Abstract
Melatonin is a pleiotropic molecule that can influence various aspects of plant performance. Recent studies have exhibited that it mediates plant defensive responses, probably through managing redox homeostasis. We tried to track the regulatory effects of melatonin on the antioxidant machinery of Linum album cell culture. To this, different concentrations of melatonin were applied, and the oxidative status of cells was investigated by measuring the levels of oxidative molecules and antioxidant agents. The results showed that H2O2 content did not change at the low melatonin levels, while it increased at the high concentrations. It can be correlated with the low melatonin dosages capacity to remove excessive amounts of H2O2, while the high melatonin dosages exhibit toxicity effects. In contrast, the NO enhancement occurred at 50 μM melatonin, proposing its role in triggering melatonin-induced defensive responses. The MDA results stated that NO led to oxidative stress in melatonin-treated cells at 50 μM melatonin. Antioxidant enzyme POD was activated by melatonin treatment, while SOD enzyme behaved reversely which can explain the changes in the H2O2 level. In addition, the analysis of the phenolics profile showed that the contents of phenolic acids, flavonoids, and lignans enhanced following an increase in PAL enzyme activity. The increased level of phenolic hormone SA can indicate that melatonin affects the defensive responses in L. album cells through a SA-dependent pathway. In general, it seems that melatonin, by modulating NO and SA levels, can induce the activity of antioxidant enzymes and the production of phenolics, especially lignans, in L. album cells.
Similar content being viewed by others
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), an indole compound derived from serotonin, is a naturally pleiotropic molecule with different physiological functions in plants and animals1,2. In plants, it is assumed as a multifunctional regulatory molecule involved in numerous physiological processes such as root formation, seed germination, photosynthesis, circadian rhythms, leaf senescence delay, fruit ripening, and defensive responses1,3,4,5.
It has been stated that melatonin may increase in plants versus abiotic and biotic stresses, which is consistent with its key roles in activating defense reactions as a signaling and antioxidant molecule. In response to adverse conditions, plants undergo changes in cellular redox status as a result of reactive oxygen species (ROS) and reactive nitrogen species (RNS) production, through apoplastic and/or subcellular organelles reactions6. Oxidative stress can damage cellular components such as membranes, proteins, carbohydrates and nucleic acids7,8. Likewise, plants can cope with oxidative stress by evolving several efficient defensive reactions which exhibit crucial roles in preserving their survival9,10. Melatonin is known to be involved in maintaining cellular and intracellular redox homeostasis to regulate plant cell physiological function in response to stress conditions11,12,13. Recent studies have suggested that this molecules can directly enhance the cell antioxidant capacity and equips plants with different degrees of tolerance to biotic and abiotic stresses, such as drought, salinity, high light intensity, herbicides, and ultraviolet radiation11,14,15. Furthermore, since mitochondria and chloroplasts have been recently known the sources of melatonin production, it can act against various ROS in these compartments16,17. Previous studies have indicated that the application of melatonin induces the antioxidant enzymes activity such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) that is considered as a general mechanism to prevent the worst impacts of ROS on the cells18. This function of melatonin suggests its role as a signaling molecule in the regulation of plants’ defensive responses by influencing downstream signaling. Regarding this hypothesis, the interactions between melatonin and different signaling molecules such as mitogen-activated protein kinase (MAPK) cascades, Ca2+ influx, hydrogen peroxide (H2O2), nitric oxide (NO), and salicylic acid (SA) was previously observed19.
Besides, melatonin apparently acts as a phytohormone due to its structure, contributing to produce secondary metabolites more efficiently20. Recent studies have clearly shown that the melatonin application can impressively affect phenolics accumulation in the different plant species such as Ocimum basilicum var purpurascens2, and Rosmarinus officinalis21. It has been reported that melatonin can affect the contents of phenolic compounds through activation of enzymes involved in phenylpropanoid pathway22. Phenolic compounds (e.g., phenolic acids, flavonoids, and lignans) are characterized as natural products, linked to increasing the antioxidant capacity of the plants and protecting them from adverse conditions23,24. These compounds are derived from the phenylpropanoid pathway, where phenylalanine and tyrosine amino acids are converted into simple phenolic acids by the activities of Phenylalanine ammonia-lyase (PAL) and Tyrosine ammonia-lyase (TAL) enzymes, respectively10. A large body of studies is postulated that plants transiently increase the production of phenolic compounds in response to stresses25,26. Previous studies have pointed out that endogenous SA, a phenolic phytohormone, regulates many features of the plant defense machinery, including the biosynthesis of secondary metabolites8. Also, it has been suggested that melatonin and SA biosynthesis pathways compete for the chorismic acid precursor, which is provided by shikimic acid route27. According to the findings of Khan et al., 2015 and Wang et al., 2018, the protective roles of melatonin are probably related to its cross-talk with SA and other signaling molecules such as H2O2 and NO, which can improve the production of secondary metabolites and the activities of antioxidant enzymes to alleviate the oxidative stress27,28,29.
