Abstract
Herein, the adsorption of methyl orange (MO), a dangerous anionic dye, from an aqueous solution was investigated using a novel magnetic nanocomposite adsorbent. A nanocomposite entitled manganese chromium-layered double oxide/cobalt spinel ferrite, (MnCr)-LDO5wt.%/CoFe2O4, which links the interlayer structural characteristics of layered double oxides (LDOs) with the magnetic properties of spinel ferrites (SFs) was synthesized using the eco-friendly co-precipitation technique. Determination of structural parameters, crystallite size, and micro-strain was done using X-ray diffraction (XRD) analysis. Transmission electron microscopy (TEM) was used to determine grain shape and size. Surface analysis was performed using X-ray photoelectron spectroscopy (XPS) to identify elements and oxidation states present in the prepared nanocomposite. Vibrating sample magnetometer (VSM) was utilized to examine the magnetic characteristic. A comprehensive comparative study about the effectiveness and durability of CoFe2O4 and (MnCr)5wt.%/CoFe2O4 as nanoadsorbents for MO was conducted. Numerous variables, including contact time, MO concentration, adsorbent dosage, and pH were tested for their effects on the adsorption removal percentages. The findings showed that the maximum removal percentage was 86.1% for 25 ppm of MO was for 0.1 g/100 mL of (MnCr)-LDO5wt.%/CoFe2O4 at pH = 3. Investigations of isotherms and kinetics were conducted under batch conditions. The Langmuir isotherm matched the experimental data, for both nanoadsorbents, quite well due to the homogeneous distribution of active sites. Adsorption kinetics data were found to be compatible with intra-particle diffusion and pseudo-second order models for CoFe2O4 and (MnCr)5wt.%/CoFe2O4, respectively. A total of five adsorption–desorption cycles were performed to determine the prepared adsorbents’ recyclable nature.
Similar content being viewed by others
Introduction
One of the most basic necessities of all living organisms on earth is access to water. As the world’s population grows and the quality of water supplies declines, water is becoming more and more scarce1. In addition, various industrial and municipal pollutants are thrown directly into rivers, oceans, and other water sources, harming water resources. Industrial effluents comprise a wide range of poisonous, mutagenic, cancer-causing, and non-recyclable compounds that harm ecosystem health. In addition to polluting water, coloured effluents from dyeing factories also hinder photosynthesis2. It is crucial that these contaminants are removed from the water in order to alleviate the rising water issue in the world. One popular and well-known azo anionic dye is methyl orange (MO). The molecules of this water-soluble organic synthetic dye include aromatic and –N=N– groups, which are very poisonous, carcinogenic, and teratogenic3,4, as well as being hazardous to the environment and creatures5,6. It also has an intense orange colour when dissolved in water. MO is employed as a simulated contaminant in the present study. Some of the processes used to remove water impurities include coagulation, flocculation, membrane filtration, reverse osmosis, ozonolysis, electrostatic precipitation, and adsorption1,2,3,4,5,6,7. Because of its ease of use and low cost, adsorption is the most widely used technology among the ones mentioned.
Lately, magnetic nanoadsorbent composites are developing as highly effective functional materials with superior micropollutant adsorption and rapid adsorption kinetics8,9. In addition to their low-cost synthesis, high surface area, high porosity, high degree of selectivity, binding specificity, and superb reuse ability8,9,10,11, magnetic nanoadsorbent composites have the ability to be separated immediately from adsorption-remediated waters in the form of magnetic nanoadsorbent-adsorbate sediment by the application of a powerful magnetic field. Layered double oxide/spinel ferrite (LDO/SF) composites are promising candidates as effective magnetic nanoadsorbents. The multilayer structure of LDO and the magnetic nature of SF have been identified as potential properties for a wide range of environmental and industrial applications. As a result, the fabrication of LDO/SF composites is critical, particularly for the adsorption of many pollutants from water, which will be blocked among the layered structure of LDO, and the presence of spinel ferrite will facilitate their easy separation from aqueous solution using only a piece of magnet8.
Layered double hydroxides (LDHs) are promising candidates for anionic dye removal due to their surface complexion and electrostatic interactions11,12. They are known as hydrotalcite-like compounds that are represented by the formula [M2+(1−z) M3+z (OH)2]z+(An−)z/n·mH2O, where M2+, M3+ are divalent and trivalent metal cations that result in the indefinite repetition of positively charged sheets (lamellas) alternating with An− ions (i.e. CO32−, OH−, Cl−, etc.), which are required to balance the net positive charges of the hydroxide layers. The molar ratio z = M3+/(M3+ + M2+) frequently falls between 0.2 and 0.410. Moreover, LDH can be transformed into LDO by calcination at 600 °C, and LDO can be transformed back into LDH after being dissolved in water. This mechanism, known as the memory effect, can increase surface area while decreasing adsorption effectiveness13. Layered double oxides (LDOs) have been widely used as anion exchangers, adsorbents, and catalysts14,15.
Spinel ferrites are compounds with the general formula MFe2O4, where M is a divalent metal ion such as Ni2+, Co2+, Mn2+, Zn2+, or another metal ion. Cobalt ferrite (CoFe2O4) is discovered to be more adaptable due to its ferromagnetic feature as well as its high electrochemical stability, making it a significant spinel material. CoFe2O4 has an inverted spinel structure, which means that ferric ions are present at tetrahedral sites while ferric and cobalt ions are present at octahedral sites. CoFe2O4 was selected for this investigation because of its high magnetic permeability, low magnetic loss, high cut-off frequency, high saturation magnetization, high curve temperature, temperature stability, low coercivity, and biodegradability16,17. Several composites comprised of ferities and LDH/LDO have been conducted17,18,19,20, including CoFe2O4/CuAl-LDH, NiFe2O4/ZnCuCr-LDH, Fe3O4/Zn–Al–Fe–La-LDH, Fe3O4/ZnCr-LDH and CoFe2O4/MgAl-LDO21. However, as far as we are aware, no other work has utilized (MnCr)-LDO5wt.%/CoFe2O4 nanocomposites as an affordable adsorbent and active photocatalyst for MO dye removal from aqueous solution.
In this study, a novel magnetic nanocomposite entitled (MnCr)-LDO5wt.%/CoFe2O4 was fabricated through the facile co-precipitation route, and its removal efficiency and photocatalytic activity for methyl orange dye were examined and compared to that of pure CoFe2O4. Numerous variables, including; the contact time, the dye concentration, the nanoadsorbent dosage, and pH, were tested for their effects on the adsorption rate. Investigations of equilibrium and kinetics were conducted under batch circumstances.
