Abstract
We sought to investigate the association between optic nerve head (ONH)/choroidal microvasculature perfusion and optic disc hemorrhage (ODH) in eyes with primary open-angle glaucoma (POAG) using swept-source optical coherence tomography angiography (SS-OCTA). A total of 266 POAG eyes (59 with a single instance of ODH, 40 with a history of recurrent ODH, and 167 eyes without ODH) with a mean follow-up of 5.4 years were included. Intradisc vessel density (VD), parapapillary choroidal VD, optic disc microvascular dropout (MvD), and choroidal microvascular dropout (CMvD), were evaluated on a 3 × 3 mm SS-OCTA image of ONH and compared between eyes with and without ODH. Recurrent ODH was defined as occurrence 1 year after first ODH detection during the total follow-up period. Logistic regression analyses were performed to investigate factors associated with ODH. The prevalence of CMvD, optic disc MvD, and β-parapapillary atrophy were not different among the no ODH, single ODH, and recurrent ODH groups. Eyes with ODH had lower intradisc VDs than those without ODH (P = 0.021), but no difference was found in intradisc VDs between the single and recurrent ODH groups (P = 0.977). Better VF MD at baseline (odds ratio [OR], 1.150; 95% confidence interval [CI], 1.055–1.254; P = 0.002) and lower intradisc VD (OR, 0.863; 95% CI, 0.812–0.918; P < 0.001) were associated with ODH occurrence. Among POAG eyes, those with ODH had lower intradisc VDs than those without ODH. POAG eyes in an earlier disease stage or those with lower intradisc VDs should be monitored for the possibility of ODH occurrence.
Similar content being viewed by others
Introduction
Primary open-angle glaucoma (POAG) is a chronic progressive optic neuropathy characterized by the loss of retinal ganglion cells and their axons, leading to visual field (VF) defects1. Although increased intraocular pressure (IOP) has been noted as the predominant risk factor for its development and progression, the potential role of reduced optic nerve head (ONH) perfusion has also been continuously investigated2,3,4. Recently, several studies using optical coherence tomography angiography (OCTA) have revealed the regional association between microvascular dropout (MvD) in the deep parapapillary layer, retinal nerve fiber layer (RNFL) defects, and decreased peripapillary vascular density (VD) in glaucomatous eyes, suggesting that microvascular changes may influence the glaucoma pathogenesis5,6,7.
Optic disc hemorrhage (ODH) is a well-known risk factor for glaucoma development and disease progression8,9,10. Recent studies have reported an association of ODH and vascular pathogenesis in glaucoma using OCTA. The prevalence of choroidal microvascular dropout (CMvD) was significantly higher in POAG eyes with ODH compared to POAG eyes without ODH11,12. Moreover, a topographic correlation has been found between CMvD and ODH location in glaucomatous ONH11,12. These findings suggest that both CMvD and ODH may share the vascular pathogenesis of glaucomatous damage. We hypothesized that ODH may be associated with the microvascular loss in ONH along with that in the parapapillary choroid represented as CMvD.
In the present study, we used swept-source (SS)-OCTA, which has the advantages of deeper penetration and higher resolution13 to assess microvascular perfusion in the ONH region of POAG eyes with and without ODH during follow-up, and we evaluated the association between ODH and microvascular changes within the optic disc.
Methods
We conducted a retrospective review of electronic medical records of patients diagnosed with POAG who underwent SS-OCTA examination at the glaucoma clinic of Asan Medical Center from May 2020 to June 2021. The study protocol and the waiver of written informed consent were approved by the institutional Review Board of Asan Medical Center and this study adhered to the tenets of the Declaration of Helsinki.
Participants
All participants underwent comprehensive ophthalmic examinations, including slit-lamp biomicroscopy, IOP measurement with Goldmann applanation tonometry, gonioscopy, refraction error measurement, central corneal thickness assessment with ultrasonic pachymetry (SP-3000; Tomey, Nagoya, Japan), dilated color fundus photography (Canon, Tokyo, Japan), ONH stereoscopic photography/red-free RNFL photography (Canon), and axial length measurement with the IOLMaster (Carl Zeiss Meditec Inc., Dublin, CA, USA), respectively. Patients were also examined using the Cirrus HD spectral-domain OCT system (Carl Zeiss Meditec Inc.), Humphrey field analyzer with Swedish Interactive Threshold Algorithm 24–2 VF testing (Carl Zeiss Meditec Inc.), and PLEX Elite 9000 version 1.7 SS-OCTA system (Carl Zeiss Meditec Inc.). IOP was measured at each visit, and the mean IOP was defined as the average of all IOP measurements during follow-up. Clinical history was collected from participants, including demographic characteristics and the presence of cold extremities, migraine, and other systemic conditions.
