Abstract
The recommended first-line treatment for Mycoplasma genitalium infections is azithromycin. However, the prevalence of macrolide resistance for M. genitalium has increased to more than 50% worldwide. In 2013, Australia introduced a resistance-guided therapy (RGT) strategy to manage M. genitalium infections. This study assesses the cost-effectiveness of the RGT approach compared to no RGT (i.e., without macrolide resistance profile test) in women, men who have sex with men (MSM), and men who have sex with women (MSW) in Australia. We constructed dynamic transmission models of M. genitalium infections in women, MSM, and MSW in Australia, each with a population of 100,000. These models compared the costs and quality-adjusted life-years (QALYs) gained between RGT and no RGT scenarios from a healthcare perspective over ten years. All costs are reported in 2022 Australian dollars (Australian $). In our model, RGT is cost saving in women and MSM, with the incremental net monetary benefit of $1.3 million and $17.9 million, respectively. In MSW, the RGT approach is not cost-effective, with an incremental cost-effectiveness ratio of -$106.96 per QALY gained. RGT is cost saving compared to no RGT for M. genitalium infections in women and MSM, supporting its adoption as the national management strategy for these two population groups.
Similar content being viewed by others
Introduction
The pooled prevalence of Mycoplasma genitalium in randomly selected samples from the general population was 1.3% (95% CI 1.0% to 1.8%, I2 41.5%) for 2007 to 2015 in higher Human Development Index (HDI) countries1. In many countries, M. genitalium was the second most prevalent bacterial sexually transmitted infection (STI) after Chlamydia trachomatis2,3. According to the third National Survey of Sexual Attitudes and Lifestyle (Natsal-3), the prevalence of M. genitalium was 1.2% and 1.3% in British men and women, respectively4. In the US, a multicentre surveillance study from sexual health clinics showed the overall prevalence of M. genitalium infection was 16.6% (95% CI 14.9–18.5%; site-specific range: 9.9–23.5%)5. In Australia, the prevalence of M. genitalium infections ranges from 1.3% to 3.9% in the community1, and 1.3% in 2017 in women6. A cross-sectional study in Australia on asymptomatic men who have sex with men (MSM) found that the prevalence rate of M. genitalium was 9.5% (95% CI 7.7% 0 11.5%)7. While M. genitalium infection is frequently asymptomatic, it can cause urethritis in men, and cervicitis, endometritis, and pelvic inflammatory disease (PID) in women, although these data are limited8,9,10. Research from Australia also suggests that M. genitalium could contribute to symptomatic proctitis, although data are conflicting between published studies11,12.
Current national and international guidelines for managing M. genitalium do not advise screening for M. genitalium in asymptomatic individuals13,14, largely because treatment is becoming increasingly challenging due to rising antimicrobial resistance (AMR)15. The recommended first-line treatment for M. genitalium infections globally has been 1 g of azithromycin, resulting in more than 10% of susceptible infections developing selected macrolide resistance16. Widespread use of 1 g azithromycin for STI syndromes, chlamydia, M. genitalium and N. gonorrhoeae is likely to have contributed to the rise in macrolide resistance in M. genitalium, particularly in MSM who have higher rates of STIs and high levels of antibiotic consumption16,17,18. In Australia, the proportion of diagnosed infections with macrolide resistance mutations (MRMs) increased from 18.8% in 2010 to 66.0% in 2016–201719. M. genitalium infections are now commonly macrolide-resistant, and the proportion of infections with MRMs ranges from 32.7 to 44.0% in women, 53.0% in men who have sex with women (MSW), and 84.2–87.0% in MSM16,17,18.
Resistance-guided therapy (RGT) involves selecting antibiotics based on the macrolide resistance profile of each infection20. This strategy was developed to reduce the empiric use of azithromycin, enabling a more precise selection of first-line antibiotics, which improves antibiotic stewardship and the cure rate of first-line treatment. If doxycycline is used to treat STI syndromes instead of azithromycin, then when the macrolide resistance profile is known, macrolide-susceptible M. genitalium infections can be effectively treated with higher doses of azithromycin. Macrolide-resistant infections can be treated with moxifloxacin as a first-line treatment16. In a 2016 study, RGT resulted in cure rates of over 96% for macrolide-susceptible infections and 92% for macrolide-resistant infections and a reduction in selected macrolide resistance to less than 4% with the higher azithromycin dose16. As the incidence of AMR in M. genitalium continues to rise, using an RGT approach will promote antibiotic stewardship and could be less expensive than presumptive approaches21. Despite this potential, no economic evaluation of RGT for M. genitalium infections exists. To address this gap, this study aims to assess the cost-effectiveness of RGT compared to no RGT, where there is no macrolide resistance profile test performed for women, MSM and MSW living in Australia.