Experimental technique
Synthesis
Materials
High purity reagents of iron chloride hexahydrate (FeCl3.6H2O, ≥ 98%, Oxford), cobalt chloride hexahydrate (CoCl2·6H2O, ≥ 97%, Loba Chemie), manganese chloride tetrahydrate (MnCl2·4H2O, ≥ 99%, Qualikems), chromium chloride hexahydrate hydrate (CrCl3·6H2O, 99.9%, Oxford), sodium bicarbonate (Na2CO3, ≥ 99% Loba Chemie), sodium hydroxide (NaOH, ≥ 98%, Loba Chemie) and methyl orange (C14H14N3NaO3S) were utilized. Throughout the synthesis, distilled water was employed as the dispersion solvent.
Preparation of MnCr-LDH and MnCr-LDO
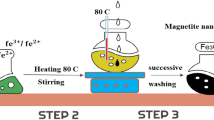
By using the low super saturation co-precipitation technique, two aqueous solutions were prepared and double titrated to 10 mL of distilled water while being stirred at 500 rpm. The first solution included 50 mL of distilled water along with 4.4527 g of MnCl2·4H2O and 1.9983 g of CrCl3·6H2O. The second solution was for 50 mL of distilled water, 3.3747 g of NaOH, and 2.9809 g of Na2CO3. After almost three hours, the titration was terminated, and the pH had reached 10. The mixture was then heated to 80 °C for 4 h. The temperature is turned off, and the mixture is aged overnight at room temperature. The solution is then centrifuged at 1000 rpm for 30 min, and the resulting precipitate is washed several times with distillated water until the pH is neutral. The precipitate was dried at 50 °C for 48 h, and finally a calcination process was performed at 600 °C for 3 h to convert the LDH to LDO.
Preparation of CoFe 2 O 4
CoFe2O4 was synthesized using a simple and environmentally friendly co-precipitation method. Separately, 25 mL of distilled water was used to dissolve 13.515 g of FeCl3·6H2O and 5.9482 g of CoCl2·6H2O. The two solutions were mixed and stirred until perfect homogeneity was achieved. The chlorides mixture solution was titrated with 3 M NaOH in 75 mL of distilled water at a very slow rate until the pH reached 13. The mixture is then heated to 80 °C and stirred at 500 rpm for 3 h. A piece of magnet was used to collect the dark brawn precipitate, which was then washed numerous times with distillated water until the pH reached 7. The precipitate was dried at 50 °C overnight before being ground and calcined at 700 °C for 3 h. This method yielded a 6 g CoFe2O4 sample.
Preparation of (MnCr)-LDO x /CoFe 2 O 4
To prepare a composite of type (MnCr)-LDOx/CoFe2O4, 100 mL beaker filled with 25 mL distilled water was used to dissolve the required amount of the prepared MnCr-LDO with a percentage x = 5 wt.% from CoFe2O4 total weight (6 g), then sonicated for 16 min. The iron and cobalt chloride precursor’s solutions were prepared and stirred at 300 rpm. The MnCr-LDO solution was mixed with the chloride precursor’s solution. Droplets of 3 M NaOH solution were used to make the pH = 13. The mixture was continuously stirred at 500 rpm and heated at 80 °C for 3 h. The precipitate was magnetically separated and washed several times with distilled water to reach neutrality. Drying the precipitate at 50 °C overnight was conducted. After that, the powder was ground and calcined at 700 °C for 3 h. A schematic representation for (MnCr)-LDO5wt.%/CoFe2O4 preparation steps is shown in Fig. 1.
Characterization
Structural characterization was achieved using the X-ray powder diffraction (XRD) technique with Shimadzu LabX 6100 Japan CuKα radiation (λ = 1.54178 Å). The transmission electron microscope (TEM) model JEOL JEM 1400 Plus was used to observe particle size and sample morphology. The XPS spectra were recorded using the Thermoscientific K-Alpha X-ray photoelectron spectrometer system, with the pass energy constant mode of energy analyzer at 200 eV (full survey XPS spectra) and 50 eV (high-resolution HR-XPS spectra). Al-kα radiation energy source was used under a pressure of 10−9 mbar. The vibrational modes were obtained by the FTIR using Bruker Vertex70 Germany (KBr technique-400:4000 wavenumbers). A vibrating sample magnetometer (VSM) model Lake Shore 7410 with a maximum applied magnetic field of 20 kOe was used to conduct the magnetic measurements. Nitrogen adsorption–desorption measurements at 77 K were performed by BELSORB III analyzer, Japan, to determine Brunauer–Emmett–Teller (BET) specific surface area and Barrett-Joyner-Halenda (BJH) average pore diameter. Prior to analysis, samples of about 0.3 g were degassed at 200 °C for 3 h.
Adsorption test
A standard MO stock solution of 25 ppm was prepared and diluted with distilled water in order to obtain different known initial concentrations, i.e., 5, 10, 15, 20, and 25 ppm. The relative absorbance of these MO concentrations was determined from a UV–Vis spectrophometer (Systronics UV–Vis spectrophometer-117) at a wavelength of 460 nm. Figure 2 depicts the variation of absorbance (A) with MO concentrations. The absorbance is shown to be linearly related to the concentration of MO. Straight line fitting was performed for the experimental data with a correlation factor of R2 = 0.99. For equilibrium and kinetic studies, this curve was utilized as a calibration curve to convert absorbance data into concentrations.
The MO adsorption experiments were conducted in batch conditions using two nanoadsorbents termed CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4. Several parameters have been examined to show their effect on MO adsorption. These parameters are the contact time, the dye concentration, the nanoadsorbent dosage, and finally the pH. For the effect of contact time; 25 ppm of MO solutions were kept in 100 mL flasks, and 0.1 g/100 mL of each nanoadsorbent was added to them. The flasks were placed on a shaker with a speed of 150 rpm, and the nanoadsorbents were magnetically separated from the solutions at different contact times (t = 10–150 min). The supernatants were analyzed by UV–Vis spectrophometer, at a wavelength of 460 nm, after each contact time. To study the effect of dye concentration, the dye concentration was varied from 5 to 25 ppm while the nanoadsorbent dosage was fixed at 0.1 g/100 mL, and the contact time was maintained at the equilibrium time of each nanoadsorbent (90 and 120 min for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4, respectively). The effect of nanoadsorbent dosage was studied by varying the dosage of CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 from 0.1 g/100 mL to 0.5 g/100 mL, and the rest of the parameters were fixed at 25 ppm MO concentration, and the collection times were their equilibrium times. The effect of pH was conducted with 25 ppm of MO solutions which were agitated with 0.1 g/100 mL of both CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 at different pH values (3–8) at 25 °C. The dye removal percentage (R %)22, the kinetic adsorption capacity of MO dye at equilibrium time, qe (mg L−1), and the kinetic adsorption capacity of MO dye at time t, qt (mg L−1), were estimated from the following relations23:
where the definition of each parameter in Eqs. (1–3) were tabulated in Table 1. The equilibrium adsorptions of MO dye from aqueous solutions were investigated using Langmuir, Freundlich, and Temkin models for isotherms. Furthermore, the kinetics of MO dye adsorption was examined using Lagergren's pseudo-first order, pseudo-second order, Elovich, and intra-particle diffusion.