For inclusion in the current study, all patients had to meet the following criteria: (1) age > 18 years; (2) presence of open-angle glaucoma by gonioscopic examination; (3) glaucomatous optic nerve damage (i.e., neuroretinal rim thinning, notching, and/or RNFL defect); (4) glaucomatous VF defect defined by Anderson’s criteria (i.e., three or more adjacent points on a pattern deviation probability map with P < 0.05 and one abnormal point with P < 0.01, a Glaucoma Hemifield Test result outside the normal limits, or a pattern standard deviation of P < 0.05 confirmed on 2 consecutive reliable VF tests)14 in ≥ 2 reliable tests (fixation loss rate < 20% and false-positive and false-negative error rates < 15%) corresponding to glaucomatous structural damage; and (5) > 3 years of follow-up with regular OCT and VF exams at 6-month intervals.
Exclusion criteria were as follows: (1) eyes with spherical refraction < − 6.0 diopters (D) or ≥ + 3.0 D and cylinder correction < − 3.0 D or ≥ + 3.0 D; (2) eyes with other intraocular or neurologic diseases that affected RNFL thickness measurements; and (3) eyes with any history of intraocular surgery other than simple cataract surgery. If both eyes of a patient met the criteria, the right eye was included.
Assessment of optic disc hemorrhage
Each ODH was evaluated using ONH stereoscopic photographs taken during the study follow-up period by two glaucoma experts (K.R.S. and J.M.K.) independently who were blinded to each patient's clinical information and VF results. The no ODH group was defined as including eyes without ODH during the whole follow-up period. The single ODH group was defined as that with eyes in which ODH only occurred once, while the recurrent ODH group was defined as that with eyes with ≥ 1 ODHs within 1 year after the initial ODH. If eyes experienced multiple ODHs at the same time, but no recurrence was found later on, these eyes were included in the single ODH group.
SS-OCTA examination
All subjects underwent SS-OCTA, which has an eye-tracking system, using the FastTrac motion correction software. It operates at a 1060-nm central wavelength and its scan speed is 100,000 A-scans per second, with an axial resolution of 6.3 µm and a transverse resolution of 20 µm in tissue to acquire volumetric scans. Its A-scan depth-penetration in tissue was 3.0 mm, and the machine included an enhanced-depth imaging function. Only qualified and reliable images were included, and those with a signal strength index of < 8, representing poor scan quality, motion artifacts, misalignment, or irregular optic disc boundaries, were excluded.
The methods of identifying parapapillary choroidal VD, intradisc VD, and whole-image VD (wiVD) have been previously introduced15,16. An 8-bit binary slab was generated based on the mean threshold algorithm of the ImageJ software (version 1.52; Wayne Rasband, National Institutes of Health, Bethesda, MD, USA), which automatically measured the threshold value as the average of the local grayscale distribution. After assigning white pixels to vessels and black pixels to the background, respectively, VDs were calculated as a percentage of vessel pixels within the ONH and β-parapapillary atrophy (β-PPA) area.
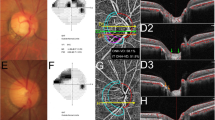
Parapapillary choroidal VD was assessed using a 3.0 × 3.0 mm ONH choroidal layer en-face image automatically generated by layer segmentation of signals from the retinal pigment epithelium to the inner border of the sclera. To measure parapapillary choroidal VD within the β-PPA zone, the margins of the β-PPA zone were manually defined, while excluding the large projecting vessels from the overlying retinal layer within the β-PPA zone on en-face scanning laser ophthalmoscopy images using ImageJ software (Fig. 1A–B, yellow line)15,16,17.