Methods
Model
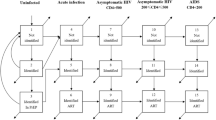
Dynamic transmission models of M. genitalium infection were constructed using TreeAge Pro Healthcare 2022 (Supplementary Fig. 1). There are three models that evaluated cohorts of 100,000 women, 100,000 MSM, and 100,000 MSW in Australia over ten years. The prevalence of M. genitalium varies in these three different population groups and is likely to be higher in specific populations whose behaviour puts them at a high risk of STI, such as MSM6. These three groups were selected to reflect the different risks of getting M. genitalium infections, the different rates of MRMs and consequent morbidity. All three models used a ten-year time horizon to capture the long-term complications of M. genitalium infections in women10. These three models used the same structure with different input parameters adjusted to each population group (Table 1). These three models were stratified by symptom status, distinguishing between symptomatic and asymptomatic M. genitalium infections (Fig. 1). The force of infection, which represents the rate at which susceptible individuals in the model became infected with M. genitalium at a given point in time, was determined as a function of the number of infectious individuals in a year (based on the M. genitalium infections incidence rate) and the population size of our cohort 22.
Treatment pathways were modelled, with only those in the symptomatic M. genitalium infection state transitioning to first-line therapy. Cohorts that failed the first-line therapy were transitioned to the second-line therapy. Cohorts in the model who did not complete treatment or were lost to follow-up during any treatment phase were transitioned to the incurable state. The incurable state was not regarded as terminal; cohorts within it could be cured, returning to an 'uninfected or susceptible state’ based on the weekly spontaneous cure rate15. Our model also allows M. genitalium infection, symptomatic or asymptomatic, to spontaneously clear without testing and treatment15,23. Figure 1 illustrates the structure of the model for symptomatic and asymptomatic M. genitalium infections in women, MSM and MSW. Cohorts in all health states in this model were subject to natural background mortality. The main parameters used in this model are shown in Table 1, and a complete list is available in Supplementary Table 1.
The model also measured M. genitalium-related complications in women, including PID and chronic pelvic pain10. A separate decision tree model was created to calculate the cumulative expected values of the probability, costs, and disutility associated with M. genitalium-related complications in women (Supplementary Fig. 2). This value was then assigned in the dynamic model with each new case of M. genitalium-related complications.
Definition of scenarios
The study presents a comparison of RGT and no RGT for the treatment of M. genitalium infections (Supplementary Table 2). We acknowledge that the reference regimen for no RGT may differ globally from 1 g of azithromycin in many low- and middle-income countries, 1.5 g in some northern European countries, to even using moxifloxacin in some sub-populations where macrolide resistance is known to be high. For our model, we defined the reference scenario as no RGT and first-line therapy as azithromycin 1 g on day 1. If a patient fails this regimen, second-line therapy comprises 400 mg of moxifloxacin daily for seven days14.
In the RGT arm, the first-line therapy is based on macrolide susceptibility. For patients with macrolide-susceptible infections, the first-line therapy consists of doxycycline 100 mg twice a day for seven days, followed by azithromycin (1 g on day one and 500 mg for the next three days, totalling 2.5 g) for four days14. In contrast, the first-line therapy for patients with macrolide-resistant infections was doxycycline 100 mg twice daily, followed by moxifloxacin 400 mg daily for seven days. The second-line therapy consists of 400 mg of moxifloxacin daily for seven days for those who are macrolide-susceptible, and minocycline 100 mg twice a day for 14 days for those with macrolide-resistant.
Cost-effectiveness analysis
The cost-effectiveness analysis was conducted from a healthcare provider’s perspective, following the Consolidated Health Economic Evaluation Reporting Standards (Supplementary Table 3)24, using a 3% annual discount rate for both costs (2022 Australian $) and units of health (quality-adjusted life-years [QALYs]). We compared the cost-effectiveness of RGT to the absence of RGT (no RGT) in treating M. genitalium infection. Transition probabilities, costs, and utilities in the model were determined using information from published literature and expert opinion (Table 1).