Linum album Kostchy Boiss., a native species of Iran, is a potent alternative source of phenolics, especially lignans, widely used as antiviral, anti-cancer, anti-tumor, and anti-inflammatory agents30. Lignans are an important subgroup of phenylpropanoids, leading to reinforce defense responses in this species31. However, till now, there are no scientific reports on the possible mechanisms by which melatonin regulates the defense responses of L. album cells. Therefore, in this study, the L. album cells were treated with different concentrations of melatonin (50, 100, and 150 μM) for 120 h. Subsequently, we analyzed the changes in the POD and SOD enzymes activities, H2O2, NO, and malondialdehyde (MDA) levels, and phenolic acids, flavonoids, and lignans quantification in the treated cells.
Results
Effect of melatonin on H2O2, NO, and MDA contents
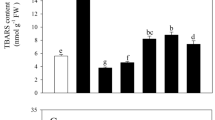
In the present study, changes in the oxidative status of L. album cells treated with different concentrations of melatonin were initially evaluated by measuring H2O2, NO, and MDA contents. Results of this experiment showed that under treatment condition, the content of H2O2 was increased at 150 µM melatonin (p ≤ 0.05), while low levels of melatonin did not significantly influence H2O2 content (Fig. 1A). In contrast, NO content was induced at low level of melatonin treatment, so that the highest amount of NO was observed in L. album cells under 50 µM melatonin compared to the control (Fig. 1B). Under melatonin treatment, the content of MDA was transiently enhanced, where the application of 50 µM melatonin induced it by 1.14-fold compared with the control (Fig. 1C). At low concentrations of melatonin, H2O2 content remained constant compared to the control. On the other hand, with a sudden increase in NO content at 50 µM melatonin, the formation of MDA was also significantly changed.
Effect of melatonin on the activity of POD and SOD enzymes
To better comprehend the effect of melatonin on enzymatic antioxidant responses, the activity of POD and SOD in the treated and untreated cells were tracked. As depicted in Fig. 2A, POD enzyme activity raised quickly after melatonin treatment compared to the control and reached its peak (approximately 2.38-fold higher than in the control cells) at 50 µM melatonin treatment. However, data showed that exposure to different concentrations of melatonin decreased the activity of SOD in the L. album cells, which was 1.35-fold less than the control sample at 150 µM melatonin (Fig. 2B).
Effect of melatonin on PAL and TAL enzyme activities
In this study, the catalytic activity of PAL and TAL enzymes in L. album cells were determined. According to the results, PAL activity in the treated cells was induced at low concentrations of melatonin treatment, which reached its peak at 100 µM (Fig. 3A). In return, different concentrations of melatonin had no significant effect on the TAL enzyme activity relative to the control group (Fig. 3B). Moreover, the activity of this enzyme decreased at the high level of melatonin compared to the control sample.
Effect of melatonin on phenolic acids and flavonoids accumulation
The pronounced effect of melatonin on the accumulation of individual phenolic acids and flavonoids were assessed through HPLC method. The results revealed that the content of caffeic acid was significantly impaired under treatment with different concentrations of melatonin in comparison with the control condition. However, the exact contrary trend was observed in the case of cinnamic acid. According to our data, the exogenous application of melatonin significantly increased cinnamic acid at 50 and 100 µM, which were by 1.50-fold and 1.60-fold compared to the control, respectively. On the other hand, the coumaric acid content in cells of L. album was raised by 50 µM melatonin treatment, but its level did not change significantly compared to the control. Moreover, ferulic acid and SA contents remarkably enhanced at 50 and 100 µM melatonin treatments, as well (Table 1).
Meanwhile, the application of melatonin decreased catechin content, while significantly increased kaempferol level compared to the control after 120 h. Also, resveratrol changes were dependent on the melatonin concentrations, and accordingly, the amount of resveratrol reached its peak at 50 and 100 µM melatonin treatments (Table 1). Therefore, 50 and 100 µM melatonin treatment was an appropriate concentration to induce phenolic acids and flavonoids in L. album cells.