Measurements of parapapillary choroidal vessel density (VD) and intradisc VD and confirmation of the presence of choroidal microvascular dropout (CMvD), and optic disc microvascular dropout (MvD) in a primary open-angle glaucoma (POAG) eye with CMvD using swept-source optical coherence tomography angiography (SS-OCTA). (A) The blue ellipse line shows the optic nerve head (ONH) margin. The yellow outline indicates the β-parapapillary atrophy (β-PPA) margins, excluding large retinal vessels, which are applied to the SS-OCTA choroidal layer en-face image. The selected area of interest, excluding large retinal vessels, within the β-PPA was converted to a region of interest (ROI), (B) then applied to the 8-bit binary slab of an SS-OCTA choroidal layer en-face image. (C) The ROI within the ONH, excluding large retinal vessels, was manually demarcated on a whole-signal mode ONH en-face image. (D) These areas of interest were used to measure the intradisc VD (orange outline) using the ImageJ software. (E) The presence of CMvD (green arrow) and optic disc MvD (yellow arrow) was detected (F) using a horizontal B-scan that showed loss of microvasculature signal in the prelaminar tissue and anterior portion of lamina cribrosa.
Intradisc VD was evaluated using whole-signal mode ONH en-face images, which were automatically generated by signals below the inner limiting membrane. The boundary of the optic disc was manually delineated as the inner margin of the peripapillary scleral ring which was identified using scanning laser ophthalmology images, and then was applied to the same position of ONH en-face images using the ImageJ software. The temporal side of the vertical line passing across the optic disc center was used for the analysis because the deep intradisc microvasculature at the nasal side of the optic disc was difficult to visualize using OCTA due to large vessels and thick neuroretinal rim (Fig. 1C,D, orange line)18,19,20.
Average wiVD was assessed using a superficial slab of the ONH en-face image, extending from the inner limiting membrane to the posterior boundary of the inner plexiform layer. For the measurement of wiVD, the circumpapillary large vessels and optic disc margin were defined manually, then were excluded using ImageJ software.
The CMvD was defined as a focal sectoral capillary dropout with a circumferential width of > 200 µm or the width of the central retinal vein, with no visible microvascular network identified on the choroidal en-face images7,21. Optic disc MvD also was defined as a complete loss of the OCTA signal within the ONH, which is ≥ 200 µm in width and ≥ 100 µm in length18. For the evaluation of an optic disc MvD, visualization of the anterior lamina cribrosa (LC) was ensured in all horizontal B-scan images in order to verify the complete microvasculature loss throughout the deep layer of the ONH (Fig. 1E,F). Poor visibility of the LC was defined as < 70% visibility of the anterior LC surface within the Bruch's membrane opening7,18. If the anterior LC portion was poorly visualized at the area with complete OCTA signal loss in the horizontal B-scan images, the eyes were excluded from further analyses18. Two independent observers (J.Y.L. and J.W.S.), who were blinded to the clinical, VF, and RNFL details of the patients, identified MvDs. Disagreements between the observers were resolved by a third adjudicator (K.R.S.).
Statistical analysis
Baseline characteristics are expressed using mean ± standard deviation values. The demographics and clinical characteristics were compared among the no ODH, single ODH, and recurrent ODH groups. For continuous variables, one-way analysis of variance with post-hoc tests (Tukey’s or Dunnett T3 methods) was used for the comparison of subgroups, as appropriate. For categorical data, chi-squared tests were performed. The kappa (κ) coefficient was calculated to evaluate inter-observer agreement in determining CMvD and intradisc MvD, respectively. Odds ratios (ORs) were calculated for the association between putative clinical factors, including OCTA-driven parameters and ODH using logistic regression analysis. Variables with P < 0.10 by univariate analyses were included in the multivariate models. To develop the final multivariate model, a backward elimination process was used and adjusted ORs with 95% confidence intervals (CIs) were calculated.
Results
A total of 301 eyes were initially included in the study. Thirty-five eyes (11.6%) with poor-quality OCTA images were excluded because the ONH margin and intradisc VD quantification could not be clearly depicted by the software. Finally, 59 POAG eyes with single ODH, 40 POAG eyes with recurrent ODH, and 167 POAG eyes without ODH during the mean follow-up period of 5.4 years were included. Inter-examiner agreements regarding the determination of the presence of CMvD and intradisc MvD were excellent (k = 0.910, P < 0.001; k = 0.904, P < 0.001, respectively).
