Abstract
Laboratory and field-based studies of the invasive mosquito Aedes albopictus demonstrate its competency to transmit over twenty different pathogens linked to a broad range of vertebrate hosts. The vectorial capacity of Ae. albopictus to transmit these pathogens remains unclear, partly due to knowledge gaps regarding its feeding behavior. Blood meal analyses from field-captured specimens have shown vastly different feeding patterns, with a wide range of anthropophagy (human feeding) and host diversity. To address this knowledge gap, we asked whether differences in innate host preference may drive observed variation in Ae. albopictus feeding patterns in nature. Low generation colonies (F2–F4) were established with field-collected mosquitoes from three populations with high reported anthropophagy (Thailand, Cameroon, and Florida, USA) and three populations in the United States with low reported anthropophagy (New York, Maryland, and Virginia). The preference of these Ae. albopictus colonies for human versus non-human animal odor was assessed in a dual-port olfactometer along with control Ae. aegypti colonies already known to show divergent behavior in this assay. All Ae. albopictus colonies were less likely (p < 0.05) to choose the human-baited port than the anthropophilic Ae. aegypti control, instead behaving similarly to zoophilic Ae. aegypti. Our results suggest that variation in reported Ae. albopictus feeding patterns are not driven by differences in innate host preference, but may result from differences in host availability. This work is the first to compare Ae. albopictus and Ae. aegypti host preference directly and provides insight into differential vectorial capacity and human feeding risk.
Similar content being viewed by others
Introduction
Mosquito blood feeding behavior is a critical determinant of pathogen transmission. Some species have innate host preferences, actively choosing to feed on one host species or class over others1,2,3. Other mosquito species are host generalists, and exhibit little to no preference for particular host species or groups. Host preference plays a role in host contact rates, interacting with external factors, such as host availability, to influence feeding patterns in the field1,4,5. Feeding patterns, in turn, influence the probability of mosquito contact with infectious host reservoirs and onward transmission to susceptible hosts6,7.
Despite the important role of host preference in pathogen transmission, this trait is not well characterized for the tiger mosquito, Aedes albopictus, a highly invasive nuisance species with the potential to transmit over twenty pathogens that infect a range of vertebrate host species8,9. Several of these pathogens share another mosquito vector, Ae. aegypti, including dengue, Zika, and chikungunya viruses. In contrast to Ae. albopictus, Ae. aegypti host preference is well characterized. In its invasive range outside of Africa, Ae. aegypti is strongly anthropophilic, preferring the odor of humans over that of other host species10,11. Within its ancestral range in Africa, Ae. aegypti is more diverse and exhibits a range of host preferences, from zoophilic (non-human preferring) to anthropophilic (human preferring)11,12,13. Human-preferring populations in and out of Africa are genetically related, sharing common mutations in several chromosomal regions11, which provides additional support for the genetic underpinnings of human preference13.
Aedes albopictus host preference has only been assessed twice to date, despite its importance as a major vector and nuisance species. In Thailand, landing catches were performed, comparing attraction of wild mosquitoes to human, pig, buffalo, dog, and chicken14. In La Réunion, preference assays were conducted with human, cow, dog, goat, and chicken, measuring choice between human and each of the non-human animals by releasing mosquitoes in an enclosure and subsequently assessing host feeding rates15. The results of both studies indicate a preference for humans over the other animals tested. However, the results from the La Réunion study may have been influenced by host defenses15. Human subjects may have avoided defending themselves since they knew they were in a scientific study while the non-human animals would likely have exhibited typical host defense behaviors that may have impacted feeding success16. Note, however, host defenses were not addressed in the text15. In La Réunion, no-choice (single host) assays were also conducted, measuring feeding on human, pig, goat, cow, dog, duck, chicken, rat, chameleon, gecko, mouse, and shrew in the absence of other hosts15. Aedes albopictus fed readily on chicken, human, dog, and cow, and significantly less often on all other hosts assessed.
While little is known about Ae. albopictus host preference, there has been robust investigation of its feeding patterns. Feeding patterns are distinct from host preference in that patterns describe mosquito-host associations in nature and are influenced by environmental and biological parameters, whereas preference describes the innate tendency of a mosquito species to choose a certain host over others1. Aedes albopictus feeding patterns have been assessed across nineteen blood meal analysis studies from across the world, the results of which exhibit a remarkably diverse range of feeding (reviewed by Fikrig and Harrington, 20211). The percent of blood meals identified as human ranged from 3.9 to 100% and the number of host species identified ranged from three to fifteen. The cause of this striking variability in feeding patterns is unknown. Methodological differences, such as blood meal analysis and collection techniques may explain some of the differences. There were also likely differences in host availability. However, only three of these studies quantified host availability17,18,19, so it is impossible to retrospectively determine the extent to which external factors drove the differences in host usage. Another possibility is that Ae. albopictus populations vary in genetically-based host preference, similar to Ae. aegypti populations within Africa, thus driving divergent feeding patterns11.
