Abstract
The possibility of coherent manipulation of optical and thermal energies in noble metal nanostructures has given birth to an enduring research arena coined by thermoplasmonics. Upon interaction with electromagnetic radiation, the energy of the produced hot electrons in metallic nanostructures is converted into heat and is transferred to the medium as a consequence of numerous relaxation processes. Gold nanorods have, often, been adopted as the classical anisotropic nanostructures owing to excellent shape-selective plasmonic tunability in the vis–NIR region. When a pair of metallic nanostructures are sufficiently close to each other to imbue electromagnetic interaction, there occurs evolution of collective plasmon modes, substantial enhancement of near field and strong squeezing of electromagnetic energy at the interparticle spatial region of the dimeric nanostructures. Recent advances in the ‘tips and tricks’ guide to assembling, even, anisotropic nanostructures in colloidal dispersions have offered the opportunity to interplay with the phenomenological plasmonic and thermal characteristics. The photothermal attributes emerging due to electromagnetic coupling of fringing fields have been explored considering parallel and perpendicular configurations of gold nanorod dimers as the prototypical systems from theoretical and experimental perspectives and their biomedical consequences have been realised in a mice model towards the photothermal apoptosis of cancerous cells.
Similar content being viewed by others
Introduction
Since its ancient usage in 1700 BC where the glowing tip of a fire drill had been used for breast cancer therapy, the exploration of heat has been borne as an emerging technique towards the hyperthermia treatment of malignant cells1. The conversion of light into heat governed by the law of conservation of energy has attracted enduring research interest that has given birth to the emergence of new subdisciplines recognized as thermoplasmonics2. During the boom of nanotechnology, wide varieties of nanomaterials that offer excellent capabilities of light harvesting and photothermal conversion, have been endowed for exploring fascinating and prospective applications3, ranging from wireless phototherapy4 to photothermal attogram spectroscopy5. Correlated rod-like nanostructures of gold have, often, been chosen as the prototypical anisotropic architectures owing to excellent shape-selective plasmonic characteristics in the vis–NIR region owing to the collective oscillations of the free conduction electrons along transverse and longitudinal directions upon interaction with electromagnetic radiation6. Moreover, the ordered arrangement of the nanostructures into numerous plausible statistical assemblies has offered the opportunity to unravel their plasmonic attributes from electromagnetic perspectives and therefore, to design a landscape towards the wide gamut of niche practical applications7. Recent advances in the ‘tips and tricks’ guide have extended the opportunity to induce aggregation amongst the rod-like nanostructures, even, in the colloidal dispersion. When a couple of metallic nanorods are proximal to each other sufficient enough to incur electromagnetic interactions, there occurs the evolution of collective plasmon modes, significant enhancement of the near field, and squeezing of light at the interparticle spatial region of the dimeric nanostructures8. The extension of the electric field some distance away at the edges of the anisotropic nanostructures is known as the fringing field which has also been coined as the ‘edge effect’ realized in a variety of nanostructures9.
The interaction of electromagnetic radiation converts the energy of the produced hot electrons into heat governed by the relaxation processes associated with the Fermi–Dirac distribution and electron–electron scattering and the converted thermal energy is transferred to the medium corresponding to the electron–phonon cooling as well as phonon–phonon scattering processes10. Several distinctive physical attributes have been exhibited by gold nanorod dimers, such as angle-resolved plasmonic photocapacitance11, two-photon photoluminescence12, the emergence of charge transfer plasmon13, plasmonic waveguiding14, and surface-enhanced Raman scattering15. The plasmonic characteristics of the assemblies of gold nanorod dimers have been exploited in a wide regime of applications, such as plasmonic sensing16,17, plasmonic mode imaging18, optical data storage19 and environmental remedies20. The interparticle coupling effect between the nanorod pairs offers extensive advantages as these exhibit the emergence of multiple plasmon modes that can be tuned by the geometry and relative orientations of the proximal nanostructures in the dimeric configurations21. Therefore, although there have been several extensive investigations to unravel the plasmonic characteristics and their plausible applications, the electromagnetic coupling of the fringing field at the edges of nanorods has remained unexplored.
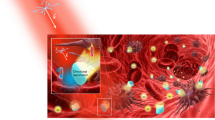
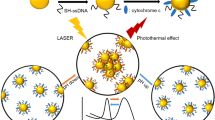
In this article, we have elucidated the photothermal apoptosis of cancerous cells in a mice model upon laser heating of the gold nanorod dimers from both theoretical and experimental perspectives. Aqueous dispersion of the gold nanorods has been synthesized through a seeded growth approach and subsequently, parallel and perpendicular configurations between the couple of nanostructures have been induced upon judicious addition of specific organic molecules in the reaction medium. The spatial and temporal variations of plasmonic and thermal characteristics as a consequence of electromagnetic coupling of fringing fields in gold nanorod dimers have been measured and to validate the experimental observations, numerical simulations based on discrete dipole approximation (DDA) technique and finite element method (FEM) have been performed. The photothermal characteristics due to the coupling of the fringing fields in the dimeric configurations have been assessed in the photothermal apoptosis of cancerous cells in a mice model to design a landscape towards plausible biomedical engineering inside the living cells.