Desorption and reusability
At room temperature, 0.1 g/100 mL each of CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 were added to 100 mL conical flasks with 25 ppm of MO solutions and shaken in an orbital incubator shaker for 90 and 120 min, respectively. The final dye concentration was determined by magnetically separating the mixture. The magnetic nanoadsorbent was recycled by being washed several times with 200 mL distilled water while being stirred constantly for 15 min. After that, a reused magnetic nanoadsorbent was added to a MO solution to initiate adsorption. A total of five adsorption–desorption cycles were performed to determine the material's recyclable nature.
Photocatalytic test
The degradation of MO solution under UV light irradiation was used to measure the photocatalytic activity of the prepared samples. The reaction mixture was agitated in the dark for 1 h in this experiment to guarantee that MO was adsorbed to saturation on the catalysts. The solutions were then exposed to UV light using a 6 W–365 nm lamp. A 0.1 g photocatalyst was added to a 50 mL MO (5 ppm) solution with a solid/water ratio of 1 g/L. At each irradiation time interval, 5 mL aliquots were extracted and magnetically separated to remove almost all of the catalysts. During the photo-degradation process, the quantities of the leftover dye were detected using UV–Vis spectrophometer by measuring the absorbance of solutions at 460 nm. The identical process was used to carry out the blank reaction, but without inserting the catalyst.
Results and discussions
Structural analysis
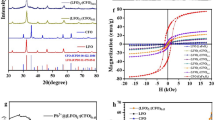
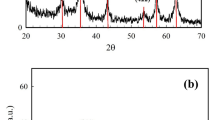
The XRD patterns for the MnCr-LDH, MnCr-LDO, CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 samples are shown in Fig. 3a–d, respectively. It is clear that the typical peaks for the hydrotalcite-like crystal structures (JCPDS: 38-0487)24 are present in the synthesized MnCr-LDH, as shown in Fig. 3a. These diffraction peaks were well indexed by the hexagonal structure and R3̅m rhombohedral symmetry25. Specifically, eight peaks occurred at 2θ of 11.97°, 23.65°, 32.82°,33.04°, 38.17°, 45.28°, 55.13 and 65.70°, correspond to (003), (006), (009), (012), (015), (018), (110), and (113) diffraction planes, which are signatures for hydrotalcite-like structures. The basal peaks at (003), (006) and (009) are often connected with the lamellar stacked layers, the (110) correlates with the organizational structure inside the lamellae, and the (012), (015) and (018) are related to the inner layers26. Figure 3b is for MnCr-LDO after heating MnCr-LDH at 600 °C for 3 h. The MnCr-LDO is still preserving the hydrotalcite-like crystal structure. The peaks become sharper especially for the basal (003) plane and this reflects the good crystallinity the sample has acquired after calcination. The lattice parameters a and c of MnCr-LDO were derived from (110) and (009) reflections, respectively, (tabulated in Table 2) according to the hexagonal unit cell symmetry (\(a\, = \,b\, \ne \,c; \, \alpha \, = \,\beta \, = \,90^\circ ,\;\gamma \, = \,120^\circ\)) using the following equation27:
where dhkl is the interplannar spacing. The estimated lattice parameter a = 2.8827 Å refers to the distance between the cations inside the structure's layers, whilst the determined value of c = 24.5331 Å is connected to layer thickness and interlayer spacing26. The lattice parameters values are very close to those estimated by Teixeira et al.26 for MgAl-LDO. The average crystallite size D of MnCr-LDO has been calculated using Debye–Scherrer equation28.
where α is the Scherrer constant (0.9), λ is the X-ray wavelength (1.5406 Å), β is full width of half maximum (FWHM) and θ is the Bragg’s angle. The micro strain \(\varepsilon\) was calculated using the formula suggested in29 as follows:
The values of D and \(\varepsilon\) are listed in Table 2. The estimated crystallite size (D = 27.2 nm) was larger than that reported for MgAl-LDO by Teixeira et al.26 (24.7 nm). Larger crystallite sizes result in narrower peaks, which are associated with more crystallinty, and this appears to be the scenario here. Furthermore, the estimated crystallite size is quite close to that determined for CoCr-LDO by Balayeva et al.30 using 0.6 M NaOH. The micro strain \(\varepsilon\) value for synthesized MnCr-LDO is found to be 1.09 × 10−3.
The XRD pattern of the synthesized CoFe2O4 (Fig. 3c) was analyzed and compared to standard sheet number JCPDS: 22-108631. Bragg reflections at 2θ of 29.93°, 35.31°, 36.95°, 42.93°, 53.33°, 56.85° and 62.52° correspond to (220), (311), (222), (400), (422), (511), and (440) planes, respectively. This validates the development of ferrite with the FCC structure and the fd3̅m space group. Furthermore, the lack of reflections at 2θ = 33°, 49° and 66° revealed that the sample is single phase and devoid of the impurity phase of α-Fe2O3. Figure 3d displays the XRD pattern of (MnCr)-LDO5wt.%/CoFe2O4 nanocomposite. The resultant nanocomposite inherited high intensity peaks related to CoFe2O4 and low intensity peaks accounted for MnCr-LDO indicating their successful incorporation. The lattice parameters of CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 were calculated according to the FCC unit cell based on the following equation31
The average crystallite sizes and micro strains for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 were calculated in the same manner discussed for MnCr-LDO according to Eqs. (5) and (6), and their values were listed in Table 2. It is obvious, from Table 2, that the addition of MnCr-LDO to CoFe2O4 has created a nanocomposite with enhanced lattice parameters, crystallite size, and reduced micro strain. The increase in lattice parameter and the reduction in micro strain could be attributed to the agglomeration of MnCr-LDO at the grain boundaries, which results in improved crystallite size that reduces the defects and overall line broadening during synthesis32. A similar lattice constant value was also observed by Reddy et al.33 and Monisha et al.31. For CoFe2O4; Monisha et al.31 have found a crystallite size of 16.7 nm, while Manouchehri et al.34 have reported an average crystallite size of 13 nm for CoFe2O4 nanoparticles coated with dimercaptosuccinic acid. Naseri et al.35 reported the crystallite size to be 20 nm for MnFe2O4 nanoparticles. Moreover, Kombaiah et al.36 have reported higher values of 56 nm and 43 nm for CoFe2O4 prepared by conventional and microwave heating method, respectively.