Effect of melatonin on lignans production
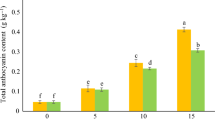
In the current work, the relative contents of four prominent lignans including secoisolariciresinol (SECO), lariciresinol (LARI), matairesinol (MATA), and podophyllotoxin (PTOX) were assayed through the HPLC system. As shown in Fig. 4A and C, the results emphasized that the SECO and MATA contents decreased at different concentrations of melatonin, so that their lowest levels were seen at 150 and 50 µM melatonin treatment, respectively. On the other hand, according to Fig. 4B, LARI content raised in all melatonin concentrations, while its level peaked at 150 µM melatonin treatment. Ultimately, PTOX enhancement occurred at 50 µM melatonin treatment, and its peak was about 1.53-fold in comparison to the control sample (Fig. 4D).
Effects of different concentrations of melatonin on secoisolariciresinol (A), lariciresinol (B), matairesinol (C), and podophyllotoxin contents in L. album cells. Means from 3 separate experiments ± SE. Means marked with the same letter were not significantly different according to Duncan's multiple range test (p value ≤ 0.05).
Metabolic profiles of L. album cells in response to melatonin treatment
For a better understanding the effects of melatonin on metabolic profiles, we analyzed the correlations amongst various metabolites through principal component analysis (PCA) (Fig. 5A). The loading plots provided by PCA showed that the metabolic profiles were successfully classified with an acceptable and excellent accuracy according to PCA results (the first component and second components were 79.7 and 17.5%, respectively). This analysis suggests clear separation based on samples at 50 and 100 µM of melatonin in the treated and untreated cells of L. album. The score graph (PC1 and PC2) of the PCA data emphasized that the separation of the sample outcomes was accomplished, and the variability of melatonin treatment (50 and 100 µM) is depicted by component 1. The response to melatonin treatment perspicuously had a significant percentage of the data set variability between different concentrations of melatonin treatment. Moreover, the metabolites provided from the tested cells were relegated on the basis of a similarity between the clusters pattern which was performed by the Pearson correlation coefficient (Fig. 5B). Enzymatic and non-enzymatic antioxidants were mostly relatively closely grouped, indicating that the observed distribution of samples is due to the concentrations of melatonin treatment (1–5). As the results, Fig. 5B explained that there are positive correlation patterns among various phenolic compounds in melatonin-treated L. album cells.
(A) Score (left) and loading (right) plots of the principal component analysis (PCA) conducted on the metabolomics data from samples treated with melatonin concentrations and the control. Each group of samples on the plot is indicated by an ellipse with 95% CI. (B) HCA map was employed for clustering of several metabolites and oxidative status according to Pearson correlations coefficient. Data are obtained from three replicates for each variation at all samples. Positive and negative correlations are described by red and blue colour, respectively. There are 5 basic clusters that are shown 1 to 5 on the picture.
Finally, Fig. 6 is described a summary of this research, and we found that the NO molecule can play a crucial role in melatonin responses in L. album cells. An increase in NO can affect H2O2 as a secondary messenger for inducing the phenylpropanoid pathway by enhancing SA production and the activity of PAL enzyme. Also, NO can provoke lipid peroxidation after melatonin treatments.
A summary of this research in which NO molecule can play a crucial role in melatonin responses in L. album cells. An increase in NO can affect H2O2 as a secondary messenger for inducing the phenylpropanoid pathway through enhancing SA production and the activity of PAL and TAL enzymes. Also, NO can provoke lipid peroxidation after melatonin treatment.
Discussion
Melatonin is a ubiquitous regulator molecule with multifunctional roles in the plant life processes, including tolerance against various environmental stresses32. Recently, there has been a large amount of literature describing that the exogenous application of melatonin can influence the plant defensive responses4,5. Despite enormous efforts to demonstrate the protective roles of melatonin in plant stress responses, the biological mechanisms of melatonin at the cellular level are not fully elucidated33. In this study, we illustrated that different concentrations of melatonin can induce enzymatic and non-enzymatic antioxidant reactions in L. album cells.
Defense mechanisms are triggered by many physiological processes, such as rising H2O2 content, increasing membrane permeability, releasing NO molecule, and producing secondary metabolites13. It is evident from the results that low concentrations of melatonin did not show significant effects on the content of H2O2, while it increased at the high dosage. These observations can be due to the inhibitory role of melatonin on the free radicals in L. album cells. This study confirms previous works on Cucumis sativus34 and Salvia reuterana35 that expressed melatonin may chiefly function as an essential regulator of ROS level, and detoxify excessive amounts of H2O2 molecule, suggesting that the low dosages of melatonin exhibiting no per-oxidative effects. In contrast, it has been reported that melatonin-induced H2O2 production can show a concentration-dependent behavior, and the high dosages of melatonin may presumably behave as a toxic element and reduce plant productivity32,36. This speculation is in agreement with our data indicating that H2O2 content can be enhanced through the high levels of melatonin in L. album cells.