The demographics and baseline clinical characteristics of subjects were compared among the no ODH, single ODH, and recurrent ODH groups in Table 1. There were no significant differences among the three groups in age, sex, central corneal thickness, axial length, baseline IOP, and mean and peak IOPs during follow-up. However, no ODH group showed lower baseline VF mean deviation (MD) and thinner baseline RNFL thickness compared to the ODH group (all P < 0.05). The time between the detection of ODH in photographs and OCTA imaging was 2.2 ± 2.4 years and 3.4 ± 2.3 years in the single and recurrent ODH groups, respectively, with no significant difference between the groups (P = 0.288).
At the time of OCTA imaging, the three groups (no ODH, single ODH, and recurrent ODH) showed similar degrees of glaucomatous damage, without a significant difference in VF MD or RNFL thickness (Table 2). The proportions of CMvD, optic disc MvD, and β-PPA were not different among them. Further, parapapillary choroidal VD and wiVD did not differ among the 3 groups. However, eyes with ODH had significantly lower intradisc VDs than those without ODH (37.29 ± 6.01% vs. 39.49 ± 6.11%; P = 0.021) despite their similar degrees of glaucoma severity. The single and recurrent ODH groups did not show a significant difference in intradisc VD.
Table 3 presents clinical variables associated with the occurrence of ODH using univariate and multivariate analyses. In the univariate analysis, earlier glaucoma stages represented by functional and structural parameters (VF MD [OR, 1.096; 95% CI, 1.043–1.151; P < 0.001] and global RNFL thickness [OR, 1.045; 95% CI, 1.023–1.068; P < 0.001]) and lower intradisc VD (OR, 0.939; 95% CI, 0.900–0.980; P = 0.004) showed associations with ODH occurrence. After multivariate analysis, a better VF MD at baseline (OR, 1.150; 95% CI, 1.055–1.254; P = 0.002) and lower intradisc VD (OR, 0.863; 95% CI, 0.812–0.918; P < 0.001) were independently associated with ODH occurrence.
We analyzed clinical factors associated with the frequency of ODH during follow-up using logistic regression analyses (Table 4). There was no difference in the VD of ONH evaluated by SS-OCTA between eyes with single and recurrent ODH. The frequency of ODHs during follow-up was only significantly associated with the baseline earlier stage of structural change in POAG eyes (global RNFL thickness, OR, 1.066; 95% CI, 1.019–1.116; P = 0.006).
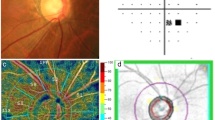
Figure 2 presents reduced intradisc VD in eyes with ODH compared to those without ODH. A left eye of a 65-year-old female patient with POAG without ODH during follow-up showed a superior hemifield scotoma with a VF MD of − 9.19 dB at baseline and an intradisc VD of 40.12%. In comparison, a 64-year-old male patient with OAG in his left eye and single ODH during follow-up and a 66-year-old male patient with OAG in his left eye and recurrent ODH during follow-up, matched for age and baseline VF severity, presented with relatively lower intradisc VDs (37.01% and 37.89%, respectively).
Representative primary open-angle glaucoma eyes with and without optic disc hemorrhage (ODH). (A) A 65-year-old female patient without ODH during follow-up in her left eye. (B) A 64-year-old male patient with a single ODH in his left eye. (C) A 66-year-old male patient with recurrent ODH during follow-up in his left eye. All these eyes presented with choroidal microvascular dropout (CMvD) in the inferotemporal area and superior visual field (VF) loss with similar degrees of damage represented by VF mean deviation (MD). On an optical coherence tomography angiography (OCTA) whole-signal mode en-face image of the optic nerve head (ONH), the blue ellipse indicates the ONH margin and the blue vertical line denotes boundaries of vessel density (VD) measurement areas within the ONH passing across the optic disc center. The boundaries of the ONH (orange outline), excluding large retinal vessels, were manually demarcated. These areas of interest were used to measure the intradisc VD. The intradisc VDs were 37.01% and 37.89% in eyes with single ODH and recurrent ODH, respectively, which were lower than that in an eye without ODH (40.12%).
Discussion
We evaluated the association between ODH and intradisc VD in POAG eyes. On SS-OCTA scans, the intradisc VD measurements were significantly lower in the ODH group than the no ODH group, whereas the parapapillary choroidal VD and wiVD values were similar between the two groups. Moreover, the occurrence of ODH was significantly associated with a lower intradisc VD based in multivariate analyses. These findings suggest that eyes with ODH may have association with the microvasculature loss within the ONH region.