Results and discussion
In this experiment, monomeric gold nanorods have been synthesized by a two-step seed-mediated growth method by following the protocol of the Murphy group22. The dimerization amongst the individual nanorods has been induced through the judicious manipulation of selective organic molecules by following the protocol of our group reported earlier11. Specifically, it has been observed that parallel (side-by-side) and perpendicular (end-to-end) dimers are formed upon addition of 1,8-diaminooctane and phloroglucinol (1,3,5-trihydroxybenzene), respectively23,24. The molecule, 1,8-diamino octane (C8H20N2) has a topological polar surface area of 52.0 nm2 with availability of lone pair of electrons of –NH2 groups; on the other hand, phloroglucinol (C6H3(OH)3) possesses a relatively large topological surface area of 60.7 nm2 and availability of lone pair of electrons of –OH groups. Moreover, the stereochemical orientation of these two types of molecules can be conceived to be distinctly different upon adsorption onto the gold surface. The delicate counterbalance between the stereochemical and electrostatic interactions of the specific organic molecules with the positively charged gold nanorods surfaces imparts dimerization selective to parallel and perpendicular orientations as governed by Deryaguin–Landau–Verwey–Overbeek (DLVO) theory25. Now, these as-synthesized gold nanorod dimers have been exploited as plausible nanostructures towards the photothermal apoptosis of cancerous cells; Fig. 1 illustrates the overall proof-of-concept.
The optical and morphological characteristics of the individual and dimers of gold nanorods have been exhibited in Fig. 2. Transmission electron micrographs (panels a–c) illustrate the monomers and parallel and perpendicular configurations of the dimers of gold nanorods, respectively. The length and width of the nanorods have been measured to be 37 ± 10 and 10 ± 2 nm, respectively with an aspect ratio of 3.7 ± 0.3. Upon addition of the specific organic linkers, the monomeric gold nanorods become assembled into parallel and perpendicular orientations with an average interparticle spacing of ca. 3.8 ± 0.3 and 5.6 ± 0.4 nm, respectively. Dark field micrographs (panels d-f) display that the plasmonic modes of the monomeric nanorods are snuffed for parallel orientations while becoming brightened corresponding to perpendicular configurations of the nanorods that occurs corresponding to the difference in the polarizations of the plasmon modes along longitudinal and transverse directions. The absorption spectral characteristics (panels g,h) reveal the modification of the localized surface plasmon bands upon addition of particular organic molecules. The appearance of two distinct absorption bands corresponding to the transverse and longitudinal plasmon modes can be ascribed to the geometry of the nanorods26. However, upon the addition of organic ligands, a significant spectral shift of the localized surface plasmon bands for the two different orientations has been observed. The salient feature of physical significance is that while a noticeable change in the longitudinal plasmon band is observed in both cases with respect to the orientation of the nanorods in the dimers, the transverse band remains almost unperturbed. A closer observation further elucidates that the longitudinal band shows a blue shift from 797 to 723 nm (ca. 74 nm) in case of the formation of perpendicular dimers while a relatively less red shift from 797 to 834 nm (ca. 37 nm) corresponding to the parallel dimers. The electromagnetic coupling between the gold nanorods is responsible for the changes in spectral positions; the relative decrease or increase in energy (relative to the longitudinal mode of a non-interacting rod) indicates the formation of dimers oriented in sidewise and orthogonal directions, respectively. The shift of the longitudinal band is due to the coupling between the plasmon modes of the individual monomers while the transverse band is not affected appreciably upon dimerization. This is because of although polarization of light perpendicular to the axis of the nanorods causes excitation of the transverse mode, the weak coupling does not impart a significant effect on the plasmon resonance in the dimeric configurations of the nanorods27.
Transmission electron micrographs of the (a) monomers, (b) parallel and (c) perpendicular orientations of the gold nanorods dimers; dark field micrographs of the (d) monomers, (e) parallel and (f) perpendicular orientations of the dimers; and changes in the absorption spectra upon inducing dimerization to (g) parallel and (h) perpendicular orientations of the dimers of the nanorods.