On the other hand, melatonin treatment at low concentration (50 µM) noticeably accelerated the changes in NO generation L. album cells. The melatonin-induced NO generation in this study can be due to the up-regulation of nitrate reductase (NR) enzyme. In addition to converting nitrate to nitrite, this enzyme can reduce nitrite to NO using the NADPH cofactor27. Melatonin may indirectly modulate defense responses by leading a further increase in NO generation. Similar results were presented in A. taliana infected by the bacterial pathogen37, where the application of melatonin has a potential regulatory role in the NO generation, establishing signal transduction for the regulation of plant defense responses.
One more attribute appraised in the present study was MDA content which known as a lipid peroxidation indicator. Based on the observed resultants, melatonin-exposed L. album cells at 50 μM exhibited a significant elevation in MDA content as a dose-dependent response. At the same time, there were no significant differences in MDA levels at high concentrations of melatonin compared to the control. Therefore, from this point of view, we found that the highest content of NO under melatonin exposure caused an increased production of MDA in L. album cells. These results are in contrast to Soleimani Aghdam et al.38, who reported that interaction of melatonin with NO corresponded to the decreased MDA content, which may partially reflect in maintaining safe membrane integrity during chilling stress. In consistent with our findings, Kim et al.39 suggested that an increase in the endogenous NO level may promote oxidative damage, and thus raise the content of MDA.
Antioxidant enzymes play a key role in the plants defense machinery against various biotic and abiotic stress conditions. Melatonin noticeably improved the activity of antioxidant enzymes. Melatonin as a regulatory molecule can activate these enzymes and lead to balance of ROS levels and reduction of cellular oxidative damage40. In this experiment, the use of melatonin led to a change in the activity of antioxidant enzymes with different patterns in L. album cells. The activity of SOD enzyme as a source of H2O2 production, decreased in response to melatonin concentrations. In contrast, POD activity increased in the melatonin-treated L. album cells, which was coincided with the increased level of NO, representing that the activity of POD probably had a direct relationship with NO level. Furthermore, these changes in the activity of antioxidant enzymes can explain the changes trend in H2O2 level. An intriguing observation arising from the earlier studies4,32,41 is that melatonin positively motivates the activities of SOD and POD via the NO-dependent pathway. It has been suggested that melatonin can induce NO signaling pathway through interacting with unknown receptors, and subsequently regulate the function of various antioxidant enzymes and transcription factors32.
To get a better insight into the underlying mechanisms of melatonin in regulating the antioxidant agents, we performed a comparative metabolome analysis to characterize its functional role in inducing phenolic compounds in L. album cells. Likewise, PAL enzyme activity substantially increased in the presence of different concentrations of melatonin as a dose-dependent response. However, melatonin had no positive effect on TAL enzyme activity compared to the control group. The responses of PAL and TAL enzymes may differ due to their usage substrates. In addition, coumaric acid can be produced through an alternative pathway in which cinnamic acid is hydroxylated under the action of the cinnamate 4-hydroxylase (C4H) enzyme42. Over-activation of PAL enzyme resulted in a better supply of cinnamic acid for feeding into the phenylpropanoid pathway that was parallel with the increase of phenolic acids, flavonoids and lignans contents in the treated cells. It is important to mention that PAL and TAL are key enzyme to trigger the phenylpropanoid pathway via the provision of cinnamic acid and coumaric acid as primary precursors into other complex phenolics10,23. In accordance with our results, Duran et al.43 documented the significant effects of melatonin on PAL enzyme activity, promoting melatonin-induced defense reactions through the accumulation of phenolic compounds in Ocimum basilicum.
Interestingly, we found that content of defensive phytohormone SA increased in L. album cells in response to melatonin treatment. Melatonin can probably cause an increase the content of this phenolic hormone through regulating two key enzymes in the SA biosynthesis; PAL as a key enzyme in the production of phenolic compounds and also ICS-1 as the main and important enzyme in the biosynthesis of SA27. Overmeyer et al.44 also achieved that melatonin treatment triggers SA-induced defense signaling pathways by up-regulating SA biosynthesis genes such as isochorismate synthase-1 (ICS-1). On the other hand, SA enhancement can be a regulatory factor to adjust PAL activity and therefore, the modification of phenolic compounds production under the effect of melatonin could be a SA-dependent response8. Therefore, melatonin may be served as an up-regulator in the defense hormones signaling pathways, particularly SA. Their interaction likely led to strengthened defensive responses, indicating that the melatonin-mediated antioxidant activation in L. album cells not only limited to the NO molecule but may also derive from a complex interaction among melatonin, SA and NO signaling pathways. These results are consistent with Samari et al.8, who reported that biotic elicitor activates specific synergistic or antagonistic cross-talk amongst multiple signaling pathways, which trigger a complex network responsible for particular responses in various plant species. Our results can support the assumption that melatonin addition would positively affect phenolics accumulation through SA induction in L. album cells. As can be seen, there are many coincidences between both melatonin and SA molecules that their actions have aroused the production of phenolic metabolites, which are related to enhancing resistance to adverse situations.