Despite the correlation between ODH and faster glaucoma progression22,23,24,25, the pathogenesis of ODH remains unclear. Recently, OCTA has been widely used to overcome the difficulty of exploring the ONH vasculature in glaucomatous eyes with ODH, and studies have reported decreased ONH-related vessel densities in them. Rao et al. reported that there was no difference in the whole en-face VD of ONH evaluated by spectral-domain OCTA between eyes with and without ODH in the early stage of glaucoma (VF MD = − 3.8 dB)26. The association between the presence of ODH history and reduced parapapillary VD in patients with normal-tension glaucoma was supported by Nitta et al.27 and Okamoto et al.28 Our results showed that the occurrence of ODH was related to lower intradisc VD in eyes with POAG. The underlying pathogenesis may not be fully elucidated in the present study, but we speculate the following scenario. Since ONH perfusion can be dependent on adjacent parapapillary choroidal circulation29,30, the presence of CMvD may indicate reduced ocular perfusion. Also, the strong spatial correlation between CMvD and optic disc MvD has been reported by previous studies18,31. Such data may partly explain the ODH group having a higher rate of patients with CMvD and lower intradisc VD.
Our study found that parapapillary choroidal VD and wiVD measurements did not differ between eyes with and without ODH. As the parapapillary choroid is closely related to ONH perfusion, insufficient blood flow within the parapapillary choroid can result in decreased blood flow to the ONH and glaucomatous damage32. In addition, diminished blood flow to the parapapillary choroid (i.e., CMvD) was associated with a great degree of glaucomatous damage33. Because eyes with and without ODH had similar glaucoma severities at the time of SS-OCTA imaging, the parapapillary choroidal VD might not have shown a difference between the two groups. However, this requires careful interpretation due to the transient nature of ODH because ODH may have occurred and been absorbed before or in the middle of CMvD and optic disc MvD development during follow-up.
In the current study, recurrent ODH occurred in 40.4% (40/99) of ODH eyes during 5.4 years. As ODH is known to precede glaucomatous changes34, recurrent ODH may be associated with more extensive glaucomatous changes. Ishida et al.35 reported that their recurrent ODH group showed more progressive VF changes than the single ODH group after a mean follow-up period of 5.6 years. We speculated that optic disc perfusion, as represented by the intradisc VD, might be more decreased in the recurrent ODH group. However, we were unable to demonstrate any significant difference between the single ODH and recurrent ODH groups with regard to intradisc VD, parapapillary choroidal VD, and wiVD. This may be due to the different mechanism among various types of ODH or due to the similar degree of glaucoma severity at the time of SS-OCTA imaging. Further studies are needed to clarify speculations about the mechanism and clinical significance of recurrent ODH in POAG patients.
Kurvinen et al. evaluated peripapillary retinal blood flow at the time of ODH detection and 6 months later using scanning laser Doppler flowmetry36. They found that reduced flow at the time of ODH and increased flow after resorption supported the vascular etiology of ODH. Park et al. compared eyes with ODH at the border of localized RNFL defects and those with ODH not related to localized RNFL defects using disc fluorescein angiography37 and found prolonged arteriovenous transit time and vessel filling defects or delayed filling in eyes with ODH accompanying RNFL defects, implying blood flow stasis at the disc margin proximal to where ODH occurred. In our study, whether reduced intradisc VD is the result or cause of ODH is unclear; we only acquired SS-OCTA images during follow-up after the technology was introduced in 2020. Nonetheless, despite OCTA’s inherent limitations in accurately representing the nature of vascular changes, we speculate that capillary occlusion and resultant hemorrhage may have led to the reduction of intradisc VD or blood flow, and subsequent stasis could have caused ODH. To evaluate the temporal relationship between parapapillary choroidal VD/intradisc VD/RNFLT changes and ODH detection, prospective studies with baseline SS-OCTA imaging are required.
Our finding is in agreement with previous studies38,39 in which the frequency of ODH increased from an early to moderate stage (i.e., higher VF MD value at baseline) and decreased with progression toward an advanced stage of glaucoma in eyes in which ODH was no longer detected, consistent with prior research that found that ODH was detected more frequently in early to moderate glaucoma than advanced glaucoma39,40.