Aedes albopictus has high levels of phenotypic variation for numerous traits, including diapause20, fecundity-size relationships21, competitive interactions21, larval growth rate22, and viral susceptibility23. It also has a large genome24 and substantial levels of genetic variation25,26,27, although the level of variation among populations is different in different parts of the world28. Aedes albopictus genetic variation has been shown to underpin phenotypic variation of several traits, including vector competence29 and diapause30,31,32. This trait variation has been credited for the impressive invasive potential of Ae. albopictus and its widespread establishment across a variety of climates and habitats33,34.
It is unclear whether such variation exists for host preference among Ae. albopictus populations; the two host preference studies conducted to date do not provide insight into the possibility that innate host preferences vary in relation to observed differences in feeding patterns. The first Ae. albopictus host preference study referenced above was conducted in Thailand14, where a blood meal analysis revealed that 100% of blood fed Ae. albopictus fed on humans, the most anthropophagic feeding pattern reported to date35. The other was conducted in La Réunion, which was the site of a chikungunya epidemic transmitted by Ae. albopictus, suggesting a high level of human feeding (although no blood-feeding pattern study has been performed to corroborate this)36,37. There has been no experimental assessment of host preference from locations where Ae. albopictus populations have lower levels of anthropophagy (human feeding), nor any comparison of host preference between discrete populations.
We investigated whether population-level variation in Ae. albopictus host preference drives the divergent feeding patterns reported around the world. Using a dual-port olfactometer to simultaneously present human and guinea pig odors, we measured the host preference of low-generation Ae. albopictus colonies derived from six populations around the world: three from populations with previously reported low levels of anthropophagy in the United States (New York17, Maryland38, and Virginia39), and three from populations with high levels of anthropophagy (Florida, USA40, Cameroon41, and Thailand35). We directly compared these colonies to anthropophilic and zoophilic Ae. aegypti colonies, thus also providing the first direct comparison of the host preferences of these two vector species.
Results
Using a dual-port olfactometer, we measured the host preference of Ae. albopictus colonies derived from three anthropophagic and three zoophagic populations, as well as one zoophilic and two anthropophilic Ae. aegypti colonies. Eight replicates were conducted across two separate experimental rounds. Additionally, in the second experimental round, we assessed biological replicate colonies of three Ae. albopictus populations (established from a site at least 1.5 km away from the primary colony collection site) and an anthropophilic Ae. aegypti transport control (to control for potential effects of transportation from the laboratory at Cornell to Princeton). For each of the colonies, we report the predicted probability of choosing human over guinea pig. We assessed whether differences in host choice existed between the colonies by analyzing the data together and for each experimental round separately.
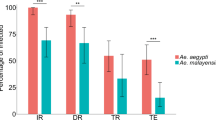
For the combined analysis, all six Ae. albopictus colonies were more likely to choose guinea pig than human. The predicted probabilities of choosing human were below 0.5, including both the anthropophagic and zoophagic Ae. albopictus colonies (Fig. 1A, Supplemental Table S1). As expected, the zoophilic Ae. aegypti colony was more likely to choose guinea pig than human and the two anthropophilic Ae. aegypti colonies were more likely to choose human than guinea pig. All Ae. albopictus colonies were clearly zoophilic, with the exception of Cameroon 1. This was the only Ae. albopictus colony with an upper confidence limit that crossed 0.5, the dividing line between whether a colony is more likely to choose human or guinea pig (predicted probability = 0.386, lower CL = 0.232, upper CL = 0.566). Because this upper confidence limit was greater than 0.5, it is the only Ae. albopictus colony for which we cannot preclude the possibility of human preference under these experimental conditions, although the predicted probability was below 0.5. Notably, Cameroon 1, and all five other Ae. albopictus colonies were still significantly less likely to choose human than the two anthropophilic Ae. aegypti colonies (α = 0.05) and did not behave significantly differently from the zoophilic Ae. aegypti (Supplemental Table S1).
This figure shows the predicted probability of choosing human for each of the colonies in: (a) the combined model, (b) the first round model, and (c) the second round model. Within each graph, colonies that do not share a letter above the upper confidence limit are statistically different (p < 0.05). The green colonies are Ae. albopictus derived from populations with previously reported low levels of anthropophagy, the orange colonies are Ae. albopictus from populations with previously reported high levels of anthropophagy, and the black are Ae. aegypti control colonies. The dashed grey line indicates a 0.5 probability of choosing human, the level at which a colony would be equally likely to choose human or guinea pig; above this line, the colony is more likely to choose human and below, guinea pig. The first and second round trials were performed with minor methodological differences, including arm presentation. The following colony abbreviations were used: NY New York, MD1 Maryland 1, VA Virginia, FL Florida, Cam1 Cameroon1, Thai1 Thailand 1, Zoophil. Zoophilic, CU anthro. Cornell anthropophilic, PU anthro. Princeton anthropophilic.
We then examined the results for each round separately due to slight technical differences between the method of human host presentation (elbow versus forearm and hand). We also added a transport control and biological replicates for three Ae. albopictus colonies in the second round to control for potential effects of transportation from the laboratory at Cornell to Princeton (ca. 4-h drive in a car with human odor) and potential founder effects, respectively. The first-round model included data from the four first-round replicates and was largely consistent with our analysis of both rounds combined with slight differences in the probability of choosing human over guinea pig (Fig. 1B, Supplemental Table S1). One notable difference in the first round alone compared with the combined model is that the Thai Ae. albopictus did not present a significantly different probability of choosing human compared with the Cornell anthropophilic Ae. aegypti colony (p = 0.103), although it remained significantly different from the Princeton anthropophilic Ae. aegypti colony (p = 0.007). All other significant and non-significant relationships remained the same as the combined model (α = 0.05). In this round, the colonies exhibited a spectrum of response rates (percent of released mosquitoes that entered a host port), ranging from 11.2 to 25.0% for the Ae. albopictus colonies and between 41.6% and 71.3% for the Ae. aegypti colonies.