The model ran in a weekly cycle (total of 520 cycles), and therefore, the annual incidence and clearance rates for M. genitalium infection were transformed into weekly rates. Cure rates for the first-, and second line for RGT and no RGT were used as transition probabilities between treatment phases. When there was no published data on M. genitalium infection, parameters from chlamydia studies were utilised as a substitute. For instance, the proportion of symptomatic and asymptomatic M. genitalium infections in women was obtained from a published chlamydia study25. Chlamydia trachomatis and M. genitalium are highly prevalent bacterial STIs with a significant co-infection rate in some populations. Although caused by entirely different microorganisms, they share similarities in pathogenesis, clinical manifestations and treatment26.
The costs evaluated in this study were from a healthcare perspective and were calculated based on the unit costs from the Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Schedule (PBS). Direct medical costs were included in the model, such as costs associated with M. genitalium diagnosis and resistance testing, treatment, and medical visits. Costs for diagnosis include one consultation and one test for M. genitalium infections, while costs for treatment include one consultation and one for receiving treatment. Costs for additional medical visits or treatment for individuals in the incurable state were not added as no data were available for this cohort. Cumulative costs for M. genitalium-related complications were obtained from published evidence and converted to Australian dollars in 2022. All costs are reported in 2022 Australian dollars. Quality adjusted life years (QALYs) gained were estimated by applying utility (health-related) weights of the various health states. Data on the utility weights associated with M. genitalium infections were limited, and for women, a utility weight of 0.96 was assumed based on a previous cost-effectiveness study on chlamydia27. This weight was derived from the possibility of stress and anxiety resulting from a positive diagnosis. For MSM and MSW, a utility weight of 0.96 was used based on a published cost-effectiveness study on M. genitalium infections in MSM15.
Sensitivity analysis
Univariate sensitivity analyses were performed to assess the impact of uncertainties associated with the model parameters. Uncertainties in this study may arise from the data through natural variation in the population, from the evaluative process through generalising from the context of one study to other contexts and patient populations or from choice of analytical method. The following variables (rate of M. genitalium infections, probability of getting tested and treated, discount rate, time horizon, proportion of asymptomatic and symptomatic M. genitalium infections, probability of complications, and all costs included in the model) were varied over plausible ranges to explore the impact of these values on the results (Supplementary Table 1). The willingness to pay threshold of $50,000 per QALY gained was used to determine whether the intervention was cost-effective. This threshold is defined as the maximum amount society is willing to pay for an extra unit of health gain. Results from the univariate sensitivity analyses are presented as tornado plots (Fig. 2a–c). In this plot, each bar represents the impact of uncertainty in each variable on the incremental cost-effectiveness ratio.
In probabilistic sensitivity analysis (PSA), multiple key input parameters were varied simultaneously across the cohort of 100,000 women, MSM and MSW over 1000 simulations. Gamma distributions were applied for treatment costs, consultation visits, tests, and cumulative costs for M. genitalium-related complications. Beta distributions were applied for treatment cure rate, utility weight and proportion of cohorts tested and treated. The results of the PSA were presented as an incremental cost-effectiveness scatter plot (Fig. 3a–c) to visualise the distribution of PSA results of RGT and no RGT.
Results
We evaluated the costs, population impact and cost-effectiveness of RGT compared to no RGT for treating M. genitalium infections in a modelled cohort of 100,000 women, 100,000 MSM and 100,000 MSW in Australia over ten years (Table 2).
Costs, effectiveness and cost-effectiveness of RGT in women
The total population being considered in this article is 300,000 (100,000 for each population group—women, MSM and MSW). Without RGT, the projected total costs of M. genitalium infections for 100,000 women over ten years were $0.75 million. The largest cost component was associated with complications from M. genitalium infections. The projected effectiveness for a cohort of 100,000 women over ten years was 996,736 QALYs. Without RGT, there were 575 M. genitalium-related cumulative complication events among 100,000 women in Australia over ten years.
Our model demonstrated that implementing RGT for treating M. genitalium infections in a cohort of 100,000 women in Australia over ten years was cost saving, resulting in lower costs and higher QALYs than no RGT. The total costs and effectiveness associated with RGT over ten years were $0.64 million and 996,765 QALYs, respectively. The net monetary benefit (NMB) of RGT at the WTP threshold of $50,000 is $1.3 million. The incremental cost-effectiveness ratio is -$3604 /QALY gained. The number of M. genitalium-related complication events over ten years was also lower by 140 compared to no RGT scenario.