It is possible that ODH and lower intradisc VD may be a marker for higher susceptibility to glaucoma progression, but their association with further neural damage remains unclear. In fact, a previous study did not reveal a difference in the incidence of hemorrhages between treated and untreated groups over time41. A recent report showed that localized VF defects were found to occur prior to ODH and to continue after the event22. In the present study, the ODH group showed less advanced disease stage at baseline compared to non-ODH group. However, at the time of OCTA imaging after similar duration of follow-up period, the ODH group showed a similar degree of disease severity. This observation suggests that ODH and/or reduced intradisc VD may be both a result of progressive glaucomatous damage and an indicator of possible subsequent progression.
The following limitations should be considered when interpreting our results. First, patients assigned to the no ODH group could have had ODH in the past when they were not monitored or in the period between study visits. Therefore, the number of ODHs may have been underestimated in some patients. To reduce this, we included only patients who had been regularly followed up for ≥ 3 years with optic disc photographs taken every 6 months. Second, factors associated with hypertension (e.g., blood pressure and use of antihypertensive medication) could have affected ODH or VD measurements. Although no difference in the proportion of patients with hypertension was found between the groups with and without ODH, further studies that include hypertension data are warranted. Third, the OCTA algorithm includes large vessels along the capillaries within ONH in the estimation of VD. To minimize the potential effects of artifacts, such as large retinal vessels, neuroretinal rim shadowing, and projections, only images with well-visualized optic disc margins and anterior LC in the horizontal B-scans that detected complete loss of a microvascular structure were included. Our results may not be generalizable to settings that use different OCT/OCTA devices; however, we used SS-OCTA as it is able to clearly visualize the intradisc microvasculature with deeper penetration and higher resolution than other approaches42,43. Lastly, the β-PPA area has been associated not only with the severity of glaucoma but also with the visibility and density of parapapillary choroidal vessels15,16. Although areal measurements were not conducted in this study, we speculated that estimation of parapapillary choroidal VD better reflected ischemic insults within the β-PPA than did β-PPA area itself. Additionally, the density of parapapillary choroidal vessels and the presence of CMvD were not significantly different between the groups with and without ODH.
In conclusion, POAG eyes with ODH showed reduced intradisc VD compared to those without ODH when measured by SS-OCTA. Eyes with POAG in an earlier disease stage or those with lower intradisc VDs should be monitored for the possibility of ODH occurrence.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
References
Miki, A. et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 121, 1350–1358. https://doi.org/10.1016/j.ophtha.2014.01.017 (2014).
Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 21, 359–393. https://doi.org/10.1016/s1350-9462(02)00008-3 (2002).
Leske, M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114, 1965–1972. https://doi.org/10.1016/j.ophtha.2007.03.016 (2007).
Leske, M. C. et al. Risk factors for incident open-angle glaucoma: The barbados eye studies. Ophthalmology 115, 85–93. https://doi.org/10.1016/j.ophtha.2007.03.017 (2008).
Lee, E. J., Lee, S. H., Kim, J. A. & Kim, T. W. Parapapillary deep-layer microvasculature dropout in glaucoma: Topographic association with glaucomatous damage. Invest. Ophthalmol. Vis. Sci. 58, 3004–3010. https://doi.org/10.1167/iovs.17-21918 (2017).
Lee, J. Y., Shin, J. W., Song, M. K., Hong, J. W. & Kook, M. S. An increased choroidal microvasculature dropout size is associated with progressive visual field loss in open-angle glaucoma. Am. J. Ophthalmol. 223, 205–219. https://doi.org/10.1016/j.ajo.2020.10.018 (2021).
Suh, M. H. et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology 123, 2509–2518. https://doi.org/10.1016/j.ophtha.2016.09.002 (2016).
De Moraes, C. G. et al. Rate of visual field progression in eyes with optic disc hemorrhages in the ocular hypertension treatment study. Arch. Ophthalmol. 130, 1541–1546. https://doi.org/10.1001/jamaophthalmol.2013.1137 (2012).
Drance, S., Anderson, D. R., Schulzer, M. & Collaborative Normal-Tension Glaucoma Study, G. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 131, 699–708. https://doi.org/10.1016/s0002-9394(01)00964-3 (2001).
Ernest, P. J. et al. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 120, 512–519. https://doi.org/10.1016/j.ophtha.2012.09.005 (2013).