Results of the second round, which included the remaining four replicates, were also consistent with the combined model (Fig. 1C, Supplemental Table S1). All nine Ae. albopictus colonies (the six original colonies and three biological replicates) were not significantly different from one another (p > 0.05). The transport control (the Princeton anthropophilic Ae. aegypti colony reared at Cornell) did not behave differently from the same colony raised at Princeton (p = 1.00), suggesting that preference behavior was not modified by the transport of mosquitoes from Cornell to Princeton and slight differences in rearing. The two Cameroonian colonies (p = 0.260 and p = 0.2434) and the zoophilic Ae. aegypti colony (p = 0.122) were not different from the Cornell anthropophilic Ae. aegypti, but were different from the Princeton anthropophilic Ae. aegypti, as were all other Ae. albopictus colonies (p < 0.01). All other significant and non-significant relationships remained the same as the combined model (α = 0.05). In this round, the colony response rates ranged from 16.5 to 39.3% for the Ae. albopictus colonies and between 24.3% and 45.8% for the Ae. aegypti colonies.
Variation by experimental subject
Individual host variation in mosquito attraction is a well-documented phenomenon42. To account for this, we examined mosquito behavioral responses across the individual experimental subjects (human and guinea pig). In the first round, a different human subject was used for each of the four replicates (Fig. 2). We detected significant differences in the predicted probability of choosing human between four of the six paired comparisons (p < 0.05) and one pair with a marginally significant difference (p = 0.0514) (GLMM, human = fixed effect, colony = random effect).
In the second round, the same human was used for all four replicates and two guinea pigs were used for two replicates each. This experimental set up allowed us to isolate the effect of guinea pig; one guinea pig was more attractive than the other (p < 0.0001; Fig. 3) (GLMM, guinea pig = fixed effect, colony = fixed effect). When stratified by guinea pig, the pairwise estimated marginal mean comparisons between colonies for each guinea pig did not change the main conclusion that the Ae. albopictus colonies were less likely to choose human than anthropophilic Ae. aegypti and behaved similarly to zoophilic Ae. aegypti. Although two guinea pigs were also used for two replicates each in the first round, each human was only paired with one guinea pig due to animal use constraints, making it difficult to isolate the effect of guinea pig, resulting in no significant difference between the two guinea pigs in the first round or combined models (p = 0.065 and p = 0.063, respectively).
Discussion
This study is the first to compare the host preference of Ae. albopictus across multiple populations, the first to characterize host preference of zoophagic Ae. albopictus populations, the first to assess Ae. albopictus host odor preference separately from other cues in a controlled laboratory setting, and the first to directly compare host preference of Ae. albopictus to that of Ae. aegypti. Our results provide important insight into the behavior of this mosquito species and can help us to evaluate the relative importance of these vector species for transmission of anthroponotic and zoonotic pathogens.
Our results do not support the hypothesis that differences in Ae. albopictus host preferences drive observed differences in feeding patterns among populations in nature. The three anthropophagic Ae. albopictus populations we tested were not more likely to choose a human host versus a guinea pig compared with the three zoophagic populations. Further, we found no significant differences between any of the Ae. albopictus populations tested, representing a broad global distribution, including several populations from across the Eastern United States, Asia and Africa. Our results contrast with findings for two other species, including variation in Ae. aegypti host preference within Africa and between African and non-African populations, measured using the same experimental design we used in our study11, and Culex annulorostris within Australia43. At a finer geographic scale, we also did not detect variation for host preference in the locations where we collected paired biological replicate colonies of Ae. albopictus within about 1.5 km of one another, similar to the absence of fine scale variation between paired Ae. aegypti colonies collected within 5–10 km of each other11. Given the wide distribution of Ae. albopictus around the world, it is possible that host preference variation exists at locations that were not included in this study. The colonies tested in our study do not encompass all genetic backgrounds identified globally44.
All Ae. albopictus we tested, regardless of geographic origin and previously reported feeding patterns, were significantly less likely to choose human over guinea pig than the previously characterized Princeton anthropophilic Ae. aegypti from Thailand and none were significantly different from the previously characterized zoophilic Ae. aegypti from Uganda11. Human preference responses between our two anthropophilic Ae. aegypti colonies varied but were not significantly different.