A detailed kinetic analysis of the plasmonic and thermal characteristics upon laser irradiation of the gold nanorod dimers has been enunciated in Fig. 3. The time-dependent photothermal experiments have been performed with continuous wave near-infrared laser (785 nm) by taking the dispersion of gold nanorod dimers in a 1 cm well-stoppered quartz cuvette. The laser irradiation has been carried out over a span of 200 min at a periodic interval of 5 min and distinct plasmonic shift of the localized surface plasmon band (panels a,b) has been observed for both the dimeric configurations of the nanorods. It is noted that both the parallel and perpendicular orientations upon laser irradiation impart a significant shift in the absorption spectrum at regular time intervals. However, while a considerable shift of the longitudinal plasmon band is observed, a mere change in the transverse mode of the spectrum is noticed in the case of both dimers. An explicit observation shows that the end-bonded orientations undergo a radical blue shift (ca. 102 nm) while the sidewise-oriented dimers show relatively less shifting (ca. 50 nm) of the longitudinal band as time progresses. Transmission electron micrographs (panels c,d) show the rupturing of the dimers upon laser exposure and reshaping into smaller architectures. It can, therefore, be anticipated that the linkage between the dimers is broken and deformation occurs upon photothermal melting of the rod-like nanostructures. This can be ascribed to the fact that following laser exposure, the photon energy is absorbed at the fringing junction of the perpendicular dimers with the maximum local electron density which is not possible for parallel dimers. The coupled monomers will, then, break and the ensuing assemblies becomes disrupted as a result of electron–phonon coupling and electron–electron scattering increasing the local temperature. Further long-term laser irradiation ruptures the crystalline structure and modifies the geometry of the nanorods under the experimental conditions. Panels (e,f) display the captured infrared images of the parallel and perpendicular dimers recorded on Fluke Connect PTi120 pocket thermal camera during photothermal treatment corresponding to time steps maintained at kinetic experiments. The thermal images recorded have been analyzed with the Fluke Connect software. A hand-written python code has been used to compute the equilibrium temperature profiles around the nanostructures. The 3D plots of the imported data represent the corresponding temperature distribution profiles of the gold nanorod dimers during the melting process. Panels (g,h) exhibit the temperature distribution profiles of the parallel and perpendicular dimers corresponding to the captured images during the photothermal treatment. The plots show the profiling from initial to the final point and the peak point indicates the maximum temperature rise in the respective process. A closer analysis reveals wider distribution of temperature profiles for perpendicular dimers than that of parallel dimers indicating larger distribution of higher temperature in the endwise orientations in comparison with sidewise orientations. It has been enumerated that the initial temperature of the process recorded is ca. 27 °C (room temperature) and final temperature raises up to 38 °C and 42 °C for parallel and perpendicular dimers, respectively. The salient feature of physical significance is that the temperature difference between the time steps is 1–3 °C, which is the feasible condition for biomedical applications. It is, therefore, apparent that the temperature distribution around the nanostructures during photothermal treatment can be manipulated by varying the relative configuration of the nanorods in the dimer geometry.
(a,b) Photothermal kinetics showing the modification in the absorption spectra; (c,d) transmission electron micrographs; (e,f) digital infrared images captured with thermal imaging camera; and (g,h) corresponding temperature distribution profiles obtained using Fluke connect software of the parallel and perpendicular orientations of the gold nanorod dimers, respectively. The marked regions with arrow head in the panels (a,b) indicate the blue shift between the initial and the final steps.
The coupling of gold nanorods shifts the maximum of the localized surface plasmon band in the absorption spectrum. The overall electric field experienced by a nanorod in the dimer is equal to the sum of the incident field and the induced field due to the adjacent nanorod28,29. As the nanorods lie in a close proximity to one another, the induced field can be thought of as the ‘near-zone’. The overall polarizability and the plasmon resonance frequency can be obtained which depends upon the dielectric constant of the material and the distance between the coupled rods in the dimer. In the present work, finite element analysis has been carried out to calculate the field enhancement using Maxwell’s equations for electromagnetic wave at the junction of the two nanorods. The field enhancement at the surface of the nanorod intermixes to obtain a higher field at the junction than the individual particles which indicates the coupling between the nanorods. The overall electric field distribution patterns have been calculated at different orientations corresponding to two different geometries of the dimers using finite element method as displayed in Fig. 4. Maxwell’s equations have been solved to calculate the electric field distributions for both the parallel and perpendicular orientations of the dimers. The field distribution patterns have been obtained corresponding to the shift in respective wavelength in the transverse and longitudinal modes during the photothermal melting experiments. A plane wave has been considered to pass through the operating xy-plane. The dimension of the nanorods has been assumed to be of length 37 ± 10 nm and width 10 ± 2 nm and average interrod spacings have been considered to be 3.8 and 5.6 nm, respectively for parallel and perpendicular orientations in consistent with transmission electron microscopic measurements. It has been observed that dimeric structures display longitudinal and transverse polarizations, which are extremely sensitive to the shift in wavelength during the photothermal melting experiments30. The change in particle size has implicitly been taken into consideration through the associated change in electric field distribution at each time step. For parallel configurations (panel a), while the longitudinal mode field patterns are at the junction between the dimers, the transverse mode field patterns are spread outwards along the width of the dimers. On the other hand, for the perpendicular orientations (panel b), corresponding to the transverse mode, the field patterns are distributed along the width of the dimers while for longitudinal mode, the fringe region experiences an intensified electric field in comparison with the residual part of the dimer. This is due to the fact that the individual plasmon oscillations on proximal particles can be increased via the respective near-field interactions resulting in coupled plasmon resonance modes, quite akin to excitonic coupling model during hybridization of molecular orbitals31. Since the distribution of electric field is more outside the dimer region corresponding to the transverse plasmon mode, the field enhancement occurs at the end of the dimers due to antibonding interaction of the monomers. Thus, the electric field upon dimerization does not change significantly that corroborates well with the negligible shift observed in transverse band. Moreover, the magnitude of the near field enhancement at the interparticle gap depends directly on the distance between the nanostructures, being greater as the gap becomes smaller8,18. Laser irradiation at higher energies produces near-field enhancement at the interparticle gaps, which is large enough to melt gold nanorod tips; thus, offering a new avenue towards perpendicular melting of gold nanorod dimers with a plasmonic response at the near-infrared region32. Therefore, it can be anticipated that plasmonic dimers can be selectively trapped and melted down, which has been analyzed in terms of a model that predicts with reasonable accuracy the relative orientations of the individual nanorods.
