). Four tests were RIF-sensitive by Xpert MTB/RIF but RIF-resistant by nucleotide MALDI-TOF MS, and the remaining two tests were vice versa, leading to the agreement of 90.5% of these two methods. Using the results of MGIT 960 culture as the references, three of cases identified as RIF-Resistant via nucleotide MALDI-TOF MS were RIF-sensitive confirmed by culture and the remaining cases were identical with those obtained from culture.
Discussion
In this study, the diagnostic capacity of nucleotide MALDI-TOF MS assay to detect MTB in BALFs from presumed PTB patients was evaluated and compared with conventional methods used in our hospital. Our results revealed that the performance of nucleotide MALDI-TOF MS assay was significantly superior to that of smear microscopy, MGIT 960 culture and Xpert MTB/RIF test not only on overall BALF samples but also smear-negative ones. In the present study, the diagnostic sensitivity of nucleotide MALDI-TOF MS was 71.5% for BALFs from PTB patients, whereas those of smear, culture and Xpert MTB/RIF were 22.6%, 40.6%, and 40.8%, respectively. Together, about 50% and 30% patients benefited from nucleotide MALDI-TOF MS compared with smear and culture or Xpert MTB/RIF, respectively.
MALDI-TOF MS has been widely used in clinical microbiology detection for decades, with mature application for the identification of NTM based on proteins19,20,21,22. However, culture of mycobacteria was required if using protein-based MALDI-TOF MS assay, providing great advantages for fast-growing mycobacteria, while rapid detection of slow-growing mycobacteria, such as MTB, cannot be achieved. Nucleotide MALDI-TOF MS assay, using nucleic acids as substrates, can solve this above problem by targeting specific nucleotide polymorphisms. In 2017, a research study from Taiwan demonstrated that the detection limit of nucleotide MALDI-TOF MS assay on mycobacterial species identification was as low as 5 copies, with well-performed sensitivity and specificity of drug resistant detection23. Wu et al., have evaluated the prediction of MTB drug resistance with nucleotide MALDI-TOF MS, using phenotype drug resistance as reference and showed that it could be a promising tool for rapid detection of drug resistance15. Nevertheless, the evaluation was conducted on the culture samples of MTB instead of clinical samples directly. Although it is featured with high throughput, speed, sensitivity, high accuracy and low cost, investigations on the application of nucleotide MALDI-TOF MS assay for PTB diagnosis in real world are still extremely limited and more research data are needed for support. To this end, we conducted this retrospective study to evaluate the diagnostic performance of nucleotide MALDI-TOF MS assay on BALFs from suspected PTB patients in Southwestern region of China with relatively large sample size. Li et al. and Yang et al. have published similar articles with very small sample size (37 and 52 BALFs respectively), likely resulting in big bias24,25. The results of various methods in their study were generally higher than those obtained from our study. The influential factors among them may be various other than the sample size, such as study site, the experimental operators.
BALF is the most common used respiratory sample type for the diagnosis of PTB besides sputum, which is also preferred by clinicians due to its closer proximity to the lesion. Multiple studies have reported the capability of different molecular tests to diagnose PTB in BALFs, such as Xpert MTB/RIF assay, SAT-TB, LAMP, next-generation metagenome sequencing, nanopore sequencing, etc., with sensitivities raging from 45.18 to 94.4%26,27,28,29,30,31. The comparatively lower results of the Xpert MTB/RIF assay in our study than those previously studies may be related to several factors, including study site, sample source, and the operator, suggesting that we need to improve the level of testing within our hospital. On the other hand, it is essential to emphasize the acquisition of high-quality specimens, since this often determines the final yield. Jiang et al., have demonstrated that attention to sputum quality can significantly increase bacteriological confirmation32. None the less, the very recent published study also showed a low sensitivity of Xpert MTB/RIF assay on BALFs to be 34.78%, lower than ours33. We can also assess the impact of BALF quality on bacteriological confirmation in the future.