Our results suggest that Ae. albopictus host preference behavior is closer to that of ancestral Ae. aegypti populations than to that of the invasive Ae. aegypti lineage that evolved to specialize in biting humans and then spread out of Africa and around the world. We can therefore conclude that Ae. albopictus is less anthropophilic than invasive Ae. aegypti. In the past, Ae. albopictus has been considered both an anthropophilic and a generalist blood feeder1; however Ae. albopictus has typically been presumed to be less anthropophilic than Ae. aegypti (e.g.45). The quantification of their relative anthropophily in this study has implications for understanding the relative threat posed by the two species, which transmit many of the same pathogens (e.g. dengue, Zika, and chikungunya) and live in similar habitats with overlapping distributions46. Aedes albopictus may only pose a comparable threat for transmission of anthroponoses in settings where humans are highly available, such as densely populated urban areas with open housing structures. It also suggests that in settings where humans are available at intermediate levels alongside other hosts, Ae. albopictus is more likely to transmit zoonotic pathogens than Ae. aegypti.
Our assays also demonstrated variation in preference for host individuals of the same species (both individual humans and individual guinea pigs). It is well-established that there is variation in mosquito attraction to different humans (reviewed by Martinez et al., 202142). It is notable that variation in human preference was observed in the first round of our experiment despite the recruitment process to select humans with a relatively high level of attractiveness to anthropophilic Ae. aegypti, which may have been expected to limit the observed variation in attraction among human hosts. We do not know whether Ae. aegypti and Ae. albopictus prefer the same individual hosts. It is possible that Ae. albopictus responds to different odor components than Ae. aegypti, which would mean that human subjects chosen to maximize the response of anthropophilic Ae. aegypti (see Methods) might create or increase the perceived difference in human preference between the two species in the first experimental round. Mosquito species can respond differently to bacterial volatiles from different host species47, which may also be the case for differences between individuals of a given host species.
Variation in intra-species attraction among non-human hosts has been reported previously, with attraction varying by physiological stage in rats48, stress hormones in zebra finches49, and body mass and metabolic rate in house sparrows50. The two guinea pig hosts used in our study were both mature females, however they were different ages, with the older of the two being the more attractive. We did not measure body mass, metabolic rate, stress hormones, or any other parameter that may have driven the difference in attraction of the two guinea pigs. Additional experiments designed to assess attraction to individual hosts may provide more insight on variation in attractiveness among individual humans and among individual non-human hosts. Many host preference studies use one or a few individuals to represent the human and non-human host species; it should be acknowledged that the individuals chosen for each species may impact the magnitude and even directionality of the measured preference.
In our study, the way human odor was presented to the mosquitoes differed between round one and two. In round one, air was passed over only the middle section of a human arm, from just below to just above the elbow (Fig. 4). In round two, air was passed over the full forearm and hand. We found that changing the arm display from the elbow to the full forearm and hand could increase the level of attractiveness of an unattractive human subject. Exposing the full forearm and hand may have increased available surface area or exposed different odors emitted by different parts of the arm. Some mosquito species exhibit preference for certain body parts, based on preference for specific microbial volatiles in those areas51, although such body part-specific preferences are not always detected52. Despite this change in odor presentation, the patterns of host attraction remained similar across the first and second rounds.
(a) The dual-port olfactometer consists of the following components: 1. the mosquito chamber, in which mosquitoes are released and allowed 10 min to acclimate prior to odor exposure; 2. funnel that allows mosquito entry into ports after the sliding door is opened but limits return of mosquitoes from the trap to the mosquito chamber; 3. mosquito trap, with a funnel on one end and a screen on the other to prevent access to hosts; 4. host chambers that hold the host for odor presentation; the method of human arm presentation pictured here was used in the second experimental round (the full forearm and hand were inserted through the far end of the tube and exposed to air flow, with the breathing tube inserted alongside the arm). An opaque panel (not pictured) divided the hosts from the mosquitoes such that they were not visible from the mosquito chamber prior to host choice; 5. wind source, which was created by blowing a carbon-filtered air source over the hosts in the first round (with a nozzle connected to each host chamber and an air outtake at the other end of the mosquito chamber; ~ 0.6 m/s windspeed), and in the second round, created by a window fan fitted to the far end of the mosquito chamber, producing air flow by sucking air from the room through the host chambers and the mosquito chamber. (b) In the first experimental round, the human arm was displayed by inserting the arm perpendicularly through the host chamber, with the hand protruding from the opposite side, such that the elbow was exposed to air flow. The breathing tube (6) was also inserted into the host chamber. Schematics modified from Metz et al. (2022)53.
In addition to the challenge of individual host variation in attractiveness, laboratory preference assays need to ensure field-relevance of the mosquito colonies. Collection and colony rearing techniques can lead to founder effects, bottlenecks, and selection54,55. We attempted to limit selection pressure on host preference by sampling Ae. albopictus population egg or larval stages, except for the Thai Ae. albopictus, which were collected via human-landing catch, potentially selecting for human preference. Despite this difference, the Thai Ae. albopictus colonies were not more likely to choose human than the other colonies when tested in the olfactometer. To avoid laboratory selection for host preference, as has been described in other species56, mosquitoes were tested within just a few generations of colony establishment (F2-F4), were fed on artificial feeders with minimal host cues, and were given ample time to blood feed (until > 90% were fed). Therefore, we expect that the laboratory colonies tested in these experiments are representative of the field populations.