Rao, H. L. et al. Choroidal microvascular dropout in primary open-angle glaucoma eyes with disc hemorrhage. J. Glaucoma 28, 181–187. https://doi.org/10.1097/IJG.0000000000001173 (2019).
Park, H. L., Kim, J. W. & Park, C. K. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125, 1003–1013. https://doi.org/10.1016/j.ophtha.2018.01.016 (2018).
Suh, M. H., Jung, D. H., Weinreb, R. N. & Zangwill, L. M. Optic disc microvasculature dropout in glaucoma detected by swept-source optical coherence tomography angiography. Am. J. Ophthalmol. 236, 261–270. https://doi.org/10.1016/j.ajo.2021.10.029 (2022).
Anderson, D. R. Automated static perimetry. (Mosby Year Book, 1992).
Park, H. Y. L., Shin, D. Y., Jeon, S. J. & Park, C. K. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual field progression in patients with glaucoma. Jama Ophthalmol. 137, 681–688. https://doi.org/10.1001/jamaophthalmol.2019.0422 (2019).
Jo, Y. H., Shin, J. W., Song, M. K., Won, H. J. & Kook, M. S. Choroidal microvasculature dropout is associated with generalized choroidal vessel loss within the beta-parapapillary atrophy in glaucoma. Am. J. Ophthalmol. 215, 37–48. https://doi.org/10.1016/j.ajo.2020.03.009 (2020).
Jonas, J. B. Clinical implications of peripapillary atrophy in glaucoma. Curr. Opin. Ophthalmol. 16, 84–88. https://doi.org/10.1097/01.icu.0000156135.20570.30 (2005).
Akagi, T. et al. Optic disc microvasculature dropout in primary open-angle glaucoma measured with optical coherence tomography angiography. PLoS ONE 13, e0201729. https://doi.org/10.1371/journal.pone.0201729 (2018).
Hasegawa, T. et al. Structural dissociation of optic disc margin components with optic disc tilting: A spectral domain optical coherence tomography study. Graef. Arch. Clin. Exp. 254, 343–349. https://doi.org/10.1007/s00417-015-3210-0 (2016).
Kimura, Y. et al. Lamina cribrosa defects and optic disc morphology in primary open angle glaucoma with high myopia. PLoS ONE https://doi.org/10.1371/journal.pone.0115313 (2014).
Kwon, J. M., Weinreb, R. N., Zangwill, L. M. & Suh, M. H. Parapapillary deep-layer microvasculature dropout and visual field progression in glaucoma. Am. J. Ophthalmol. 200, 65–75. https://doi.org/10.1016/j.ajo.2018.12.007 (2019).
De Moraes, C. G. V. et al. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest. Ophth. Vis. Sci. 50, 4727–4733. https://doi.org/10.1167/iovs.09-3446 (2009).
Gracitelli, C. P. et al. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS ONE 9, e105611. https://doi.org/10.1371/journal.pone.0105611 (2014).
Medeiros, F. A. et al. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology 117, 2061–2066. https://doi.org/10.1016/j.ophtha.2010.02.015 (2010).
Pradhan, Z. S. et al. Progressive vessel density reduction on OCT angiography in glaucoma eyes with disc hemorrhages. Ophthalmol. Glaucoma 5, 421–427. https://doi.org/10.1016/j.ogla.2021.11.001 (2022).
Rao, H. L. et al. Optical coherence tomography angiography vessel density measurements in eyes with primary open-angle glaucoma and disc hemorrhage. J. Glaucoma 26, 888–895. https://doi.org/10.1097/IJG.0000000000000758 (2017).
Nitta, K., Sugiyama, K., Wajima, R., Tachibana, G. & Yamada, Y. Associations between changes in radial peripapillary capillaries and occurrence of disc hemorrhage in normal-tension glaucoma. Graef. Arch. Clin. Exp. 257, 1963–1970. https://doi.org/10.1007/s00417-019-04382-3 (2019).
Okamoto, Y. et al. Longitudinal changes in superficial microvasculature in glaucomatous retinal nerve fiber layer defects after disc hemorrhage. Sci. Rep. 10, 22058. https://doi.org/10.1038/s41598-020-79151-y (2020).