Costs, effectiveness and cost-effectiveness of RGT in MSM
Without RGT, the projected total costs of M. genitalium infections over ten years for a cohort of 100,000 MSM were $2.22 million, with the largest cost component being the costs for diagnosis. The projected effectiveness for a cohort of 100,000 MSM with no RGT was 1,026,662 QALYs gained.
Our model demonstrated that implementing RGT for treating M. genitalium infections in a cohort of 100,000 MSM in Australia over ten years was cost saving, resulting in lower costs and higher QALYs than no RGT. The total costs and effectiveness associated with RGT over ten years were $2.07 million and 1,026,974 QALYs, respectively. The NMB of RGT at the WTP threshold of $50,000 is $17.9 million. The incremental cost-effectiveness ratio was -$432.94/QALY gained.
Costs, effectiveness and cost-effectiveness of RGT in MSW
Without RGT, the projected total costs of M. genitalium infections over ten years for a cohort of 100,000 MSW amounted to $0.45 million, with the largest cost component being the costs of diagnosis. The projected effectiveness for a cohort of 100,000 MSW with no RGT was 991,267 QALYs gained over ten years.
Our model demonstrated that implementing RGT for treating M. genitalium infections in a cohort of 100,000 MSW in Australia over ten years was not cost-effective at the WTP of $50,000, with an incremental cost-effectiveness ratio of -$196.96 per QALY gained. The total costs and effectiveness associated with RGT over ten years were $0.45 million and 991,234 QALYs, respectively, for a cohort of 100,000 MSW in Australia.
Sensitivity analysis
Tornado plots were used to present the results of the univariate sensitivity analyses in Fig. 2a–c. The incremental cost-effectiveness ratio (ICER) for comparing RGT to no RGT for M. genitalium infections in women (Fig. 2a) was most sensitive to several factors: the utility during M. genitalium infections, the utility when experiencing M. genitalium-related complications, and the costs associated with M. genitalium-related complications.
(a) Univariate sensitivity analysis of the Incremental Cost-Effectiveness Ratio of no RGT compared to RGT for M. genitalium infections in the cohort of 100,000 women. Black bars correspond to the effect of the low value in the sensitivity analysis, and grey bars correspond to the high value in the sensitivity analysis. This figure includes only the 12 variables with the highest uncertainty values. (b) Univariate sensitivity analysis of the Incremental Cost-Effectiveness Ratio of no RGT compared to RGT for M. genitalium infections in the cohort of 100,000 MSM. Black bars correspond to the effect of the low value in the sensitivity analysis, and grey bars correspond to the high value in the sensitivity analysis. This figure includes only the 12 variables with the highest uncertainty values. MSM, Men who have sex with men. (c): Univariate sensitivity analysis of the Incremental Cost-Effectiveness Ratio of no RGT compared to RGT for M. genitalium infections in the cohort of 100,000 MSW. Black bars correspond to the effect of the low value in the sensitivity analysis, and grey bars correspond to the high value in the sensitivity analysis. This figure includes only the 12 variables with the highest uncertainty values. MSW, Men who have sex with women.
The ICER for comparing RGT to no RGT for M. genitalium infections in MSM (Fig. 2b) was most sensitive to the cure rate of first-line therapy in no RGT, the cost of first-line therapy in RGT for those macrolides susceptible, and cost of second-line therapy in no RGT. . For MSW (Fig. 2c), the ICER for comparing RGT to no RGT for M. genitalium infections was most sensitive to the cure rate of first-line therapy in no RGT, the cost of first-line therapy in RGT for those macrolides susceptible, and the probability of getting treatment.
Figure 3a–c illustrate the results of the probability sensitivity analysis. Figure 3a demonstrates that RGT has a 100% probability of being cost-effective in women, while for MSM, RGT has an 88.2% probability of being cost-effective at the given willingness-to-pay threshold. Figure 3C shows that RGT has 60.7% probability of being cost-effective in MSW at the given WTP threshold (WTP = $50,000).