Electric field distribution patterns calculated through finite element method corresponding to the longitudinal and transverse polarizations at the representative wavelength maxima observed during the photothermal transformations of the (a) parallel and (b) perpendicular orientations of the gold nanorod dimers.
The manoeuvring of highly efficient and biocompatible plasmonic colloidal nanostructures has fostered numerous plausible applications in biomedical contexts, especially, the global demand for revolutionary improvement in the cancer therapeutics33. The controlled and confined photothermal ablation of tumour tissues leaving the healthy ones intact can be achieved through miniature manipulation of optical and thermal energies in plasmonic nanostructures34. The emergence of squeezing of fringing fields in gold nanorod dimers can be employed as efficient photothermal manipulation for the detection of apoptosis within carcinogenic cells. To explore the plausible biomedical applications of the dimeric nanostructures, the plasmonic and thermal attributes should be correlated at the nanoscale. Considering the coherence and unidirectional flow of the continuous wave laser beam towards the dimeric nanostructures, it can be perceived that conductive heat generated by the resistive field can be approximated as unidirectional heat flow across the surface. The temperature distribution, \(T\left( x \right)\) governed by heating power density, \(q\left( x \right)\) can be calculated by the Poisson equation as35,
where \(k_{x}\) is the thermal conductivity of gold (= 318 W/m K). Thus, the heat power density, \(q\left( x \right)\), along the x-axis can be expressed as,
The quantity of plasmonic heat, \(Q_{ext}\), governed by the process of resistive light–matter interaction with electromagnetic radiation can be represented as,
where σ is the optical conductivity of the materials, \(j\) the current, \(\varepsilon\) the optical dielectric constant corresponding to the angular frequency \(\left( \omega \right)\) of the incident wave, and \(E\) and \(E^{*}\) are the respective components of real and imaginary fields considering a plane wave interaction with the electromagnetic radiation that can be solved using finite element method corresponding to the nanostructures. Figure 5 depicts the plot of \(Q_{ext}\) as a function of time for the two conceived dimers corresponding to the entire laser wavelength maxima; the patterns show exponential decay with increase in time which indicates that the amount of generated plasmonic heat decreases as the time progresses. We have considered the modified form of Drude model for the calculation of optical conductivity where the wavelength shift of the dimers during laser irradiation has been taken into account36. The optical conductivity has been derived from the values of dielectric constant and electrical conductivity, calculated from Drude equation. The perpendicular dimers being orthogonal to each other, the hotspot region holds the maximum electron density. When an external force (laser) is applied, the hotspot region absorbs the incident photon energy more compared to the parallel arrangement and thus, the orthogonal position is more susceptible to heat absorption and its dissipation to the surroundings. Thus, the shift in the longitudinal band is greater in perpendicular dimers than in parallel dimers and also the overall heat released is greater in case of perpendicular dimers than in parallel dimers during laser irradiation. The salient feature of biological significance is that the perpendicular geometry releases more heat during the melting process as compared the parallel configuration and therefore, endwise orientations can act as efficient photothermal agents between the two-specific dimeric configurations under consideration.