The goal of our study was to understand, for the first time, levels of Ae. albopictus anthropophily across global geographic isolates. Our experimental design exposed a small but consistent part of human hosts to mosquitoes and was conducted in a controlled laboratory setting. We chose to use guinea pig as the non-human host because it is one of the most common hosts used in Ae. aegypti host odor preference assays, allowing us to assess Ae. albopictus host preference using a host that elicits replicable, divergent behavior between the Ae. aegypti control colonies. Domesticated guinea pigs have been dispersed by trade throughout much of the world from their native origin in South America57, resulting in an overlapping geographic distribution with the globally invasive Ae. albopictus. Although guinea pig has never been reported in Ae. albopictus blood meal contents, it is unknown whether guinea pigs were present at the study sites. As such, it is possible that guinea pigs serve as a natural host in the field, but it has yet to be demonstrated. Further research is needed to understand the probability of choosing a human over more relevant non-human animals in field settings given large variation in host sizes and the need to include a range of hosts naturally available to Ae. albopictus. Our results are in contrast to the two previous assessments of Ae. albopictus host preference, which tested the full human body versus various non-human animals and concluded that Ae. albopictus prefer humans to non-human animals14,15. In the latter study, host defenses may have contributed to the conclusion of human preference. Additional Ae. albopictus host preference assays should be conducted using different experimental techniques and host animals to better understand this trait and tease out potential artifacts of experimental design.
Here, we demonstrated for the first time that Ae. albopictus host preference is not likely to be the driver of the highly variable feeding patterns reported in the literature for this species. The Ae. albopictus colonies that we tested were consistently zoophilic in contrast to the highly anthropophilic invasive lineage of Ae. aegypti, further supporting our understanding that Ae. aegypti has a higher capacity to transmit arboviruses between humans than Ae. albopictus.
Methods
Colony establishment
Based on previously reported Ae. albopictus blood meal analysis studies, three populations with high levels of anthropophagy and three populations with low levels of anthropophagy were selected. The high anthropophagy populations included Cameroon (99.4% anthropophagy, defined as the percent of all identified bloodmeals identified as human)41, Thailand (100% anthropophagy, including 5.7% that fed on both a human and non-human animal)35, and Florida, USA (91% anthropophagy)40. The low anthropophagy populations, all from the eastern United States, included New York (32.2%)17, Maryland (13.6%)38, and Virginia (7.3%)39. Three of these populations (Cameroon, Thailand, and Maryland) included a biological replicate (a second colony from a site at least 1.5 km away from the primary colony collection site). The collections took place at roughly the same sites as the blood meals were collected in previous studies, except for Thailand because sites could not be reached due to COVID travel restrictions (see Supplemental document S1 for site details). However, time gaps did exist between the blood meal and colony collections, ranging from 18 to 2 years (Thailand and Virginia, respectively). Populations may have evolved in the intervening time, potentially reducing the concordance between population traits that produced the feeding patterns and the host preferences observed here.
Most collections were conducted with oviposition traps, black buckets treated with an attractant (water infused with dog food, hay, or other organic material, depending on site) and lined with paper towel or seed germination paper to collect eggs. At least ten traps were distributed at least 100 m apart, except for the Florida site, which was in a scrapyard that was not sufficiently large for such distant spacing. Collections were conducted over 2–3 weeks and egg sheets were removed multiple times per week. The collections that were not conducted with oviposition traps included Thailand, Cameroon, and one of the Maryland biological replicates (Maryland 2). In Thailand, collections were conducted with human landing catches, which were performed prior to other collections and could not be repeated with the standardized collection methods (designed to avoid selection on host preference) due to COVID travel restrictions. In Cameroon and the Maryland biological replicate, larval collections were conducted. Larvae were collected from at least 10 containers at least 100 m apart.
The control colonies were established prior to this study. The zoophilic Ae. aegypti was originally collected in Uganda (ZIK in Rose et al. 2020)11, and both the Princeton (T51 in Rose et al. 2020)11 and Cornell58 anthropophilic Ae. aegypti were originally collected in Thailand.
Mosquito rearing
In the case of oviposition collections, egg sheets were sent directly to Cornell University, where they were maintained in an environmental chamber until hatching (28 °C, 71.9% ± 9.5% relative humidity, 10 h light, 10 h dark, and 2 h dusk/dawn). Egg sheets were soaked in water for 20 min and then vacuum-hatched and provided with a pinch of pulverized fish food (medium Cichlid Gold™ fish food pellets; Hikari, Himeji, Japan). Within 24 h, larvae were transferred from the hatch flask to rearing trays, with 200 larvae, 1 L of distilled water, and 7 fish food pellets in each. Upon pupation, mosquitoes were transferred to cups and placed in cages. Adult females were blood fed using an artificial feeding system, with human blood (Valley Biomedical, Winchester, VA, USA), with sausage casing as the piercing membrane (DCW Casing LLC, Mount Vernon, NY, USA). Colonies were fed at approximately 7, 14, and 21 days post-eclosion. To avoid selection during the feeding process, colonies were monitored to ensure high rates of feeding (> 90%) and were fed a second day if sufficient feeding levels were not reached. Three days post-feeding, oviposition cups were placed into the cage, treated with strained larval water to induce improved egg-laying59 and dirt in the case of the Ugandan colony. Three days later, oviposition cups were removed and egg sheets were dried until slightly damp and then maintained in the environmental chamber. All colonies derived from oviposition traps were maintained in colony in this form until they were used in the preference assays in generation F2, except for the primary Maryland colony, which was F3 in the second experimental round.