In the current study, it was found that the profile of phenolic acids was robustly increased by melatonin treatment in L. album cells, but then declined with further increasing melatonin concentrations. The contents of cinnamic acid, coumaric acid, and ferulic acid reached their maximum at 50 and 100 μM melatonin treatments. The decrease in caffeic acid was since it may act as a prerequisite molecule in lignans biosynthesis. Also, our data showed a rapid increase in the contents of antioxidant compounds kaempferol and resveratrol in response to melatonin exposure, while the catechin level decreased. Several pieces of evidence in various literatures explained that exogenous melatonin treatment drastically induced secondary metabolites production derived from phenylalanine and tyrosine such as lignins, flavonoids, and lignans in different plant species4,43. Ultimately, we observed that melatonin can affect lignans accumulation differently in L. album cells. Based on our results, it appears that LARI is the lignan that is intensively accumulated in response to different concentrations of melatonin. In contrast, PTOX increased only at low concentration of melatonin, and the reduction of SECO and MATA contents as its precursor, in this concentration can be justified. The induction of lignans by elicitors has also been investigated in cell cultures of L. album in response to various elicitors10,12,13,45.
The HCA and PCA results further affirm that the melatonin can change the accumulation of phenolics, which peak at 50 and 100 µM in a dose-dependent manner. The PCA results revealed an overview of the differences between melatonin-treated and untreated cells. We observed a clear separation between the treatment parameters along the PC1, which consists 79.7% of the total variation. In accordance with these results, the increase in the contents of NO and SA, as well as the phenolic compounds, especially lignans under melatonin effect illustrates that the production of phenolic compounds can be significantly associated with the high levels of NO and SA. In this sense, it appears that L. album cells can respond to melatonin through the elevation of enzymatic and non-enzymatic defense mechanisms.
In summary, our observations confirm that melatonin can trigger complex network signaling pathways to strengthen antioxidant elements in L. album cells (Fig. 7). Based on our results, melatonin induces the NO and SA production as the regulatory molecules. It can be suggested that these regulatory agents in relationship to each other positively influences antioxidant enzymes activity and phenolic compounds production including phenolic acids, flavonoids and lignans in L. album treated cells. However, there is a need for more investigation to complete the attempt accomplished here to illustrate the detailed mechanism of melatonin in the regulation of defensive responses in this plant.
Materials and methods
Site description and collection of plant materials
All plant experiments were performed per the relevant institutional, national, and international guidelines and legislation. The plant materials were collected during June 2019 from wild-grown plants in the Sohanak region (35°480′N, 51°32′E, and altitude of 1900 m) placed in Tehran province, Iran. The permissions to harvest L. album plants in Tehran were provided. Besides they were discussed and approved by the Research Ethics Committee of Tarbiat Modares University. The formal identification of the L. album was done by Dr. Shahrokh Kazempour-Osaloo, a professor of plant systematic. A voucher specimen of L. album was kept at the Herbarium of department of Plant Biology of Tarbiat Modares University. The mature seeds were cultivated in MS medium for providing aseptic-plants as a source of explants.
Cell culture and treatment
To provide L. album cell culture, sterile leaf-explant was applied as described in our previous report46. Briefly, MS basal medium47 fortified with 3% sucrose, NAA (2 mg L−1), and kinetin (0.4 mg L−1) solidified with agar (0.8%) was employed to culture the cells at 25 °C in the dark condition. The pH of all media was adjusted to 5.6 with NaOH (1 M) before autoclaving. Callus culture was regularly passage into fresh medium once a month. Suspension culture was prepared by transferring fragile calli into liquid MS medium (as mentioned above, but without agar, 30 mL in 100 mL Erlenmeyer flasks). All media were placed on a rotary shaker (100 rpm) at 25 °C in continuous dark condition. 2 g of cells were regularly sub-cultured every 8 days on the same medium based on a presentive growth curve (Data not shown).
Different concentrations of melatonin (0, 50, 100, and 150 µM) were utilized as treatment. Melatonin was dissolved in ethanol. After autoclaving, melatonin was added to the culture medium through filter-sterilized (2.2 µm) under aseptic condition (day of treatment). All samples were harvested after 5 days, and immediately chilled in –80 °C.