The efficacy and efficiency of monomeric gold nanorods have been realized in numerous well-known photothermal processes, such as, combinational theranostics37 and photothermal ablation of malignant cells38. El-Sayed group39 employing with dark-field imaging technique have reported that gold nanorods act as potential photothermal agents upon laser irradiation that causes carcinogenic cell death with a dramatic impact of the laser power fluence. In the present work, the plausible application of apoptosis induction in cancerous cells of Swiss-albino LACA mice with two different dimeric aggregations of gold nanorods has been enunciated. The macrophages from the kidney have been isolated by following the method demonstrated by Sengupta group40. The gold nanorod dimers were internalized inside the LACA cells and the assembled nanostructures were observed to be, adequately, dispersed at the specific concentrations for, at least, 72 h that before performing the cell culture experiments. The photothermal efficiency of the coupled gold nanorod dimers towards the apoptosis of cancerous cells has been summarized in Fig. 6. Panels (a,b) display the transmission electron micrographs of the carcinogenic cells after the injection of the nanostructures (1.0 µM). Then, a closer look at the micrographs shows the incorporation of nanostructures within the cell. Now, the corresponding fluorescent staining images with DAPI and annexin-V-FITC assays (ca. both control and treated) have been performed to detect the presence of apoptotic cells. It has been known that DAPI is a blue fluorescent dye (\({\lambda }_{ex}\hspace{0.17em}\sim \hspace{0.17em}340 \text{nm}\); \({\lambda }_{em}\hspace{0.17em}\sim \hspace{0.17em}488 \text{nm}\)) that can be used for staining and fluorescence imaging of various cells. Annexin V is a protein that has a strong affinity towards PS (phosphatidylserine), a glycerophospholipid that moves from the inner to the outer leaflet of the cytoplasmic membrane during the development of apoptosis in the cells and fluorescently labelled Annexin V (Annexin V-FITC) (\({\lambda }_{ex}\hspace{0.17em}\sim \hspace{0.17em}494 \text{nm}\); \({\lambda }_{em}\sim 518 \text{nm}\)) can be utilized to detect apoptotic cells. Panels (c,e,g) and panels (d,f,h) are the fluorescent micrographs recorded in a Nikon Eclipse TS100 fluorescence microscope under control, treatment with parallel and perpendicular dimers corresponding to staining of DAPI and Annexin-V-FITC conjugates as can be recognized by the appearance of bright blue and green spots, respectively. A closer analysis of the images has been fluorescent dye (\({\lambda }_{ex}\sim 340 \text{nm}\); \({\lambda }_{em}\sim 488 \text{nm}\)) that can be used for staining and fluorescence imaging of various cells. Annexin V is a protein that has a strong affinity towards PS (phosphatidylserine), a glycerophospholipid that moves from the inner to the outer leaflet of the cytoplasmic membrane during the development of apoptosis in the cells and fluorescently labelled Annexin V (Annexin V-FITC) (\({\lambda }_{ex}\sim 494 \text{nm}\); \({\lambda }_{em}\sim 518 \text{nm}\)) can be utilized to detect apoptotic cells. Panels (c,e,g) and panels (d,f,h) are the fluorescent micrographs recorded in a Nikon Eclipse TS100 fluorescence microscope under control, treatment with parallel and perpendicular dimers corresponding to staining of DAPI and Annexin-V-FITC conjugates as can be recognized by the appearance of bright blue and green spots, respectively. A closer analysis of the images has been performed before and after injection of the dimeric nanostructures for the calculation of the percentage of apoptotic cell death. The number of spots obtained has been processed through the ImageJ software. Panel i shows the % enumeration of apoptotic cell death for the treated cells of kidney macrophages. It has been resolved that the % count of apoptotic cell death is maximum for treated (85 ± 3%) in case of perpendicular dimers rather than parallel (45 ± 2%) as compared to control (15 ± 0.7%) experiments. At the point of junction of the perpendicular dimers, the electric field is enhanced due to interaction of the fringing field of the monomers. As a result, the dimers of endwise orientations with enhanced local electron density at the fringing region absorb more photon energy in comparison with the parallel dimers and therefore, larger heat release in the surroundings. Thus, the perpendicular dimers cause a higher count rate of apoptotic cell deaths than parallel dimers. Therefore, a local temperature rise within the cells induces apoptosis and can serve as efficient photothermal nanostructures for % damage of the apoptotic cells.
Photothermal treatment of the gold nanorod dimers for detection of apoptosis: (a,b) transmission electron micrographs; fluorescent microscopic images upon (c,e,g) DAPI stained and (d,f,h) Annexin-V-FITC stained images under control and treated with parallel and perpendicular orientations of gold nanorod dimers upon laser irradiation inside cellular environment; and (i) percentage calculation (mean ± SEM) of the detection of apoptotic cells upon treatment.
In conclusion, we have elucidated the effect of the electromagnetic coupling of the fringing field in gold nanorod dimers towards the photothermal apoptosis in malignant cells in a mice model from both theoretical and experimental perspectives. The gold nanorod dimers with parallel and perpendicular orientations offer the pragmatic attribution of the fringing field to probe the photothermal generation of heat at the nanoscale. Aqueous dispersion of gold nanorods has been synthesized through a seeded growth approach; the dimerization amongst the pair of nanostructures at two specific angular orientations has been induced upon the addition of molecular linkers. The kinetics of the modification of optical characteristics upon laser-induced melting of the gold nanorod dimers has been investigated. The plasmonic characteristics as a consequence of electromagnetic interaction amongst the neighbouring nanorods have been elucidated through the discrete dipole approximation technique and finite element method. The salient feature of biomedical importance is that the fringing field gold nanorod dimers are effective in inducing apoptosis in cancerous cells. Rational design of dimeric nanostructures can render plausible optothermal manipulation in a mouse model to imbue a landscape plausible in the realm of biomedical engineering inside the living cells.