TEM and BET analysis
Figure 4a–c depicts TEM micrographs at scale of 100 nm together with the size distribution histograms of MnCr-LDO, CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 nanoparticles, respectively. The particle sizes and their distribution were made by “Image J” and Origin Lab software. The average particle sizes, listed in Table 2, were found to be 32 nm, 15.5 nm and 19 nm for MnCr-LDO, CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 nanoparticles, respectively. These values agree well with the average crystallite sizes estimated from XRD data. It is clear, from Fig. 4a, that MnCr-LDO demonstrated particle morphologies of irregular hexagonal particles. Moreover, a cubic morphologies appeared for CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 nanoparticles as shown in Fig. 4b,c. Similar cubic particles with size of 25 nm were observed for CoFe2O4 by Kumar et al.37.
Figure 5a,b show the obtained BET isotherm and BJH pore size distribution (insets) for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 samples, respectively. The surface textural parameters of N2 adsorption–desorption analysis were listed in Table 2. The CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 had BET-specific surface areas (SBET) of 27 m2/g and 30 m2/g, respectively. The (MnCr)-LDO5wt.%/CoFe2O4 composite sample had a BJH adsorption average pore diameter (dP) of 33 nm and a pore volume of 0.2264 cm3/g, which were greater than the pure CoFe2O4 sample, which had a dP of 26 nm with a pore volume of 0.1654 cm3/g. Similar value of pore diameter was observed by Deng et al.21 for CoFe2O4/MgAl-LDO. As a result, the (MnCr)-LDO5wt.%/CoFe2O4 composite is more effective nanoadsorbent for MO adsorption rather than pure CoFe2O4.
FTIR and VSM analysis
Figure 6a shows the FTIR spectra of MnCr-LDO, CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 samples. The composite has an essentially similar FTIR spectrum to that of MnCr-LDO and CoFe2O4. A broad band appeared in the wavenumber range of 3558–3421 cm−1. This broad band was ascribed to OH− groups38.The intensity of this band was reduced for the composite sample, as shown in Fig. 5a. Peaks at 1637 cm−1 were attributed to H2O molecule vibrations. This peak decreased for the composite sample. This reduction is mainly connected with the heat treatment performed on the composite sample which can result in water evaporation. The peak at 1388 cm−1 showed the vibration mode of CO32−39. The lower band frequencies between 1000 and 400 cm−1 correspond to the lattice vibration of metal–oxygen bonding to the layers of the LDO and CoFe2O438. The vibration modes of the carbonate anions were also illustrated at 962 cm−1 peak38. The presence of such vibrations reveals the intercalation of carbonate ions into the interlayer space of MnCr-LDO. Figure 6b shows the VSM curves for the prepared samples. It is clearly seen, from the inset of Fig. 6b, that MnCr-LDO has dominated paramagnetic behavior coupled with weak ferromagnetic behavior that has been seen from the tiny hysteresis loop appeared in the low field region. On the other hand, the strong ferromagnetic nature of the pure CoFe2O4 and the (MnCr)-LDO5wt.%/CoFe2O4 samples were confirmed by the large hysteresis loops which are recorded as fingerprint for hard ferrites materials.
XPS analysis
X-ray photoelectron spectroscopy (XPS) studies were carried out to examine the surface chemical composition and oxidation state of the produced (MnCr)-LDO5wt.%/CoFe2O4 nanocomposite. The wide range survey XPS spectrum (Fig. 7a) confirmed the elemental existence of Mn, Cr, Co, Fe, O and C in the prepared (MnCr)-LDO5wt.%/CoFe2O4 nanocomposite. Two spin–orbit doublets were used to match the Mn 2p core-level spectra (Fig. 7b). The Mn2+ 2p3/2 and Mn2+ 2p1/2 peaks are situated at 641.08 eV and 651.08 eV, respectively, with their satellites at 643.38 eV and 653.28 eV23,24. Figure 7c shows the Cr 2p spectrum. The Cr3+ 2p1/2 and Cr3+ 2p3/2 have two wide peaks at 587.68 eV and 576 eV, respectively3. The binding energies of Cr 2p at 578.98 eV and 576.58 eV can be assigned to the Cr–OH and Cr–O, respectively25. The XPS spectrum of Co 2p (Fig. 7d) depicted two main peaks at ∼ 795.38 and ∼ 780.08 eV, which correspond to Co2+ 2p1/2and Co2+ 2p3/2, respectively30. Co 2p satellites appeared at 784.78, 789.28, 801.28 and 803.98 eV. Moreover, the spectrum of Fe3+ 2p (Fig. 7e) exhibits strong peaks located at around 724.58 and 7011.08 eV, which are assigned to Fe3+ 2p1/2 and Fe3+ 2p3/2, respectively, indicating a 3+ valency of Fe ions [31–34]. In the O 1s spectrum (Fig. 7f), the binding energy peaks are located at 529.48 and 531.38, corresponding to the characteristic bands of oxygen in the M–O lattice, surface hydroxyl group and bound water, respectively25. The C 1s spectrum (Fig. 7g) can be deconvoluted into three main peaks with the highest binding energy at 287.68 eV corresponding to O–C=O in CO32−. This further confirms the existence of CO32− in the interlayer of MnCr-LDO. As a result, XPS investigations clearly supported the production of the (MnCr)-LDO5wt.%/CoFe2O4 nanocomposite.
Adsorption analysis
Effect of contact time
One of the essential factors in adsorption processes is contact time, and it is crucial to build procedures for industrial applications that are both efficient and affordable. In order to better understand this, the impacts of contact time on the adsorption of MO by CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 adsorbents were investigated. Figure 8a shows MO removal percentage at different contact times (10–150 min) for 25 ppm dye concentration. As a primary impression, (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbent recorded higher MO removal percentages than pure CoFe2O4 for most applied contact times. This result is mainly due to the presence of MnCr-LDO inside the matrix of CoFe2O4 which offered more active vacant sites for the adsorption process owing to its layered structure. Also, it can be seen, from Fig. 8a, that the adsorption was particularly quick at first due to the nanoadsorbents' high number of unoccupied active sites. After a while, adsorption gradually increased due to increasing saturation of the active sites, until it reached a flat plateau, i.e. equilibrium region40. For CoFe2O4, increasing contact time increased MO dye adsorption until it remained constant after 60 min with a maximum percentage of dye removal of 41.61%. On the other hand, increasing contact time using (MnCr)-LDO5wt.%/CoFe2O4, increased MO dye adsorption to reach 56.64%, after that it remained constant at contact time ˃ 120 min, which is higher than that of CoFe2O4.
Effect of initial dye concentration
To overcome the mass transfer resistance of metal ions between the aqueous and solid phases, the initial concentration of MO dye is crucial. Both CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 were used to study the sorption of MO dye at concentrations ranging from 5 to 25 ppm. Figure 8b indicates clearly that the percentage of removal for both nano-sorbents decreases with increasing concentration. When the concentration of MO increased, the efficiency with which they could be removed from the sport's surface diminished. More binding sites are accessible when the concentration of MO is low to begin with. The number of ions vying for biomass binding sites, however, increased as concentration rose42.