In Thailand, the adult female mosquitoes captured through human landing catch were brought to the lab and blood fed using human blood via an artificial feeder. The egg sheets derived from these feedings were sent to Cornell University (F1). The Thai Ae. albopictus used in experiments were generation F3. In Cameroon, the collected larvae were brought to the lab, reared, and blood fed using rabbit blood (live and via artificial feeder). They were maintained in colony in Cameroon for one more generation and F2 eggs were sent to Cornell University. The Cameroonian Ae. albopictus used in experiments were generation F3 and F4. For the second biological replicate from Maryland, larvae were shipped in water to Cornell University. The Ae. albopictus from the Maryland biological replicate colony was generation F2 in the experiments. In all cases, upon arrival at Cornell University, the colonies were maintained as described above until preference assays were performed.
The zoophilic Ae. aegypti eggs were sent to Cornell from a colony at Princeton and reared as described above. The Cornell anthropophilic Ae. aegypti were acquired directly from eggs derived from the Cornell colony, which is maintained similarly to the methods described above, except for blood feeding, which is typically conducted with a live human and periodically with a live chicken. Mosquitoes reared for experiments were reared in the same fashion as the Ae. albopictus colonies. The Princeton anthropophilic Ae. aegypti were reared at Princeton (F14), with slight differences in rearing protocol: eggs were hatched in deoxygenated water and fed Tetramin Tropical Tablets fish food (Spectrum Brands, Inc) ad libitum until pupation, then transferred to cages. In the second experimental round, the Princeton colony was reared at Cornell as well as Princeton as a transport control—eggs were brought to Cornell and reared alongside the other colonies.
At about five days post-eclosion, colonies were transported to Princeton in a heated car for preference assays (except for the Princeton Ae. aegypti, which were already located there). No mortality was noticed. The colonies were given approximately 50 h to acclimate in the rearing chamber at 28 °C (71.9% ± 9.5% RH) before preference assays commenced.
Preference assays
First-round
Preference assays were performed in a dual-port olfactometer (Fig. 4A), using a similar methodology as previous studies11,13. Between 50 and 175 females were released in the mosquito chamber and allowed to acclimate for approximately ten minutes. Only the first and second replicate of the two anthropophilic Ae. aegypti colonies were conducted with fewer than 98 females; all others included 98–175 females. After acclimation, the ports were opened and the wind source was turned on, moving carbon-filtered air over the hosts and into the mosquito chamber, presenting the mosquitoes with a human and guinea pig odor plume emanating from the respective ports. The human odor source included the elbow of the human, inserted perpendicularly through two holes in the host chamber, and breath, exhaled from the nose every 30 s through a breathing tube (Fig. 4B). The full guinea pig was presented in the other host chamber and allowed to breathe normally. Guinea pig was chosen as the non-human host because it is one of the most common hosts used to assess Ae. aegypti host odor preference, eliciting replicable, divergent behavior between the control colonies11. The mosquitoes were given ten minutes to fly upwind and enter one of the port traps, where they were prevented from accessing the host via a screen and inhibited from flying back into the mosquito chamber via a funnel. At the conclusion of the ten minutes, the number of mosquitoes that entered each port was counted.
The first round of replicates was conducted over four days. Each day, the six primary Ae. albopictus colonies, the zoophilic Ae. aegypti, and the two anthropophilic Ae. aegypti colonies were tested. The order of testing was rotated so that the colonies would be tested at different times of day. The side on which the human and guinea pig were presented was swapped after approximately half of the replicates were completed each day.
Potential human subjects were tested for attractiveness level prior to inclusion using the two anthropophilic Ae. aegypti colonies; the goal of this study was to measure the relative anthropophily of the colonies, so we wanted to include attractive humans to maximize the potential dynamic range of anthropophily observed. We selected four of seven tested human subjects; two were excluded due to low levels of attraction and one due to illness during the first round. Each of the four human subjects were used for one full day of replicates (all nine colonies tested in this round). These participation of humans in olfactometer trials using these methods was approved and monitored by the Princeton University Institutional Review Board (protocol 8170). All participants provided informed consent before participating.
Two guinea pigs were used and rotated after each day of experiments. The guinea pigs periodically defecated or urinated during the trial; when this occurred, the soiled protective sheet at the bottom of both host chambers were removed and replaced with new sheets. The use of guinea pigs in olfactometer trials was approved and monitored by the Princeton University Institutional Animal Care and Use Committee (protocol 1998–20). All methods were carried out in accordance with the corresponding guidelines and regulations.