Materials and methods
Reagents and instruments
All the reagents used were of analytical reagent grade. Hydrogen tetrachloroaurate (HAuCl4.3H2O), cetyltrimethylammonium bromide (CTAB), silver nitrate (AgNO3), L-ascorbic acid, sodium borohydride (NaBH4), 1,8-diaminooctane (C8H20N2), phloroglucinol (C6H3(OH)3), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), histopaque-1077, Dulbecco’s phosphate buffered saline (DPBS), RPMI (Roswell Park Memorial Institute) 1640 medium, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) and fatal bovine serum (FBS) were purchased from Sigma-Aldrich and were used as received. Annexin V-FITC conjugates (green) were purchased from Invitrogen and were used without further purification. Male Swiss Albino LACA (Laboratory Animal Centre A-strain) mice of 6–7 weeks old having a body weight of 20 ± 2 g were purchased to perform animal research from Pasteur Institute, Shillong, India (License No.: 34/DR/1966). The mice were accommodated in polycarbonate cages at 22 ± 2 °C temperature, 85% relative humidity and 12 h light–dark cycles with standard rodent food and water ad libitum. All experimental protocols were followed as approved by the Institutional Ethical Committee (IEC/AUS/2013-019 dated March 20, 2013) of the Assam University, Silchar. All methods were carried out in accordance with relevant guidelines and regulations and were reported in accordance with ARRIVE guidelines for the reporting of animal experiments. HPLC water was used throughout the course of this investigation.
Absorption spectra were measured in a PerkinElmer Lambda 750 UV–vis–NIR spectrophotometer by taking the sample in 1 cm quartz cuvette. Transmission electron microscopy (TEM) was performed on a JEOL JEM-2100 microscope with a magnification of 200 kV. Samples were prepared by placing a drop of solution on a carbon-coated copper grid and dried overnight under a vacuum. Laser heating was carried out with a continuous wave near-infrared diode laser (Model: ML-III-785-1 W, Changchun New Industries Optoelectronics Technology Co. Ltd., China) of wavelength 785 nm and output power 1 Watt by taking the sample in 1 cm quartz cuvette. Dark-field imaging was carried out using Olympus GX 51F inverted optical microscope by illuminating the sample with a halogen light source (U-LH100-3). The infrared images were recorded on Fluke Connect PTi120 pocket thermal camera and the captured images were analysed by Fluke Connect software. Fluorescence microscopic images of the cells stained with DAPI (excitation: 350 nm, detection: 470 nm) were recorded in a Nikon Eclipse TS100 fluorescence microscope and analysed using ImageJ software packages. Different simulation techniques, viz., finite element method (FEM) has been performed using COMSOL Multiphysics software package41 and the scattering coefficients have been calculated using discrete dipole approximation scattering (DDSCAT) code42. The optical constants of bulk gold provided by Johnson and Christy have been used in all the calculations43. A hand-written Python code has been used to compute equilibrium temperature profiles of the nanostructures44.
Synthesis of gold nanorods
Gold nanorods were prepared by following the recipe of the Murphy group22. The process comprises two steps:
-
Preparation of gold seeds For the preparation of monodispersed seed, 2.5 mL Au(III) solution (0.25 mM) was added to the 2.5 mL CTAB solution (0.2 M). A dropwise addition of 60 µL NaBH4 (0.1 M) to the solution causes a rapid reduction of Au(III) in the stirring condition. A clear change of colour from yellow to pale brown indicates the formation of gold seeds. The colloidal dispersion was incubated for 3–4 h before the synthesis of the nanorods.
-
Preparation of gold nanorods For the preparation of the growth solution, 0.5 mL of Au(III) solution (10 mM) was added to the 5 mL solution of CTAB solution (0.2 M), the colour of the solution was dark yellow. A mild reducing agent, 80 μL L-ascorbic acid (0.1 M) was added dropwise for the reduction of the gold solution and the yellow colour became colourless. Then, 120 μL of AgNO3 solution (10 mM) was added followed by the addition of 12 µL of seed dispersion. The total volume of the solution was maintained at 10 mL. The colour of the dispersion became blue indicating the formation of gold nanorods. The solution was aged for 24 h to settle for further application.
Formation of parallel and perpendicular aggregation of gold nanorods dimers
The assemblies to parallel and perpendicular orientations of the gold nanorod dimers have been induced by following the protocol of our group reported earlier23. An aliquot of 2.0 mL of aqueous dispersion of the gold nanorods was purified by repeated centrifugation and dispersion and subsequently, dispersed to 1.0 mL ethanol by sonication. Then, aggregation was induced through the dropwise addition of 1.0 mL ethanolic dispersion of gold nanorods to 1.0 mL (0.4 mL ethanol + 0.6 mL THF) of 1,8-diamino octane (0.1 mM) or 1.0 mL (0.4 mL ethanol + 0.6 mL THF) of phloroglucinol (0.1 mM) leading to the formation of parallel or perpendicular dimers, respectively. The colour of the dispersion changed to dark blue for both the dimers. The dimeric assemblies of the gold nanorods were found to be stable, at least, for a couple of days.