Our results revealed that nucleotide MALDI-TOF MS exhibited improved sensitivity and accuracy over culture and Xpert MTB/RIF in both all suspected TB patients as well as smear-negative ones. Financially, the cost of MGIT 960 culture and nucleotide MALDI-TOF MS in this study was similar, at USD 40–50 and 60 per sample, respectively, being lower than Xpert MTB/RIF, with a cost of USD 100. In view of the fact that BALF is more difficult to obtain, requires specialised personnel handling and equipment, relative to sputum, eliciting more preciousness, we recommend that nucleotide MALDI-TOF MS method can be prioritized for BALF samples from patients with high suspicion of TB on the basis of the consent of the patient.
In our study, 14 cases were found to be nucleotide MALDI-TOF MS assay-negative but culture-positive. Finally, eight of them (57.1%) were clinically diagnosed with PTB and others were diagnosed with non-PTB. These false negatives may be due to insufficient nucleic acid or PCR inhibitors in BALFs34. On the other hand, 85 patients were found to be nucleotide MALDI-TOF MS assay-positive but culture-negative, 83 of which were diagnosed with PTB. This occurred might have several reasons. First, positive culture required live bacilli and the treatment history of patients might lead to negative cultures. Second, successful cultures require various factors including reagents, instruments, storage and expertise in operation35. In terms of the turnaround time of MGIT 960 culture and nucleotide MALDI-TOF MS assay, the detection time of results obtained from culture in our hospital ranged from 14 to 50 days where less than one day for nucleotide MALDI-TOF MS assay.
We here also analyzed the consistency of nucleotide MALDI-TOF MS assay and Xpert MTB/RIF for rifampicin sensitivity with a good agreement between the two assays. Rifampicin susceptibility culture results using MGIT 960 system, were all available for the six discordant samples. We observed that all samples discordant with culture results were RIF-resistant using nucleotide MALDI-TOF MS assay. Several reasons might be responsible for this inconsistence. MGIT 960 drug susceptibility testing was prone to yield false sensitive results for samples with minimal inhibitory concentrations (MICs) for rifampicin close to cut-off value36. Heteroresistance should also be considered as a factor that might influence the outcomes since nucleotide MALDI-TOF MS assay has ability to detect low-level heterogeneous drug resistance with the limit of 0.1%37. Certainly, technical errors of nucleotide MALDI-TOF MS assay leading to false resistance cannot be ruled out, after all, the results of the other two methods were consistent.
Our study has several limitations. First, it was a retrospective study conducted at a single center and some clinical data were missing. Consequently, our study did not account for some potential confounding factors that might influence TB diagnosis, such as HIV coinfection, life styles or other antibiotic use. Failure to control for these factors could impact the diagnostic tests’ accuracy. Nevertheless, using the available information we have attempted to analyse the influence of confounding factors we included on false positives and negatives using nucleotide MALDI-TOF MS. In order to obtain much more precise evaluation of performance of nucleotide MALDI-TOF MS assay, prospective and multicenter studies should be designed in future. Second, smear microscopy and nucleotide MALDI-TOF MS assay were performed on all of the samples, whereas MGIT 960 culture and Xpert MTB/RIF were not always done, thus resulting in some biases because of the missing data. For example, in this study, all 41 MTB-negative cases detected by Xpert MTB/RIF were clinically diagnosed as non-PTB cases, which resulted in 100% specificity and PPV. A bias was introduced by the fact that 28 non-TB cases were missing from the Xpert MTB/RIF results. Third, bronchoscopy was invasive and not acceptable to all patients, which was performed after carefully considered to be necessary to obtain BALFs, such as a high suspicion of PTB. This resulted in the majority of enrolled patients diagnosed as PTB, with bias in terms of specificity. Finally, in terms of the inconsistent samples for rifampicin susceptibility identified with nucleotide MALDI-TOF MS and Xpert MTB/RIF, we didn’t perform sequencing to further validate.
Conclusion
In summary, this study has shown nucleotide MALDI-TOF MS assay of BALFs is an accurate tool for the early diagnosis of PTB with high sensitivity and comparable specificity, which can provide more effective guidance for the treatment of PTB.
Data availability
All the data and material were true and available. The data is available from the corresponding author upon reasonable request.
References
World Health Organization. Global tuberculosis report 2022 (World Health Organization, 2022).
Van Cutsem, G. et al. Infection control for drug-resistant tuberculosis: Early diagnosis and treatment is the key. Clin. Infect. Dis. 62, S238–S243 (2016).