Determination of H2O2, NO and, MDA contents
The accumulation of H2O2 was assayed according to Velikova et al.48 method. Briefly, 0.2 g of the fresh cells was extracted with 3 mL of 0.1% (w/v) trichloroacetic acid (TCA) on ice. Then, all samples were centrifuged at 12,000 rpm for 15 min, 4 °C. Subsequently, the supernatant (0.5 mL) was mixed with 0.5 mL of 100 mM phosphate buffer (pH, 7.0) and 1 mL of KI (0.1 M) in HCl (1 M) allowed standing for 1 h in the dark condition. The absorption was measured at 390 nm. The calculation of H2O2 content was done based on a standard curve, and the values were presented as μmol H2O2 g−1 FW.
The NO content was determined by measurement of nitrite concentration in vivo using Griess reagent49. 0.5 g of fresh cell was ground in 3 mL of 100 mM cool PO4−3 buffer (pH 7). The homogenates were centrifuged at 10,000 rpm for 15 min at 4 °C. Subsequently, the aliquot of samples (0.2 mL) was inoculated with 1.8 mL of PO4−3 buffer (pH 7) and 0.2 mL of Griess reagent (1% sulfanilamide and 0.1% N-1-naphthyl ethylenediamine dihydrochloride in 5% phosphoric acid solution) at 25 °C for 15 min. NO content was measured based on the absorbance of samples at 540 nm, and the concentration of NO was assessed by a sodium nitrite standard curve.
The formation of malondialdehyde (MDA), a lipid peroxidation marker, was performed based on Stewart and Bewley48 method with slight modificationx. In summary, 1 mL of cell extracts (in 0.1% TCA) and 0.5% thiobarbituric acid (in 20% TCA) were incubated for 30 min at 90 °C. Ultimately, the absorbance of mixtures was determined at 532 and 600 nm, and finally, the MDA accumulation was illustrated as μmol g−1 FW.
Quantification of the activity of SOD and POD enzymes
Soluble proteins were quantified according to Bradford (1976) method51. SOD (EC 1.15.1.1) activity was assayed based on the inhibition of nitroblue tetazolium (NBT) reduction52. Also, POD activity (EC 1.11.1.7) was analyzed based on the quantity of guaiacol oxidation by H2O2 at 470 nm53.
Measurement of PAL and TAL enzyme activities
PAL (4.3.1.5) and TAL (4.3.1.23) enzyme activities were analyzed using the method of Beaudoin-Eagan and Thorpe54 with some modifications. PAL activity was measured at 290 nm according to the formation of cinnamic acids, and TAL activity was assayed at 390 nm by the production of p-coumaric acid. The activity of PAL and TAL enzymes was estimated as µmol cinnamic acid and p-coumaric acid mg−1 protein min−1.
Analysis of phenolic compounds by using HPLC
The compositions of phenolic acids were analyzed by Owen et al.55 method. At first, 0.2 g of fresh cell samples was extracted using methanol solvent in a crucible. After centrifuging at 12,000 rpm, the solvent of each sample was evaporated under ambient condition. The residue was solved in 4 mL acetonitrile. Subsequently, all samples were extracted three times with n-hexane (3 mL). The residue was resolved in 0.5 mL methanol to detect the phenolic acids through the HPLC system (Agilent Technologies 1260 infinity, USA). A C18 column (Perfectsil Target ODS-3 (5 μm), 250 × 4.6 mm; MZ Analysentechnik, Mainz, Germany) was applied for the stationary phase. The eluent system was comprised of 2% acetic acid in water (A) and methanol (B) with a gradient system, as explained by Tashackori et al.56. For phenolic acids quantification, a UV detector was set at 278 and 300 nm.
Individual flavonoids determination was performed based on Keinnen et al.57 method. Briefly, 0.2 g of fresh cell were ground to a fine powder, and then homogenized with 1.5 mL of 40% aqueous methanol supplemented with 0.5% acetic acid. These reaction mixtures were allowed to stand for 12 h, and then centrifuges at 13,000 rpm for 12 min. The supernatant was injected into HPLC system. In this analysis, the gradient mobile phase was composed of A: 0.5% phosphoric acid in deionized water and B: acetonitrile. To detect flavonoids, a UV detector was set at 254, 280, 300 and 350 nm25.
To evaluate lignans contents, 0.2 g of dried cell were extracted by methanol (80% v/v), as previously defined46. The extract was dissolved in 0.5 mL of methanol before HPLC analysis. The HPLC system was equipped with a C18-ODS3, 5 μm (250 × 4.6 mm) column. The elution solvent was acetonitrile and water with a gradient system following Ahmadian Chashmi et al.58.