Second round
The second experimental round was performed largely the same as the first round with several exceptions. In the second round, personnel constraints required one human to serve as the human subject for all four replicates, with the added benefit of removing one potential source of variation. However, the human subject in question was deemed relatively unattractive based on the recruitment assays that were conducted prior to the first round and was excluded from that round. Based on anecdotal experience, a pilot was conducted comparing two forms of arm display: the elbow as in the first round (Fig. 4B) versus the full forearm and hand (Fig. 4A). This demonstrated an increase in the probability of choosing human using the full forearm and hand compared with the elbow, which was later confirmed by a small trial (Supplemental document S2). Changing the arm presentation also required changing the airflow system. The arm was inserted through the back of the human host chamber, preventing the connection of the carbon-filtered air system. Instead, the fan attached to the far end of the mosquito chamber was alone responsible for drawing air from the room over the hosts and through the mosquito chamber, as done in a previous study13. This may have caused more mixing of host odors; however, efforts were made to reduce this phenomenon: the air exchange in the room holding the dual-port olfactometer is extremely high (multiple exchanges per hour) and the hosts were removed from the room for at least 15 min between each replicate, limiting the accumulation of host odors in the room.
The other difference in the second round was that the three biological replicate colonies and a transport control were included in addition to the nine colonies tested in the previous round. As a result, thirteen colonies were tested each day. The transport control was added to assess whether the transport from Cornell to Princeton impacted the host seeking behavior. The Princeton anthropophilic Ae. aegypti were reared at both Princeton and Cornell.
Data analysis
A Generalized Linear Mixed Model using Template Model Builder (glmmTMB)60 with a beta-binomial distribution was employed to evaluate the contribution of several factors to the predicted probability of choosing human for each of the analyses described below (the combined, first round, and second round models that seek to evaluate the effect of colony and the human model, which seeks to evaluate the effect of human). Post hoc analyses were conducted by calculating the estimated marginal means of the effects by using emmeans61. A predicted probability of choosing human equivalent to 0.5 indicates no preference between human and guinea pig; between 0.5 and 1 indicates a preference for human and between 0 and 0.5 indicates a preference for guinea pig. Analyses were conducted using R version 4.1.162. Graphs were created using ggplot63.
Combined model
The data for the six primary Ae. albopictus colonies, the zoophilic Ae. aegypti and the two anthropophilic Ae. aegypti colonies were combined across eight replicates conducted in the first and second experimental rounds, which we refer to as the combined model. In this model, colony, guinea pig, and side of human host chamber were included as fixed effects and with random effects of human and date.
First-round model
In this model, colony, guinea pig, and side of human host chamber were included as fixed effects and human as a random effect. Each human was only tested one day in the first round, so date was excluded from this analysis.
Second-round model
In this model, colony, guinea pig, and side of human host chamber were included as fixed effects and date as a random effect. Only one human was tested in the second round, so human was excluded from this analysis.
Human model
To evaluate the differential attraction to each of the four humans in the first round, human and side of human host chamber were included as fixed effects and colony as a random effect. We did not include guinea pig in this analysis, because each human was only tested against one guinea pig, resulting in nesting of this data.
Data availability
Data are deposited in Cornell University Library’s institutional repository, eCommons (https://ecommons.cornell.edu) for preservation and access via the world wide web without restriction at the following https://doi.org/10.7298/k8wf-bh80.
References
Fikrig, K. & Harrington, L. C. Understanding and interpreting mosquito blood feeding studies: the case of Aedes albopictus. Trends Parasitol. 37, 959–975 (2021).
Takken, W. & Verhulst, N. O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453 (2013).
Wolff, G. H. & Riffell, J. A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 221, jeb157131 (2018).
Yan, J. et al. Understanding host utilization by mosquitoes: Determinants, challenges and future directions. Biol. Rev. 96, 1367–1385 (2021).
Lyimo, I. N. & Ferguson, H. M. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 25, 189–196. https://doi.org/10.1016/j.pt.2009.01.005 (2009).
Thongsripong, P., Hyman, J. M., Kapan, D. D. & Bennett, S. N. Human–Mosquito Contact: A Missing Link in Our Understanding of Mosquito-Borne Disease Transmission Dynamics. Ann. Entomol. Soc. Am. (2021).
Simpson, J. E. et al. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B Biol. Sci. 279, 925–933 (2012).
Gratz, N. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18, 215–227 (2004).
Pereira-dos-Santos, T., Roiz, D., Lourenço-de-Oliveira, R. & Paupy, C. A systematic review: Is Aedes albopictus an efficient bridge vector for zoonotic arboviruses?. Pathogens 9, 266 (2020).
McBride, C. S. Genes and odors underlying the recent evolution of mosquito preference for humans. Curr. Biol. 26, R41–R46 (2016).
Rose, N. H. et al. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 30, 3570–3579 (2020).
Gouck, H. Host preferences of various strains of Aedes aegypti and A. simpsoni as determined by an olfactometer. B World Health Organ 47, 680 (1972).
McBride, C. S. et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227 (2014).
Sullivan, M. F., Gould, D. J. & Maneechai, S. Observations on host range and feeding preferences of Aedes albopictus (Skuse). J. Med. Entomol. 8, 713–716. https://doi.org/10.1093/jmedent/8.6.713 (1971).
Delatte, H. et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector-Borne Zoonot 10, 249–258. https://doi.org/10.1089/vbz.2009.0026 (2010).
Edman, J. D. & Scott, T. W. Host defensive behavior and the feeding success of mosquitos. Insect Sci. Appl. 8, 617–622. https://doi.org/10.1017/s1742758400022694 (1987).