Effect of adsorbent dosage
One of the most critical parameters for rapid and efficient dye removal is the size and amount of adsorbent, which must be optimized. The adsorbent dosage is an important parameter in adsorption studies because it determines the capacity of the adsorbent for a given initial concentration of dye solution. The effect of the adsorbent dosage was studied by varying the sorbent amounts from 0.1 to 0.5 g/100 mL. This effect was studied at an initial concentration of 25 ppm of MO dye. It may be observed from Fig. 8c that the removal percentage of MO increased rapidly with the increase in adsorbent dosage until 0.5 g/100 mL for both nano sorbents. This can be attributed to increase adsorbent surface area and the availability of more adsorption sites with increasing adsorbent dosage. For the same amount of nanosorbent (0.5 g/100 mL), the percentage of dye removal using CoFe2O4 was 55%, which is lower than that obtained using (MnCr)-LDO5wt.%/CoFe2O4 which is 65%.
Effect of pH
Solution pH plays an important role in dye adsorption. Figure 8d displays the effect of solution pH on MO dye adsorption. 25 ppm of MO solutions were agitated with 0.1 g/100 mL of both CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 at different pH values (3–8) at 25 °C. A decrease in the removal percentage of MO dye was found with increasing pH for both nanosorbents. Moreover, the increase in the dye removal percentage is higher for (MnCr)-LDO5wt.%/CoFe2O4 (86.1%) than that for CoFe2O4 (72.8%) at pH = 3. Due to the electrostatic attraction that occurs when the pH is 3, positively charged surface sites on the adsorbent favor the adsorption of dye anions. As a result, the adsorption capacity in an acidic medium is high for MO due to excess positive sites arises from acid activation. The adsorption capacity decreases when pH ˃ 3 as a result of OH groups and MO anionic ions adsorption competition on the active sites24. Consequently, CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 capacities for MO removal were optimized by many factors, i.e., acidic media (pH = 3) showed good results but increasing MO dye initial concentration decreased the removal % of the dye for both adsorbents.
Adsorption isotherms
The adsorption isotherm is a correlation between the quantity of adsorbate (MO dye) taken from the liquid phase per unit mass of adsorbent at a constant temperature and pH. The development of an adsorption system necessitates the use of adsorption isotherms. For accurate prediction of adsorption parameters and quantitative comparison of adsorption behavior across various adsorbent systems, a thorough mathematical description of equilibrium adsorption capacity is crucial. Parameters from equilibrium isotherms may shed light on the adsorbent's surface characteristics, affinity, and sorption process. The optimal conditions for developing adsorption systems depend on identifying the best correlation coefficients (R2) of equilibrium curves. The equilibrium data were examined using three isotherms termed; Langmuir, Freundlich, and Temkin. These isotherm models were represented according to the following relations41,42:
where qm represents the maximum amount of adsorbed dye, qe and Ce defined as previously mentioned in Table 1, KL represents the Langmuir constant, KF and nF represent the Freundlich constants, and kT is the Temkin constant.
The Langmuir model states that monolayer adsorption occurs on an adsorbent with a surface that is structurally homogeneous. The binding sites in this instance have a similar propensity for adsorption43. Linear plots of \(\frac{1}{{C}_{e}}\) versus \(\frac{1}{{q}_{e}}\) are shown in Fig. 9a for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbents. The lines were fitted according to Langmuir model, Eq. (8), and the fitting parameters (qm and KL) and correlation coefficients (R2) were easily determined and listed in Table 3 for both nanoadsorbents. As seen from Table 3, the correlation coefficient (R2) in case of (MnCr)-LDO5wt.%/CoFe2O4 is higher than that of CoFe2O4. On the other hand, the maximum monolayer adsorption capacity for dye removal was qm = 14.258 mg/g and Langmuir constant was KL = 1.512 for (MnCr)-LDO5wt.%/CoFe2O4 and qm = 10.537 mg/g and KL = 1.497 for CoFe2O4. The essential properties of the Langmuir isotherm may be described by the dimensionless constant RL, also known as the equilibrium parameter. It may be computed using the equation below44:
By substituting for KL and the initial dye concentration Co, the equilibrium parameters RL were estimated and were listed in Table 3 for both nanoadsorbents. As a result, the nature of the adsorption process may be characterized by the value of RL as being un-favorable for RL greater than unity, linear for RL equals unity, favorable for RL greater than zero and less than unity, or irreversible for RL equals zero44 Table 3 indicated that both adsorbents have RL values between 0 and 1, suggesting that MO dye is adsorbed to them favorably.
The empirical Freundlich model posits that the more strongly bound sites are used up first and that the binding strength diminishes with the increasing degree of site occupancy; this is done on the basis of the multilayer development of adsorbate on the heterogeneous solid surface of the adsorbent45. The plots of lnqe aganist lnCe for the adsorption of MO onto CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 are shown in Fig. 9b. The intercept and slope of the linear plots reflect the extent of adsorption and the degree of nonlinearity between the dye solution concentration and the adsorption, respectively. The Freundlich parameters (\({n}_{F},\) KF, and R2) are listed in Table 3. The value of nF varies with the heterogeneity of the adsorbent, and for a favorable adsorption process, the value of nF should be less than 10 and higher than unity. As can be seen from Table 3 that the value of nF and KF obtained was 3.663 and 5.619 for CoFe2O4, and 3.154 and 7.380 for (MnCr)-LDO5wt.%/CoFe2O4 indicating a favorable adsorption process. The less than unity values of \(\frac{1}{{n}_{F}}\) referred to satisfactory adsorption process. The values of correlation coefficients (R2) are 0.926 and 0.930 for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4, respectively. These R2 values showed that Freundlich isotherm was not proper to designate the data of the experiment.