Statistical analysis
This experiment was done based on a completely randomized design with three replications independently. To analyze all data, SPSS 25 software was employed. Duncan's multiple range test was applied for determining statistical differences between means (p value ≤ 0.05). In addition, a data-matrix prepared which its rows represented samples and columns represented metabolites. To access more information about the metabolic profiling, the Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) was computed through the algorithm embedded in the web-based software (http://www.metaboanalyst.ca).
Data availability
The data supporting the findings of this study are available from the corresponding authors, upon request.
References
Arnao, M. B. & Hernández-Ruiz, J. Melatonin: a new plant hormone and/or a plant master regulator?. Trends Plant Sci. 24(1), 38–48 (2019).
Nazir, M. et al. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 25(5), 1072 (2020).
Arnao, M. B. & Hernández-Ruiz, J. Is phytomelatonin a new plant hormone?. J. Agron. 10(1), 95 (2020).
Ahammed, G. J. et al. Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology 110(5), 999–1009 (2020).
Jahan, M. S. et al. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 197, 110593 (2020).
Reiter, R. J. et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74(21), 3863–3881 (2017).
Das, K. & Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2, 53 (2014).
Samari, E. et al. Interactions between second messengers, SA and MAPK6 signaling pathways lead to chitosan-induced lignan production in Linum album cell culture. Ind. Crops Prod. 177, 114525 (2022).
Zhao, H. et al. Antioxidant enzyme responses induced by whiteflies in tobacco plants in defense against aphids: catalase may play a dominant role. PLoS ONE 11(10), e0165454 (2016).
Tashackori, H. et al. Physiological, biochemical, and molecular responses of Linum album to digested cell wall of Piriformospora indica. Physiol. Mol. Biol. Plants 27(12), 2695–2708 (2021).
Arnao, M. B., Cano, A. & Hernández-Ruiz, J. Phytomelatonin: an unexpected molecule with amazing performances in plants. J. Exp. Bot. 73, 5779–5800 (2022).
Fakhari, S. et al. Hydrogen sulfide directs metabolic flux towards the lignan biosynthesis in Linum album hairy roots. Plant Physiol. Biochem. 135, 359–371 (2019).
Samari, E. et al. Chitosan-induced phenolics production is mediated by nitrogenous regulatory molecules: NO and PAs in Linum album hairy roots. Plant Cell Tissue Organ Cult. 140(3), 563–576 (2020).
Ahammed, G. J. et al. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 259, 113957 (2020).
Zhang, L. et al. Production of transgenic Nicotiana sylvestris plants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. In Vitro Cell. Dev. Biol. 48(3), 275–282 (2012).
Zheng, X. et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 7(1), 1–12 (2017).
Tan, D. X. & Reiter, R. J. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin res. 2(1), 44–66 (2019).
Sharif, R. et al. Melatonin and its effects on plant systems. Molecules 23(9), 23–52 (2018).
Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 105(2), 376–391 (2021).
Zhang, N. et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 7, 197 (2016).
Coskun, Y., Duran R.E. & Kilic S. Striking effects of melatonin on secondary metabolites produced by callus culture of rosemary (Rosmarinus officinalis L.). Plant Cell Tissue Organ Cult. 138(1), 89–95 (2019).
Ahammed, G. J. et al. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 265, 109205 (2020).
Khodamoradi, S. et al. Changes in phenolic compounds production as a defensive mechanism against hydrogen sulfide pollution in Scrophularia striata. Plant Physiol. Biochem. 177, 23–31 (2022).
Sagharyan, M. et al. Optimization of callus induction with enhancing production of phenolic compounds production and antioxidants activity in callus cultures of Nepeta binaloudensis Jamzad (Lamiaceae). Iran. J. Biotechnol. 18(4), e2621 (2020).
Zafari, S. et al. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. PPB. 99, 11–20 (2016).
Shahkarami, P. et al. Piriformospora indica induces phenylethanoid glycosides production and defense responses in Scrophularia striata cell culture. Plant Cell Tissue Organ Cult. 149, 381–395 (2022).
Hernández-Ruiz, J. & Arnao, M. B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. J. Agron. 8(4), 33 (2018).
Khan, M. I. R. et al. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462 (2015).
Wang, Y., Reiter, R. J. & Chan, Z. Phytomelatonin: a universal abiotic stress regulator. J. Exp. Bot. 69(5), 963–974 (2018).
Fuss, E. Lignans in plant cell and organ cultures: an overview. Phytochem. Rev. 2(3), 307–320 (2003).