Isolation of splenic macrophages
The isolation of macrophages from the kidney has been carried out by following the procedure demonstrated by Sengupta group40. The kidneys were isolated from the LACA mice followed by instant suspension in ice-cold Alsever’s solution and subsequent saturation using frosted glass slides. A single-cell suspension was achieved by aspiration through the sterilized Pasteur pipette. The suspension stored in sterile tubes was put in ice and the cell debris was allowed to equilibrate. The histopaque-1077 (3.0 mL) was used to extract the supernatant layer followed by centrifugation at 1500 rpm continued for 15 min and the entire process was performed thrice. The interface enriched with leukocyte was collected, an ice-cold DPBS solution was utilized for washing and the process was carried out twice. The resuspension of the cell pellet was performed in RPMI-1640 as the cell culture medium bearing 20 mM HEPES (pH ~ 7.2) and 5% FBS in the suspension. The whole solution was allowed to adhere to the culture plate for 1 h at 37 °C in a 5% CO2 incubator. The macrophages were adhered and collected by repeated aspiration with a sterilized Pasteur pipette and the non-adhered macrophages were removed by repetitive washing. The complete cell suspension was, again, washed off and finally, resuspension was pursued in the media (RPMI + FBS) at a density of 106 cells/mL and then, stored in ice. A batch of greater than 95% cells was observed to be viable as confirmed by trypan blue staining.
Internalization of gold nanorod dimers in LACA cells
The collected kidney tissues (500 mg) were treated with both types of nanorod assemblies at the dose of 4 mg/kg b. w. assuming all the monomeric nanorods have been converted to dimers. The assembled nanostructures were observed to be, adequately, dispersed at the particular concentrations for, at least, 72 h that was sufficient for performing the cell culture experiments.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ades, F., Tryfonidis, K. & Zardavas, D. The past and future of breast cancer treatment−from the papyrus to individualised treatment approaches. ecancer 11(746), 1–11 (2017).
Baffou, G., Cichos, F. & Quidant, R. Applications and challenges of thermoplasmonics. Nat. Mater. 19, 946–958 (2020).
Cui, X. et al. Photothermal nanomaterials: A powerful light-to-heat converter. Chem. Rev. 123, 6891–6952 (2023).
Sun, B., Teo, J., Wu, J. & Zhang, Y. Light conversion nanomaterials for wireless phototherapy. Acc. Chem. Res. 56, 1143–1155 (2023).
Pitruzzello, G. Photothermal attogram spectroscopy. Nat. Photonics 17, 837–837 (2023).
Zheng, J. et al. Gold nanorods: The most versatile plasmonic nanoparticles. Chem. Rev. 121, 13342–13453 (2021).
Ghosh, S. K. & Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 107, 4797–4862 (2007).
Pramod, P. & George Thomas, K. Plasmon coupling in dimers of Au nanorods. Adv. Mater. 20, 4300–4305 (2008).
Kumar, A. & Ganguli, S. Theoretical foundation of a scheme for the study of dielectrics by analyzing the distortion in fringing field. Rev. Sci. Instrum. 91(095101), 1–6 (2020).
Jauffred, L., Samadi, A., Klingberg, H., Bendix, P. M. & Oddershede, L. B. Plasmonic heating of nanostructures. Chem. Rev. 119, 8087–8130 (2019).
Pal, S. K., Bardhan, D., Sen, D., Chatterjee, H. & Ghosh, S. K. Angle-resolved plasmonic photocapacitance of gold nanorod dimers. Nanoscale Adv. 5, 1943–1955 (2023).
Lu, X., Punj, D. & Orrit, M. Two-photon-excited single-molecule fluorescence enhanced by gold nanorod dimers. Nano Lett. 22, 4215–4222 (2022).
Fontana, J. et al. Rise of the charge transfer plasmon: Programmable concatenation of conductively linked gold nanorod dimers. ACS Photonics 3, 904–911 (2016).
Huang, C.-P., Yin, X.-G., Kong, L.-B. & Zhu, Y.-Y. Interactions of nanorod particles in the strong coupling regime. J. Phys. Chem. C 114, 21123–21131 (2010).
Zhong, L. et al. Rational design and SERS properties of side-by-side, end-to-end and end-to-side assemblies of Au nanorods. J. Mater. Chem. 21, 14448–14455 (2011).
Kawawaki, T., Zhang, H., Nishi, H., Mulvaney, P. & Tatsuma, T. Potential-scanning localized plasmon sensing with single and coupled gold nanorods. J. Phys. Chem. Lett. 8, 3637–3641 (2017).
Beuwer, M. A. & Zijlstra, P. Correlative microscopy of single self-assembled nanorod dimers for refractometric sensing. J. Chem. Phys. 155(044701), 1–9 (2021).