Despite the Langmuir and Freundlich isotherms, which assume that sorption free energy is independent of surface coverage, the Temkin isotherm model takes into consideration interactions between adsorbents and metal ions to be adsorbed46,47,48. Figure 9c shows the plot of qe versus ln Ce for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbents. The data were linearly fitted according to Temkin model (Eq. 10). Temkin parameters (bT and KT) were determined from the slope and intercept of the plots and their values together with R2 were listed in Table 3. The applicability of the isotherm equation to describe the adsorption process was judged by the correlation coefficients, R2 values. The adsorption isotherm models fitted the data in the order of:
Langmuir ~ Freundlich > Temkin in case of CoFe2O4 suggested the homogenous distribution of active sites beside increasing the probability of formation multilayers of MO dye on to the magnetic CoFe2O4 nanoadsorbent so that the first charged adsorbent layer hinder from multiple layer adsorption, the whole process is schematically shown in Fig. 10a. The value of R2 is low (0.905) in the case of CoFe2O4, indicating poor agreement and efficiency of the Temkin isotherm in interpreting the experimental data. For (MnCr)-LDO5wt.%/CoFe2O4 the order is Langmuir > Temkin > Freundlich. The above order revealed that the equilibrium data are better fitted by the three-parameter models in case of (MnCr)-LDO5wt.%/CoFe2O4 but for CoFe2O4 two-parameter models were better fitted. In case of (MnCr)-LDO5wt.%/CoFe2O4 the Langmuir equation represents the adsorption process very well (R2 > 0.98) addition to Temkin (R2 > 0.97). The fact that the Langmuir isotherm fits the experimental data very well may be due to homogenous distribution of active sites and the formation of monolayer of MO dye over the magnetic (MnCr)-LDO5wt.%/CoFe2O4nanoadsorbent, as shown in Fig. 10b. Table 4 displays the maximum adsorption capacities for adsorbents reported in other literatures.
Adsorption kinetics
Adsorption kinetics research is useful in estimating adsorption rates and mechanisms56. The kinetics of MO adsorption onto the synthesized nanoparticles was examined using four kinetic models: pseudo-first order, pseudo-second order, Elovich, and intra-particle diffusion.
The pseudo-first-order model indicates that physical adsorption is the dominant process. The linearized pseudo-first-order equation is given by the following relation57:
where qe and qt are previously defined in Table 1, while k1 (min−1) is the rate constant.
Figure 11a depicts the experimental adsorption data obtained for different time interval at initial concentration of 25 ppm MO dye solution fitted to pseudo-first order kinetic model. The model parameters (k1 and qe) were determined from the slope and the intercept and were listed in Table 5. The low value of correlation coefficients for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 adsorbents and the non-reasonable difference between the experimental and calculated adsorption capacity (qe) of first-order kinetic showed that this model fail to interpret the experimental data.
According to the pseudo-second order model, chemisorption is the main phenomenon. Electron sharing or electron transfer between adsorbent and adsorbate occurs in this process56. The following equation gives the mathematical expression for the model as follows58
where k2 is the rate constant of the pseudo-second-order kinetic equation and qt, qe, and t are the same as defined in Table 1. Linear graphs are obtained from the plot of \(\frac{t}{{q}_{t}}\) against t as shown in Fig. 11b. The fitting parameters (qe and k2) are calculated from the slope and the intercept. Adsorption experiments for CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 were found to be consistent with a pseudo-second order kinetic model, with rate constants of 3 × 10−4 (g/mg.min) and 50 × 10−4 (g/mg.min), respectively, as shown by the experimental results in Table 5.
The Elovich model may be used to characterize second order kinetics and is typically applicable for systems with a heterogeneous adsorbing surface58. The Elovich model equation does not suggest a specific mechanism for the adsorption process59. The following equation gives the model's linear form as follows60:
where α (mg/g. min) is the initial adsorption rate, while β (g/mg) represents the amount of surface coverage and is the activation energy of chemisorption. These coefficients were obtained from the plot of qt versus ln t, as shown in Fig. 11c, and were tabulated in Table 5. The activation energy for chemisorption (g/mg) and the amount of surface covering β are responsible for the initial sorption rate α (mg/g.min). Adsorption of MO by CoFe2O4 follows the Elovich model, as shown by high R2 value (0.961). On the other hand it is not suitable for describing experimental data for (MnCr)-LDO5wt.%/CoFe2O4 adsorbent (R2 = 0.832).
The intra-particle diffusion model is commonly used for porous materials, and the physical adsorption phenomenon involves the diffusion of adsorbate into pores of varying sizes. Diffusion via intra-particle diffusion is the rate-limiting step in the transfer of adsorbate molecules/ions from the bulk solution to the adsorbent solid surface. The following equation represents the diffusion through the intraparticle diffusion model60:
where kint represents the intra-particle diffusion rate constant and C represents the intercept constant.
A plot of qt versus t1/2 is shown for both nanoadsorbents in Fig. 11d. The experimental data were fitted according to intra-particle diffusion model. The slope, which is equivalent to qt, intercept, that gives C, and R2 values were tabulated in Table 5 for CoFe2O4, and (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbents. The results indicated that MO adsorption on CoFe2O4 obeyed the intra-particle diffusion model with high R2 values of 0.990. On the other hand, this model is not suitable in case of (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbent as it showed low R2 value of 0.934. These data imply that the physical adsorption process dominates MO removal via CoFe2O4 nanodsorbent, whereas both physical and chemical adsorption processes influence MO removal via (MnCr)-LDO5wt.%/CoFe2O4 nanodsorbent, as schematically illustrated in Fig. 10b.
Reusability analysis
A reuse study of CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 nanoadsorbents for MO adsorption was carried out in order to verify the possibility of regeneration and reuse of the adsorbent material, an important factor for the replicability and economy of the process. Thedesorption of methyl orange was investigated, and the results are shown in Fig. 12. The results presented in Fig. 12 demonstrated that CoFe2O4 and (MnCr-LDO)5wt.%/CoFe2O4 showed good adsorptive capacity even after five adsorption–desorption cycles. In all cycles, an external magnetic field was used to recover the adsorbent, thus reducing the abrupt mass losses that occur in other recovery processes of the adsorbent material.
Photocatalytic study
The degradation of MO under UV light was used to gauge the composite catalyst's photocatalytic activity. The removal efficiencies for blank and (MnCr)-LDO5wt.%/CoFe2O4 nanocomposite catalyst are shown in Fig. 13 as a function of irradiation time. About 46% of MO was decomposed on (MnCr)-LDO5wt.%/CoFe2O4 within 120 min, demonstrating the predicted performance of the composite in photocatalysis as compared to the poor degradation of MO in the blank test (6.5%). The following two aspects were probably responsible for the improvement of photocatalysis: First, photocatalytic processes depend greatly on adsorption capacity. Second, (MnCr)-LDO5wt.%/CoFe2O4 exhibits a unique linked nanostructure, i.e. the presence of MnCr-LDO affects the photocatalytic activity owing to the development of mixed metal oxide and their composites20,23. Additionally, any transferred charge carriers have a greater chance of becoming accessible to either oxidants or reductants in the solution21,22.