Suzuki, S. & Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 53(4), 273–284 (2007).
Pardo-Hernández, M., López-Delacalle, M. & Rivero, R. M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 9(11), 1078 (2020).
He, H. & He, L. F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant. 170(2), 218–226 (2020).
Wang, L. et al. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54(1), 19–27 (2016).
Bidabadi, S. S., VanderWeide, J. & Sabbatini, P. Exogenous melatonin improves glutathione content, redox state and increases essential oil production in two Salvia species under drought stress. Sci. Rep. 10(1), 1–12 (2020).
Posmyk, M., Kontek, R. & Janas, K. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf. 72(2), 596–602 (2009).
Shi, H. et al. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 59(1), 102–108 (2015).
Aghdam, M. S. et al. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Technol. 148, 184–191 (2019).
Kim, T. Y., Jo, M. H. & Hong, J. H. Protective effect of nitric oxide against oxidative stress under UV-B radiation in maize leaves. Int. J. Environ. Sci. 19(12), 1323–1334 (2010).
Altaf, M. A. et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 11(2), 309 (2022).
Kaur, H. et al. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 10(11), e1049788 (2015).
Marchiosi, R. et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 19(4), 865–906 (2020).
Duran, R. E., Kilic, S. & Coskun, Y. Melatonin influence on in vitro callus induction and phenolic compound production in sweet basil (Ocimum basilicum L.). In Vitro Cell. Dev. Biol. 55(4), 468–475 (2019).
Overmyer, K., Vuorinen, K. & Brosché, M. Interaction points in plant stress signaling pathways. Physiol. Plant. 162(2), 191–204 (2018).
Tashackori, H. et al. Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta 248(5), 1289–1306 (2018).
Yousefzadi, M. et al. Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol. Lett. 32(11), 1739–1743 (2010).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15(3), 473–497 (1962).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151(1), 59–66 (2000).
Kaur, G. et al. Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10(9), e0138713 (2015).
Stewart, R. R. & Bewley, J. D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 65(2), 245–248 (1980).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976).
Cakmak, I. & Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98(4), 1222–1227 (1992).
Pandolfini, T., Gabbrielli, R. & Comparini, C. Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L.. Plant Cell Environ. 15(6), 719–725 (1992).
Beaudoin-Eagan, L. D. & Thorpe, T. A. Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 78(3), 438–441 (1985).
Owen, R. et al. Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem. Toxicol. 41(5), 703–717 (2003).
Tashackori, H. et al. Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tissue Organ Cult. 127(1), 187–194 (2016).
Keinänen, M., Oldham, N. J. & Baldwin, I. T. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J. Agric. Food Chem. 49(8), 3553–3558 (2001).
Ahmadian-Chashmi, N. et al. Analysis of 6-methoxy podophyllotoxin and podophyllotoxin in hairy root cultures of Linum album Kotschy ex Boiss. MCRE. 22(2), 745–752 (2013).
Acknowledgements
The authors are grateful to Tarbiat Modares University (Tehran, Iran) for their financial support and laboratory facilities. This research is also supported by the Iranian Biotechnology Development Council, grant number 980302.
Author information
Authors and Affiliations
Contributions
S.E. performed the experiments, analyzed data and wrote the first draft. M. Sharifi designed and supervised the study. F.G. and B.M.S. were the study advisors. E.S. advised the study and edited the final manuscript. M. Sagharyan analyzed data and wrote the first and final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esmaeili, S., Sharifi, M., Ghanati, F. et al. Exogenous melatonin induces phenolic compounds production in Linum album cells by altering nitric oxide and salicylic acid. Sci Rep 13, 4158 (2023). https://doi.org/10.1038/s41598-023-30954-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30954-9
This article is cited by
-
Melatonin as a Key Factor for Regulating and Relieving Abiotic Stresses in Harmony with Phytohormones in Horticultural Plants — a Review
Journal of Soil Science and Plant Nutrition (2024)
-
Exogenous Melatonin Alleviates Cadmium Toxicity in Wheat (Triticum turgidum L.) by Modulating Endogenous Nitric Oxide and Hydrogen Sulfide Metabolism
Journal of Soil Science and Plant Nutrition (2024)
-
Impact of phloroglucinol and indoleamines on growth and accumulation of podophyllotoxin in the roots of Hyptis suaveolens (L.) Poit
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Molecular basis of salicylic acid–phytohormone crosstalk in regulating stress tolerance in plants
Brazilian Journal of Botany (2024)
-
Crosstalk between melatonin and nitric oxide restrains Cadmium-induced oxidative stress and enhances vinblastine biosynthesis in Catharanthus roseus (L) G Don.
Plant Cell Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.