Huang, J.-S. et al. Mode imaging and selection in strongly coupled nanoantennas. Nano Lett. 10, 2105–2110 (2010).
Zijlstra, P., Chon, J. W. M. & Gu, M. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature 459, 410–413 (2009).
Wang, L. et al. Side-by-side and end-to-end gold nanorod assemblies for environmental toxin sensing. Angew. Chem. Int. Ed. 49, 5472–5475 (2010).
Halas, N. J., Lal, S., Chang, W.-S., Link, S. & Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 111, 3913–3961 (2011).
Jana, N. R., Gearheart, L. & Murphy, C. J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 105, 4065–4067 (2001).
Umar, A. & Choi, S.-M. Aggregation behavior of oppositely charged gold nanorods in aqueous solution. J. Phys. Chem. C 117, 11738–11743 (2013).
Liu, J. et al. End-to-end and side-by-side assemblies of gold nanorods induced by dithiol poly(ethylene glycol). Appl. Phys. Lett. 104(253105), 1–3 (2014).
Verwey, E. J. W. & Overbeek, J. T. G. Theory of the Stability of Lyophobic Colloids (Elsevier, 2006).
Gans, R. Über die form ultramikroskopischer goldteilchen. Ann. Phys. 342, 881–900 (1912).
Tabor, C., van Haute, D. & El-Sayed, M. A. Effect of orientation on plasmonic coupling between gold nanorods. ACS Nano 3, 3670–3678 (2009).
Biring, S., Wang, H.-H., Wang, J.-K. & Wang, Y.-L. Light scattering from 2D arrays of monodispersed Ag-nanoparticles separated by tunable nano-gaps: Spectral evolution and analytical analysis of plasmonic coupling. Opt. Exp. 16(15312), 1–9 (2008).
Biring, S. Tuning of particle plasmon resonances in binary dielectric medium. Phys. Lett. A 376, 125–127 (2011).
Funston, A. M., Novo, C., Davis, T. J. & Mulvaney, P. Plasmon coupling of gold nanorods at short distances and in different geometries. Nano Lett. 9, 1651–1658 (2009).
Nordlander, P., Oubre, C., Prodan, E., Li, K. & Stockman, M. I. Plasmon hybridization in nanoparticle dimers. Nano Lett. 4, 899–903 (2004).
Slaughter, L. S., Wu, Y., Willingham, B. A., Nordlander, P. & Link, S. Effects of symmetry breaking and conductive contact on the plasmon coupling in gold nanorod dimers. ACS Nano 4, 4657–4666 (2010).
Searson, P. C. The cancer moonshot, the role of in vitro models, model accuracy, and the need for validation. Nat. Nanotechnol. 18, 1–3 (2023).
Kollipara, S. P., Chen, Z. & Zheng, Y. Optical manipulation heats up: Present and future of optothermal manipulation. ACS Nano 17, 7051–7063 (2023).
Poisson, S. D. Probabilité des jugements en matière criminelle et en matière civile, précédées des règles générales du calcul des probabilités 1781–1840 (Bachelier, 1837).
Debnath, D. & Ghosh, S. K. Optical constants of noble metals at the nanoscale within the framework of the Drude free-electron conduction model: Implications for liquid crystal sensing. ACS Appl. Nano Mater. 5, 1621–1634 (2022).
Gu, X., Timchenko, V., Yeoh, G. H., Dombrovsky, L. & Taylor, R. The effect of gold nanorods clustering on near-infrared radiation absorption. Appl. Sci. 8(1132), 1–16 (2018).
Li, X. et al. In vitro and in vivo photothermal cancer therapeutic effect of mushroom-glucan modified gold nanorods. J. Agric. Food Chem. 66, 4091–4098 (2018).
Huang, X., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
Chakraborty, B. et al. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 13, 191–205 (2016).
Yee, K. Numerical solution of initial boundary value problems involving Maxwell’s equations in isotropic media. IEEE Trans. Antennas Propag. 14, 302–307 (1966).
Draine, B. T. & Flatau, P. J. Discrete-dipole approximation for scattering calculations. J. Opt. Soc. Am. A 11, 1491–1499 (1994).
Johnson, P. B. & Christy, R. W. Optical constants of the noble metals. Phys. Rev. B 6, 4370–4379 (1972).
Acknowledgements
We gratefully acknowledge financial support from DST-SERB, New Delhi (Project No.: EMR/2016/006842).
Author information
Authors and Affiliations
Contributions
S.K.G. contributed to the conception, design and drafting of the manuscript along with critical review. D.B. and D.S. performed the experiments, carried out numerical simulation and prepared the initial draft. D.B. and N.M. carried out the biological experiment and data analysis. M.S. contributed to the conception and design of the biological part of the MS. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bardhan, D., Maity, N., Sen, D. et al. Photothermal manipulation of the fringing field in gold nanorod dimers towards the apoptosis of cancerous cells. Sci Rep 14, 21292 (2024). https://doi.org/10.1038/s41598-024-62898-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62898-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.