Conclusions
In summary, a novel nanocomposite termed (MnCr)-LDO5wt.%/CoFe2O4 was successfully prepared via the co-precipitation route. Its removal efficiency for methyl orange dye was examined and compared to that of pure CoFe2O4. Several parameters have been examined to show their effect on the MO adsorption process. These parameters were the contact time, the dye concentration, the nanoadsorbent dosage, and pH. The findings showed that the maximum removal percentage was 86.1% for 25 ppm of MO by 0.1 g/100 mL of (MnCr)-LDO5wt.%/CoFe2O4 at pH = 3. Investigations of isotherms and kinetics were conducted under batch conditions. The Langmuir isotherm matched the experimental data for both nanoadsorbents, quite well due to the homogeneous distribution of active sites. Adsorption kinetics data were found to be compatible with intra-particle diffusion and pseudo-second order models for CoFe2O4 and (MnCr)5wt.%/CoFe2O4, respectively. These findings implied that MO removal through CoFe2O4 nanodsorbent is dominated by the physical adsorption process, while both physical and chemical adsorption processes were influencing MO removal by (MnCr)-LDO5wt.%/CoFe2O4 nanodsorbent. Furthermore, the magnetic characteristics of CoFe2O4 and (MnCr)-LDO5wt.%/CoFe2O4 aided in the reuse process by removing the requirement for time-consuming and expensive effective methods. A total of five adsorption–desorption cycles were performed to determine the prepared adsorbents’ recyclable nature.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Nizamuddin, S. et al. Iron oxide nanomaterials for the removal of heavy metals and dyes from wastewater. In Nanoscale Materials in Water Purification 447–472 (2019).
Kouhbanani, M. A. J., Beheshtkhoo, N., Taghizadeh, S., Amani, A. M. & Alimardani, V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv. Natural Sci. Nanosci. Nanotechnol. 10(1), 015007 (2019).
Bai, Y.-N. et al. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard. Mater. 388, 121753 (2020).
Haque, M. M., Haque, M. A., Mosharaf, M. K. & Marcus, P. K. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 28(1), 793–804 (2021).
Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 4, 22–26 (2012).
Mittal, J. Permissible synthetic food dyes in India. Resonance 25(4), 567–577 (2020).
Zhang, Y. et al. An aspirated in-syringe device fixed with ionic liquid and β-cyclodextrin-functionalized CNTs/TiO2 for rapid adsorption and visible-light-induced photocatalytic activity. New J. Chem. 43(24), 9345–9353 (2019).
Daud, M. et al. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 288, 110989 (2019).
Theiss, F. L., Ayoko, G. A. & Frost, R. L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods: A review. Appl. Surf. Sci. 383, 200–213 (2016).
Richetta, M., Medaglia, P., Mattoccia, A., Varone, A. & Pizzoferrato, R. Layered double hydroxides: Tailoring interlamellar nanospace for a vast field of applications. J. Mater. Sci. Eng. 6(4), 2169–2222 (2017).
Yun, S. K. & Pinnavaia, T. J. Layered double hydroxides intercalated by polyoxometalate anions with Keggin (α-H2W12O406-), Dawson (α-P2W18O626-), and Finke (Co4(H2O)2(PW9O34)210-) structures. Inorg. Chem. 35(23), 6853–6860 (1996).
Zubair, M., Daud, M., McKay, G., Shehzad, F. & Al-Harthi, M. A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 143, 279–292 (2017).
Li, M. et al. Uniformly coating ZnAl layered double oxide nanosheets with ultra-thin carbon by ligand and phase transformation for enhanced adsorption of anionic pollutants. J. Hazard. Mater. 397, 122766 (2020).
He, X., Qiu, X., Hu, C. & Liu, Y. Treatment of heavy metal ions in wastewater using layered double hydroxides: A review. J. Dispers. Sci. Technol. 39(6), 792–801 (2018).
Vithanage, M., Ashiq, A., Ramanayaka, S. & Bhatnagar, A. Implications of layered double hydroxides assembled biochar composite in adsorptive removal of contaminants: Current status and future perspectives. Sci. Total Environ. 737, 139718 (2020).
Giakisikli, G. & Anthemidis, A. N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. Anal. Chim. Acta 789, 1–16 (2013).
Palza, H., Delgado, K. & Govan, J. Novel magnetic CoFe2O4/layered double hydroxide nanocomposites for recoverable anionic adsorbents for water treatment. Appl. Clay Sci. 183, 105350 (2019).
Zhang, H. et al. From waste to waste treatment: Mesoporous magnetic NiFe2O4/ZnCuCr-layered double hydroxide composite for wastewater treatment. J. Alloys Compd. 819, 153053 (2020).
Xu, Y. et al. Effects of amphoteric polycarboxylate dispersant (APC) and acetone formaldehyde sulfite polycondensate (AFS) on the rheological behavior and model of oil well cement pastes. Colloids Surf. A Physicochem. Eng. Asp. 569, 35–42 (2019).
Chen, D. et al. Magnetic Fe3O4/ZnCr-layered double hydroxide composite with enhanced adsorption and photocatalytic activity. Chem. Eng. J. 185, 120–126 (2012).
Deng, L., Shi, Z., Peng, X. & Zhou, S. Magnetic calcinated cobalt ferrite/magnesium aluminum hydrotalcite composite for enhanced adsorption of methyl orange. J. Alloys Compd. 688, 101–112 (2016).
Dahdouh, N., Amokrane, S., Murillo, R., Mekatel, E. & Nibou, D. Removal of methylene blue and basic yellow 28 dyes from aqueous solutions using sulphonated waste poly methyl methacrylate. J. Polym. Environ. 28, 271–283 (2020).
Kesornsit, S., Sajomsang, W., Gonil, P. & Chairat, M. Adsorption kinetics and thermodynamic studies of lac dye on polydopamine-coated silk yarn. Cellul. Chem. Technol. 54, 799–810 (2020).
Asiabi, H., Yamini, Y. & Shamsayei, M. Using cobalt/chromium layered double hydroxide nano-sheets as a novel packed in-tube solid phase microextraction sorbent for facile extraction of acidic pesticides from water samples. New J. Chem. 42(12), 9935–9944 (2018).
Mamat, M., Abdullah, M. A. A., Kadir, M. A., Jaafar, A. M. & Kusrini, E. Preparation of layered double hy-droxides with different divalent metals for the adsorption of methyl orange dye from aqueous solutions. Chem. Eng. 9(6), 1103–1111 (2018).
Teixeira, T. P. F., Aquino, S. F., Pereira, S. I. & Dias, A. Use of calcined layered double hydroxides for the removal of color and organic matter from textile effluents: kinetic, equilibrium and recycling studies. Braz. J. Chem. Eng. 31, 19–26 (2014).
Hahn, T. et al. International tables for crystallography volume A1: Symmetry relations between space groups. In Dinamarca (Springer 2005).
Scherrer, P. Bestimmung der grösse und der inneren struktur von kolloidteilchen mittels röntgenstrahlen. Nachr. Ges. Wiss. Göttingen Math. Phys. Kl. 1918, 98–100 (1918).
Khan, J. et al. Synthesis and characterization of structural, optical, thermal and dielectric properties of polyaniline/CoFe2O4 nanocomposites with special reference to photocatalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 109, 313–321 (2013).