Yamazaki, S., Inoue, Y. & Yoshikawa, K. Peripapillary fluorescein angiographic findings in primary open angle glaucoma. Br. J. Ophthalmol. 80, 812–817. https://doi.org/10.1136/bjo.80.9.812 (1996).
Hayreh, S. S. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br. J. Ophthalmol. 53, 721–748. https://doi.org/10.1136/bjo.53.11.721 (1969).
Song, M. K., Shin, J. W., Lee, J. Y., Hong, J. W. & Kook, M. S. Choroidal microvasculature dropout is spatially associated with optic nerve head microvasculature loss in open-angle glaucoma. Sci. Rep. 11, 15181. https://doi.org/10.1038/s41598-021-94755-8 (2021).
Yin, Z. Q., Millar, T. J., Beaumont, P. & Sarks, S. Widespread choroidal insufficiency in primary open-angle glaucoma. J. Glaucoma 6, 23–32 (1997).
Jo, Y. H., Kwon, J., Shon, K., Jeong, D. & Kook, M. S. Greater severity of glaucomatous damage in eyes with than without choroidal microvasculature dropout in open-angle glaucoma. Invest. Ophth. Vis. Sci. 60, 901–912. https://doi.org/10.1167/iovs.18-26298 (2019).
Siegner, S. W. & Netland, P. A. Optic disc hemorrhages and progression of glaucoma. Ophthalmology 103, 1014–1024. https://doi.org/10.1016/s0161-6420(96)30572-1 (1996).
Ishida, K., Yamamoto, T., Sugiyama, K. & Kitazawa, Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am. J. Ophthalmol. 129, 707–714. https://doi.org/10.1016/s0002-9394(00)00441-4 (2000).
Kurvinen, L., Harju, M., Saari, J. & Vesti, E. Altered temporal peripapillary retinal flow in patients with disc hemorrhages. Graefes Arch. Clin. Exp. Ophthalmol. 248, 1771–1775. https://doi.org/10.1007/s00417-010-1441-7 (2010).
Park, H. Y., Jeong, H. J., Kim, Y. H. & Park, C. K. Optic disc hemorrhage is related to various hemodynamic findings by disc angiography. PLoS ONE 10, e0120000. https://doi.org/10.1371/journal.pone.0120000 (2015).
Jonas, J. B., Martus, P., Budde, W. M. & Hayler, J. Morphologic predictive factors for development of optic disc hemorrhages in glaucoma. Invest. Ophthalmol. Vis. Sci. 43, 2956–2961 (2002).
Jonas, J. B. & Xu, L. Optic disk hemorrhages in glaucoma. Am. J. Ophthalmol. 118, 1–8. https://doi.org/10.1016/s0002-9394(14)72835-1 (1994).
Ozturker, Z. K., Munro, K. & Gupta, N. Optic disc hemorrhages in glaucoma and common clinical features. Can. J. Ophthalmol. 52, 583–591. https://doi.org/10.1016/j.jcjo.2017.04.011 (2017).
Bengtsson, B., Leske, M. C., Yang, Z., Heijl, A. & Group, E. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 115, 2044–2048. https://doi.org/10.1016/j.ophtha.2008.05.031 (2008).
Akil, H. et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br. J. Ophthalmol. https://doi.org/10.1136/bjophthalmol-2016-309816 (2017).
Yoshikawa, Y. et al. Glaucomatous vertical vessel density asymmetry of the temporal raphe detected with optical coherence tomography angiography. Sci. Rep. 10, 6845. https://doi.org/10.1038/s41598-020-63931-7 (2020).
Funding
Kyung Rim Sung reports research support from Carl Zeiss Meditec. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Conception and study design: K.R.S., K.E.K., J.M.K., Data analysis: J.Y.L., K.R.S., J.W.S., K.E.K., J.M.K., First draft of manuscript: J.Y.L., K.R.S., J.W.S., K.E.K., J.M.K., Preparing Figures and Tables 1, 2, 3 and 4: J.Y.L., K.R.S., K.E.K. Revising and writing manuscript: J.Y.L., K.E.K., J.M.K., All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.Y., Sung, K.R., Shin, J.W. et al. Reduced intradisc vessel density is associated with optic disc hemorrhage in eyes with primary open-angle glaucoma. Sci Rep 13, 1281 (2023). https://doi.org/10.1038/s41598-023-28288-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28288-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.