(a) Incremental cost-effectiveness scatterplot of 1000 samples comparing RGT to no RGT for M. genitalium infections in the cohort of 100,000 women with a willingness to pay threshold of $50,000/QALY gained. The green circle shows the 95% confidence interval. (b) Incremental cost-effectiveness scatterplot of 1000 samples comparing RGT to no RGT for M. genitalium infections in the cohort of 100,000 MSM with a willingness to pay threshold of $50,000/QALY gained. The green circle shows the 95% confidence interval. MSM, Men who have sex with men. (c) Incremental cost-effectiveness scatterplot of 1000 samples comparing RGT to no RGT for M. genitalium infections in the cohort of 100,000 MSW with a willingness to pay threshold of $50,000/QALY gained. The green circle shows the 95% confidence interval. MSW, Men who have sex with women.
Discussion
In this cost-effectiveness analysis, using RGT for treating M. genitalium infections in women and MSM proved to cost less, be more effective, and was associated with lower rates of complications compared to the no RGT. In MSW, while the use of RGT for determining the treatment of M. genitalium infections was not cost-effective, the PSA shows that there is a probability of 60.7% RGT could be cost-effective at the willingness-to-pay (WTP) threshold of $50,000. Our model also predicted that RGT would lead to fewer M. genitalium infections in women and MSM over ten years. This means that fewer infections are transmitted and fewer return visits and antibiotics are used, and indicates that RGT is likely to have additional, broader population benefits in slowing the future rise and spread of AMR in both target and non-target pathogens. For the MSW cohort, RGT did not result in fewer M. genitalium infections, which might be caused by the lower testing rate among this population group. Our findings support current Australian guidelines that RGT should be the recommended management strategy for M. genitalium infections and should prompt countries with similar parameters to adopt RGT to reduce healthcare costs and improve antibiotic stewardship.
Our univariate sensitivity analyses revealed the factors that could significantly impact the cost-effectiveness of RGT. Among women, two of the most influential factors for the ICER was associated with M. genitalium-related complications. Previous meta-analyses have demonstrated a twofold increased risk of cervicitis, PID, spontaneous abortion, and infertility in women infected with M. genitalium, which results in higher costs and a greater disease burden for affected women10. Our model only considered PID and chronic pelvic pain as M. genitalium-related complications in women due to data availability. While our model provides valuable insights, we acknowledge the need for more evidence around M. genitalium-related complications in women. Some complications remain areas of debate within the field, and further research is essential to address these uncertainties. Among MSM and MSW, the cure rate of the first-line therapy in no RGT has the most significant impact on the ICER. This highlights the likelihood of RGT being more cost-effective in these two populations with the increasing rate of macrolide resistance globally in the future.
Reducing antibiotic usage in treating M. genitalium is essential for addressing the global public health crisis of rising AMR among STIs19. M. genitalium has rapidly accumulated high rates of AMR, making it increasingly challenging to treat, especially against the backdrop of already scarce treatment options28. Our findings demonstrated that RGT was a cost-saving intervention that can contribute to slowing the rise of AMR by reducing antibiotic misuse and overuse. Studies from various settings have shown that RGT is easy to implement and tends to have high adherence, providing value for money2,20. However, widespread adoption of this strategy is hindered by the limited availability of diagnostic technology, associated costs, and treatment options for M. genitalium infections globally29.
Our study assumed a static antibiotic resistance profile over a ten-year time horizon, while a meta-analysis has shown a plateau in macrolide resistance in M. genitalium in Australia since 201730. This interesting trend in macrolide resistance coincides with a reduction in using 1 g of azithromycin for M. genitalium, C. trachomatis, and STI syndromes in Australia, as well as the introduction of RGT for M. genitalium. Future studies on the cost-effectiveness analysis of RGT for M. genitalium infections may benefit from using a dynamic antibiotic resistance profile, expanding the analysis to a societal perspective, obtaining more robust estimates for variables that were identified as influential in our sensitivity analyses (e.g., for incidence of M. genitalium-related complications in women, probability of getting treatment), and assessing the epidemiological impact of antibiotic stewardship on the transmission dynamics of resistant M. genitalium. Next-generation RGT strategies that include fluoroquinolone resistance targets are also being developed, making it necessary to evaluate their cost-effectiveness across different sub-populations and geographical regions28,31.