Yoshida, S., Tsuyuguchi, K., Kobayashi, T., Shimatani, Y. & Inoue, Y. Effect of sputum quality on Mycobacterium avium-intracellulare complex lung disease diagnosis and treatment initiation according to disease type. Diagn. Microbiol. Infect. Dis. 104(3), 115773. https://doi.org/10.1016/j.diagmicrobio.2022.115773 (2022).
Hanrahan, C. F. et al. Xpert MTB/RIF as a measure of sputum bacillary burden: Variation by HIV status and immunosuppression. Am. J. Respir. Crit. Care Med. 189, 1426–1434 (2014).
Martinez, L. et al. Cytomegalovirus acquisition in infancy and the risk of tuberculosis disease in childhood: A longitudinal birth cohort study in Cape Town, South Africa. Lancet Glob. Health 9, e1740–e1749 (2021).
Wu, Q. et al. The role of Xpert MTB/RIF using bronchoalveolar lavage fluid in active screening: Insights from a tuberculosis outbreak in a junior school in eastern China. Front. Public Health 11, 1–9 (2023).
McWilliams, T. et al. Induced sputum and bronchoscopy in the diagnosis of pulmonary tuberculosis. Thorax 57, 1010–1014 (2002).
Pan, X. et al. A comprehensive evaluation of Xpert MTB/RIF assay with bronchoalveolar lavage fluid as a single test or combined with conventional assays for diagnosis of pulmonary tuberculosis in China: A two-center prospective study. Front. Microbiol. https://doi.org/10.3389/fmicb.2018.00444 (2018).
Kim, J.-Y. et al. Impact of treatment on long-term survival of patients with Mycobacterium avium complex pulmonary disease. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciad108 (2023).
Zeka, A. N., Tasbakan, S. & Cavusoglu, C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J. Clin. Microbiol. 49, 4138–4141 (2011).
World Health Organization. Module 3: Diagnosis WHO consolidated guidelines on tuberculosis Rapid diagnostics for tuberculosis detection (World Health Organization, 2020).
Hu, Y. et al. Comparison of the diagnostic performance of meltpro and next-generation sequencing in determining fluoroquinolone resistance in multidrug-resistant tuberculosis isolates. J. Mol. Diagn. 25, 342–351 (2023).
Mediavilla-Gradolph, M. C. et al. Use of MALDI-TOF MS for identification of nontuberculous mycobacterium species isolated from clinical specimens. Biomed. Res. Int. 2015, 854078 (2015).
Hsu, K.-H. et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS One 10, e0120852 (2015).
Wu, X. et al. Prediction of mycobacterium tuberculosis drug resistance by nucleotide MALDI-TOF-MS. Int. J. Infect. Dis. 121, 47–54 (2022).
Neuschlova, M., Vladarova, M., Kompanikova, J., Sadlonova, V. & Novakova, E. Identification of mycobacterium species by MALDI-TOF mass spectrometry. Adv. Exp. Med. Biol. 1021, 37–42 (2017).
National Health and Family Planning Commission of the People’s Republic of China. Diagnostic Criteria for Tuberculosis (WS 288–2017). Electronic Journal of Emerging Infectious Diseases 3, (2018).
Shi, J. et al. Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) in the detection of drug resistance of Mycobacterium tuberculosis in re-treated patients. Tuberculosis 135, 102209 (2022).
Epperson, L. E. et al. Evaluation of a Novel MALDI Biotyper algorithm to Distinguish Mycobacterium intracellulare From Mycobacterium chimaera. Front. Microbiol. 9, 1–6 (2018).
Chen, X.-F. et al. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analysis for the identification of pathogenic microorganisms: A review. Microorganisms 9, 1536 (2021).
Genc, G. E. et al. Evaluation of MALDI-TOF MS for identification of nontuberculous mycobacteria isolated from clinical specimens in mycobacteria growth indicator tube medium. New Microbiol. 41, 214–219 (2018).
Rodríguez-Sánchez, B. et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nontuberculous mycobacteria from clinical isolates. J. Clin. Microbiol. 53, 2737–2740 (2015).
Su, K. Y. et al. Rapid sputum multiplex detection of the M. tuberculosis complex (MTBC) and resistance mutations for eight antibiotics by nucleotide MALDI-TOF MS. Sci. Rep. 7, 1–10 (2017).