Balayeva, O. O. et al. Cobalt chromium-layered double hydroxide, α-and β-Co (OH)2 and amorphous Cr(OH)3: Synthesis, modification and characterization. Heliyon 5(11), e02725 (2019).
Monisha, P., Priyadharshini, P., Gomathi, S. S. & Pushpanathan, K. Influence of Mn dopant on the crystallite size, optical and magnetic behaviour of CoFe2O4 magnetic nanoparticles. J. Phys. Chem. Solids 148, 109654 (2021).
Soler, M. A. et al. Aging investigation of cobalt ferrite nanoparticles in low pH magnetic fluid. Langmuir 23, 9611–9617 (2007).
Reddy, M.P. et al. A facile hydrothermal synthesis, characterization and magnetic properties of mesoporous CoFe2O4 nanospheres. J. Magn. Magn. 388, 40–44 (2015).
Manouchehri, S. & Ghasemian, Z. Synthesis and characterization of cobalt zinc ferrite nanoparticles coated with DMSA. Chem. Xpress 2(3), 147–152 (2013).
Naseri, M. G., Saion, E. B., Ahangar, H. A., Hashim, M. & Shaari, A. H. Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 323(13), 1745–1749 (2011).
Kombaiah, K. et al. Comparative investigation on the structural, morphological, optical, and magnetic properties of CoFe2O4 nanoparticles. Ceram. Int. 43, 7682–7689 (2017).
Kumar, Y., Sharma, A. & Shirage, P. M. Impact of different morphologies of CoFe2O4 nanoparticles for tuning of structural, optical and magnetic properties. J. Alloys Compd. 778, 398–409 (2019).
Gidado, S. M. & Akanyeti, İ. Comparison of remazol brilliant blue reactive adsorption on pristine and calcined ZnAl, MgAl, ZnMgAl layered double hydroxides. Water Air Soil Pollut. 231(4), 146 (2020).
Sun, J. et al. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 532, 474–484 (2018).
Benadjemia, M., Millière, L., Reinert, L., Benderdouche, N. & Duclaux, L. Preparation, characterization and methylene blue adsorption of phosphoric acid activated carbons from globe artichoke leaves. Fuel Process. Technol. 92(6), 1203–1212 (2011).
Wang, L., Li, J., Wang, Y., Zhao, L. & Jiang, Q. Adsorption capability for Congo red on nanocrystalline MFe2O4 (M = Mn, Fe Co, Ni) spinel ferrites. Chem. Eng. J. 181, 72–79 (2012).
Bendini, A. et al. Preliminary evaluation of the application of the FTIR spectroscopy to control the geographic origin and quality of virgin olive oils. J. Food Qual. 30(4), 424–437 (2007).
Yi, X. et al. Highly efficient removal of uranium (VI) from wastewater by polyacrylic acid hydrogels. RSC Adv. 7(11), 6278–6287 (2017).
Ojedokun, A. T. & Bello, O. S. Kinetic modeling of liquid-phase adsorption of Congo red dye using guava leaf-based activated carbon. Appl. Water Sci. 7, 1965–1977 (2017).
Liu, F. et al. High response and selectivity of single crystalline ZnO nanorods modified by In2O3 nanoparticles for n-butanol gas sensing. Sens. Actuators B Chem. 277, 144–151 (2018).
Shahbeig, H., Bagheri, N., Ghorbanian, S. A., Hallajisani, A. & Poorkarimi, S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J. Modell. Simul. 9(4), 243–254 (2013).
Hasan, S. H., Ranjan, D. & Talat, M. Agro-industrial waste ‘wheat bran’ for the biosorptive remediation of selenium through continuous up-flow fixed-bed column. J. Hazard. Mater. 181, 1134–1142 (2010).
Mashkoor, F. & Nasar, A. Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J. Mol. Liq. 274, 315–327 (2019).
Zaghouane-Boudiaf, H., Boutahala, M. & Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem. Eng. J. 187, 142–149 (2012).
Ni, Z. M., Xia, S. J., Wang, L. G., Xing, F. F. & Pan, G. X. Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: Adsorption property and kinetic studies. J. Colloid Interface Sci. 316(2), 284–291 (2007).
Jiang, T., Liang, Y. D., He, Y. J. & Wang, Q. Activated carbon/NiFe2O4 magnetic composite: A magnetic adsorbent for the adsorption of methyl orange. J. Environ. Chem. Eng. 3(3), 1740–1751 (2015).
Yarahmadi, P., Movadedi, M. & Salavti, H. ZnO-MgFe2O4/(Mg)-or (Zn)-Al-LDH composites: Adsorption efficiency, kinetics, and adsorption isotherm for Congo Red removal. Phys. Chem. Res. 9(2), 311–325 (2021).
Zhao, D., Yang, B. & Nan, Z. Synthesis of uniform Co NPs with high saturation magnetization and investigation on removal of methyl orange from aqueous solution by Co/MWCNTs composite. Mater. Res. Bull. 68, 126–132 (2015).
Robati, D. et al. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng. J. 284, 687–697 (2016).
Tanhaei, B., Ayati, A., Lahtinen, M. & Sillanpää, M. Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of methyl orange adsorption. Chem. Eng. J. 259, 1–10 (2015).
Inyinbor, A. A., Adekola, F. A. & Olatunji, G. A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 15, 14–27 (2016).
Parmar, J. et al. Imatinib cation based MOIFs with [Fe(CN)5NO]2−, [Fe(CN)6]3− [Fe(CN)6]4− as adsorbent of organic pollutants, and their protein interaction study. J. Mol. Struct. 1288, 135801 (2023).
Milenković, D. D. et al. Removal of copper (II) ion from aqueous solution by high-porosity activated carbon. Water SA 39(4), 515–522 (2013).
Mezenner, N. Y. & Bensmaili, A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J. 147, 87–96 (2009).
Singh, M., Dosanjh, H. S. & Singh, H. Surface modified spinel cobalt ferrite nanoparticles for cationic dye removal: Kinetics and thermodynamics studies. J. Water Process Eng. 11, 152–161 (2016).
Acknowledgements
This work was performed in the superconductivity and metallic glasses laboratory, Faculty of Science, Alexandria University, Alexandria, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.R.: conceptualization; material synthesis; formal analysis; validation; visualization; methodology; writing-original draft; writing—review & editing. A.I.A.: investigation; visualization; data curation; formal analysis; supervision and writing-original draft. M.E.: writing-original draft; investigation; methodology and data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rekaby, M., Abou-Aly, A.I. & El-khatib, M. Preparation and characterization of a novel nanocomposite based on MnCr-layered double oxide and CoFe2O4 spinel ferrite for methyl orange adsorption. Sci Rep 13, 18006 (2023). https://doi.org/10.1038/s41598-023-45136-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45136-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.