Our study has several limitations that warrant consideration. First, the absence of publications on utility weights for individuals with M. genitalium infections and related complications prompted us to derive these values from studies on C. trachomatis, given the similarities in pathogenesis and clinical presentations of both infections32. We subjected these assumptions to sensitivity analyses to assess their potential impact on our conclusions. While they did not significantly affect our results in MSM and MSW, they were most influential in women, indicating that lower utility will have a significant impact on decreasing the ICER. Second, our study's findings may not be universally applicable, as the availability of molecular assays for resistance testing and alternative medications may vary across different regions. It would be beneficial to conduct economic evaluations tailored to specific settings and their unique management recommendations. Lastly, our model only calculated M. genitalium-related complications among cohorts of women, as limited evidence is available on the longer-term impact of M. genitalium infections among MSM and MSW. If complications such as epididymitis occurred, it would further favour using RGT in managing M. genitalium infections in MSM and MSW.
In conclusion, our study demonstrates that RGT is a cost-saving intervention, offering greater effectiveness and lower costs for women and MSM compared to no RGT. In MSW, RGT has probability of 60.7% to be cost-effective at the WTP threshold of $50,000. Future economic evaluations should be conducted in diverse settings with varying prevalence profiles and other subpopulations to validate and expand upon our findings.
Data availability
No datasets were generated during the current study. Data inputs were obtained from published studies or publicly available sources and are reported within this article and its supplementary material.
References
Baumann, L. et al. Prevalence of Mycoplasma genitalium in different population groups: Systematic review and meta-analysis. Sex. Transm. Infect 94, 254–261 (2018).
Read, T. R. H. et al. Impact of screening on the prevalence and incidence of Mycoplasma genitalium and its macrolide resistance in men who have sex with men living in Australia: A mathematical model. EClinicalMedicine https://doi.org/10.1016/J.ECLINM.2021.100779/ATTACHMENT/1F5B9325-A64E-4904-B53A-4136AB5C1992/MMC1.DOCX (2021).
Oakeshott, P. et al. Is Mycoplasma genitalium in women the ‘new chlamydia?’ A community-based prospective cohort study. Clin. Infect. Dis. 51, 1160–1166 (2010).
Sonnenberg, P. et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int. J. Epidemiol. https://doi.org/10.1093/ije/dyv194 (2015).
Manhart, L. E. et al. Mycoplasma genitalium in the US (MyGeniUS): Surveillance data from sexual health clinics in 4 US regions. Clin. Infect. Dis. 77, 1449–1459 (2023).
Baumann, L. et al. Prevalence of Mycoplasma genitalium in different population groups: Systematic review and meta-analysis. Sex. Transm. Infect. 94, 255–262 (2018).
Read, T. R. H. et al. Symptoms, Sites, and Significance of Mycoplasma genitalium in Men Who Have Sex with Men. Emerg. Infect. Dis. 25, 719–727 (2019).
Manhart, L. E., Broad, J. M. & Golden, M. R. Mycoplasma genitalium: Should we treat and how?. Clin Infect Dis 53, S129–S142 (2011).
McGowin, C. L. & Anderson-Smits, C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 7, e1001324 (2011).
Lis, R., Rowhani-Rahbar, A. & Manhart, L. E. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin. Infect. Dis. 61, 418–426 (2015).
Bissessor, M. et al. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin. Microbiol. Infect 22, 260–265 (2016).
Ong, J. J. et al. Clinical characteristics of anorectal Mycoplasma genitalium infection and microbial cure in men who have sex with men. Sex. Transm. Dis. 45, 522–526 (2018).
Jensen, J. S. et al. 2021 European guideline on the management of Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 36, 641–650 (2022).
Ong, J. J. et al. Australian sexually transmitted infection (STI) management guidelines for use in primary care 2022 update. Sex Health 20, 1–8 (2023).
Ong, J. J. et al. Impact of screening on the prevalence and incidence of Mycoplasma genitalium and its macrolide resistance in men who have sex with men living in Australia: A mathematical model. EClinicalMedicine 33, 100779. https://doi.org/10.1016/j.eclinm.2021.100779 (2021).
Read, T. R. H. et al. Clinical infectious diseases outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: A prospective evaluation. Clin. Infect. Dis. 68, 554–560 (2019).
Latimer, R. L. et al. The clinical indications for testing women for Mycoplasma genitalium. Sex. Transm. Infect. 98, 277–285 (2022).
Shilling, H. S. et al. Chlamydia trachomatis and Mycoplasma genitalium prevalence and associated factors among women presenting to a pregnancy termination and contraception clinic, 2009–2019. Sex. Transm. Infect. 98, 115–120 (2022).