Li, B. et al. Performance evaluation and clinical validation of optimized nucleotide MALDI-TOF-MS for mycobacterial identification. Front. Cell Infect. Microbiol. https://doi.org/10.3389/fcimb.2022.1079184 (2022).
Yang, H. et al. A rapid, accurate, and low-cost method for detecting Mycobacterium tuberculosis and its drug-resistant genes in pulmonary tuberculosis: Applications of MassARRAY DNA mass spectrometry. Front. Microbiol. https://doi.org/10.3389/fmicb.2023.1093745 (2023).
Bai, W. et al. Assessing the utility of the Xpert Mycobacterium tuberculosis/rifampin assay for analysis of bronchoalveolar lavage fluid in patients with suspected pulmonary tuberculosis. J. Clin. Lab. Anal. https://doi.org/10.1002/jcla.24154 (2022).
Fan, L. et al. A comprehensive evaluation of a loop-mediated isothermal amplification assay for the diagnosis of pulmonary tuberculosis in children using bronchoalveolar lavage fluid. Infect. Drug Resist. 15, 975–987 (2022).
Wu, Z. et al. The diagnostic value of the thermostatic amplification of ribonucleic acid in bronchoalveolar lavage fluid in smear-negative pulmonary tuberculosis. Front. Public Health https://doi.org/10.3389/fpubh.2022.830477 (2022).
Xu, P. et al. Next-generation metagenome sequencing shows superior diagnostic performance in acid-fast staining sputum smear-negative pulmonary tuberculosis and non-tuberculous mycobacterial pulmonary disease. Front. Microbiol. https://doi.org/10.3389/fmicb.2022.898195 (2022).
Zhu, N., Zhou, D. & Li, S. Diagnostic accuracy of metagenomic next-generation sequencing in sputum-scarce or smear-negative cases with suspected pulmonary tuberculosis. Biomed. Res. Int. https://doi.org/10.1155/2021/9970817 (2021).
Liu, Z. et al. Diagnostic value of a nanopore sequencing assay of bronchoalveolar lavage fluid in pulmonary tuberculosis. BMC Pulm. Med. 23, 77 (2023).
Jiang, Q. et al. A randomised controlled trial of stepwise sputum collection to increase yields of confirmed tuberculosis. Int. J. Tuberc. Lung Dis. 23, 685–691 (2019).
Cao, J. et al. EBUS-GS with the GeneXpert MTB/RIF assay for diagnosis of Mycobacterium tuberculosis infection of isolated pulmonary nodules. Eur. J. Med. Res. 28, 370 (2023).
Meyer, A. J. et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS One 12(7), e0180572 (2017).
Luo, J. et al. Biological interpretation of the sporadic sputum smear-positive-culture-negative outcome for patients with tuberculosis undertaking treatments. Front. Public Health https://doi.org/10.3389/fpubh.2023.1064512 (2023).
Bokop, C., Faye, L. M. & Apalata, T. Analysis of discordance between genotypic and phenotypic assays for rifampicin-resistant Mycobacterium tuberculosis Isolated from healthcare facilities in mthatha. Pathogens 12, 909 (2023).
Mosko, M. J. et al. Ultrasensitive detection of multiplexed somatic mutations using MALDI-TOF mass spectrometry. J. Mol. Diagn. 18, 23–31 (2016).
Acknowledgements
The authors acknowledged the staff at Chongqing Tuberculosis Control Institute, Chongqing Public Health Medical Center. This research was funded by Chongqing medical scientific research project, grant number 2023MSXM0321.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.J. and T.L.; Data curation, T.L.; Formal analysis, L.L. and M.X.; Funding acquisition, L.J.; Investigation, M.X. and J.L.; Methodology, L.L., M.X., J.L., J.X., J.T. and C.H.; Resources, T.L.; Software, J.T. and C.H.; Supervision, T.L.; Validation, L.J. and T.L.; Writing—original draft, L.J. and J.X.; Writing—review and editing, T.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, L., Xin, J., Liang, L. et al. Enhanced diagnosis of pulmonary tuberculosis through nucleotide MALDI-TOF MS analysis of BALF: a retrospective clinical study. Sci Rep 14, 18416 (2024). https://doi.org/10.1038/s41598-024-66178-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66178-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.