Machalek, D. A. et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: A systematic review and meta-analysis. Lancet Infect. Dis. 20, 1302–1314 (2020).
Durukan, D. et al. Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline–2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: Efficacy and tolerability. Clin. Infect. Dis. 71, 1461–1468 (2020).
Unemo, M. & Jensen, J. S. Antimicrobial-resistant sexually transmitted infections: Gonorrhoea and Mycoplasma genitalium. Nat. Rev. Urol. 14, 139–152 (2017).
Walker, J. et al. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin. Infect. Dis. 56, 1094–1100 (2013).
Horner, P. J. & Martin, D. H. Mycoplasma genitalium Infection in Men. J. Infect. Dis. 216, S396 (2017).
Husereau, D. et al. (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BM Med. 2023, 20 (2022).
Owusu-Edusei, K., Chesson, H. W., Gift, T. L., Brunham, R. C. & Bolan, G. Cost-effectiveness of chlamydia vaccination programs for young women. Emerg. Infect. Dis. 21, 960 (2015).
Ljubin-Sternak, S. & Meštrović, T. Chlamydia TRACHOMATIS and genital mycoplasmas: Pathogens with an impact on human reproductive health. J. Pathog. 2014, 1–15 (2014).
Ong, J. J. et al. Chlamydia screening for pregnant women aged 16–25 years attending an antenatal service: A cost-effectiveness study. BJOG An. Int. J. Obstet. Gynaecol. 123, 1194–1202 (2016).
Sweeney, E. L., Bradshaw, C. S., Murray, G. L. & Whiley, D. M. Individualised treatment of Mycoplasma genitalium infection-incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect. Dis. 22, 367–370 (2022).
Fernández-Huerta, M., Serra-Pladevall, J., Pich, O. Q., Barberá, M. J. & Espasa, M. From resistance-guided to risk-guided antimicrobial therapy in Mycoplasma genitalium. Sex. Transm. Dis. 47, 409–411 (2020).
Chua T. Evolving antimicrobial resistance in Mycoplasma genitalium: an updated global systematic review and meta-analysis. 2023.
Vodstrcil, L. A. et al. Combination therapy for Mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve cure. Clin. Infect. Dis. 75, 813–823 (2022).
Borgogna, J. L. C. et al. The association of Chlamydia trachomatis and Mycoplasma genitalium infection with the vaginal metabolome. Sci. Rep. 10, 3420 (2020).
Xu, X. et al. Modelling the multiple anatomical site transmission of Mycoplasma genitalium among men who have sex with men in Australia. Sci. Rep. 11, 1–8 (2021).
Doyle, M. et al. Nonquinolone options for the treatment of Mycoplasma genitalium in the era of increased resistance. Open Forum. Infect. Dis. https://doi.org/10.1093/ofid/ofaa291 (2020).
Ong, J. J. et al. Cost-effectiveness of testing for Mycoplasma genitalium among men who have sex with men in Australia. Sex. Transm. Infect. 99(6), 398–403. https://doi.org/10.1136/sextrans-2022-055611 (2023).
Acknowledgements
This work is funded through the Australian National Health and Medical Research Council Emerging Leadership Investigator Grant (GNT1193955).
Funding
JO receives funding from the Australian National Health and Medical Research Council Emerging Leadership Investigator Grant (GNT1193955). CB and CKF receive funding from the Australian National Health and Medical Research Council Leadership Investigator Grant (GNT1173361 and GNT1172900 respectively). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
R.A., J.O. and L.Z. conceptualised and designed the study. R.A. designed the model, wrote the first draft of the manuscript and revised the subsequent drafts with the input from J.O. and L.Z. C.B. and L.V. provided input into the input parameters and data analysis. R.A., J.O., L.Z., C.B., C.K.F. and L.V. reviewed and revised the manuscript and approved the final draft. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
RA, LZ and LV declare no competing interests. JO, CB and CKF reports financial support was provided by Australian National Health and Medical Research Council. CB receives federal funding from an Australian Research Council Industrial Research Transformation Hub grant and her institution has received diagnostic kits for research on M. genitalium from both Speedx pty ltd and Cepheid Pty Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adawiyah, R.A., Bradshaw, C.S., Vodstrcil, L.A. et al. Cost-effectiveness of resistance-guided therapy for Mycoplasma genitalium in Australia. Sci Rep 14, 12856 (2024). https://doi.org/10.1038/s41598-024-63056-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63056-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.