Fikrig, K. et al. The effects of host availability and fitness on Aedes albopictus blood feeding patterns in New York. Am. J. Trop. Med. Hyg. 106, 320–331. https://doi.org/10.4269/ajtmh.21-0157 (2022).
Richards, S. L., Ponnusamy, L., Unnasch, T. R., Hassan, H. K. & Apperson, C. S. Host-feeding patterns of Aedes albopictus (Diptera : Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J. Med. Entomol. 43, 543–551. https://doi.org/10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2 (2006).
Gomes, A. C., Silva, N. N., Marques, G. & Brito, M. Host-feeding patterns of potential human disease vectors in the Paraiba Valley Region, State of Sao Paulo Brazil. J. Vector Ecol. 28, 74–78 (2003).
Leisnham, P., Towler, L. & Juliano, S. Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Ann. Entomol. Soc. Am. 104, 1309–1318 (2011).
Leisnham, P., Lounibos, L., O'meara, G. & Juliano, S. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology 90, 2405–2413 (2009).
Armbruster, P. & Conn, J. E. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 99, 1234–1243 (2006).
Gubler, D. J. & Rosen, L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am. J. Trop. Med. Hyg. 25, 318–325 (1976).
Chen, X.-G. et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl. Acad. Sci. 112, E5907–E5915 (2015).
Stone, C. M. et al. Spatial, temporal, and genetic invasion dynamics of Aedes albopictus (Diptera: Culicidae) in Illinois. J. Med. Entomol. 57, 1488–1500 (2020).
Guo, S. et al. Genetic diversity of Aedes albopictus populations in the coastal areas of southern China, using the microsatellite markers. Zhonghua liu Xing Bing xue za zhi= Zhonghua Liuxingbingxue Zazhi. 40, 992–996 (2019).
Motoki, M. T. et al. Population genetics of Aedes albopictus (Diptera: Culicidae) in its native range in Lao People’s Democratic Republic. Parasite Vector 12, 1–12 (2019).
Sherpa, S., Rioux, D., Pougnet-Lagarde, C. & Després, L. Genetic diversity and distribution differ between long-established and recently introduced populations in the invasive mosquito Aedes albopictus. Infect. Genet. Evol. 58, 145–156 (2018).
Vega-Rúa, A. et al. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun. Biol. 3, 1–13 (2020).
Boyle, J. H. et al. A linkage-based genome assembly for the mosquito Aedes albopictus and identification of chromosomal regions affecting diapause. Insects 12, 167 (2021).
Poelchau, M. F., Reynolds, J. A., Elsik, C. G., Denlinger, D. L. & Armbruster, P. A. Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B- Biol. Sci. 280, 20130143 (2013).
Armbruster, P. A. Photoperiodic diapause and the establishment of Aedes albopictus (Diptera: Culicidae) in North America. J. Med. Entomol. 53, 1013–1023 (2016).
Tabbabi, A. Global invasion and phenotypic plasticity of the Asian tiger mosquito Aedes (Stegomyia) albopictus (Skuse)(Diptera: Culicidae), an invasive vector of human diseases: Review of the problem and the evidence. J. Middle East N. Afr. Sci. 10, 1–7 (2018).
Duong, C.-V., Kang, J.-H., Nguyen, V.-V. & Bae, Y.-J. Genetic diversity and population structure of the Asian Tiger mosquito (Aedes albopictus) in Vietnam: Evidence for genetic differentiation by climate Region. Genes 12, 1579 (2021).
Ponlawat, A. & Harrington, L. C. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 42, 844–849 (2005).
Delatte, H. et al. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite (Paris, France) 15, 3–13 (2008).
Tsetsarkin, K. A., Vanlandingham, D. L., McGee, C. E. & Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3, e201 (2007).
Goodman, H., Egizi, A., Fonseca, D. M., Leisnham, P. T. & LaDeau, S. L. Primary blood-hosts of mosquitoes are influenced by social and ecological conditions in a complex urban landscape. Parasite Vector 11, 218 (2018).
Little, E. A. et al. Host interactions of Aedes albopictus, an invasive vector of arboviruses, in Virginia, USA. Plos. Neglect. Trop. Dis. 15, e0009173 (2021).
Stenn, T., Peck, K. J., Pereira, G. R. & Burkett-Cadena, N. D. Vertebrate Hosts of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus (Diptera: Culicidae) as Potential Vectors of Zika Virus in Florida. J. Med. Entomol. 56, 10–17. https://doi.org/10.1093/jme/tjy148 (2019).
Kamgang, B., Nchoutpouen, E., Simard, F. & Paupy, C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasite Vector 5, 4. https://doi.org/10.1186/1756-3305-5-57 (2012).
Martinez, J., Showering, A., Oke, C., Jones, R. T. & Logan, J. G. Differential attraction in mosquito–human interactions and implications for disease control. Philos. T Roy. Soc. B 376, 20190811 (2021).
Williams, C. R., Kokkinn, M. J. & Smith, B. P. Intraspecific variation in odor-mediated host preference of the mosquito Culex annulirostris. J. Chem. Ecol. 29, 1889–1903 (2003).
Goubert, C., Minard, G., Vieira, C. & Boulesteix, M. Population genetics of the Asian tiger mosquito Aedes albopictus, an invasive vector of human diseases. Heredity 117, 125–134 (2016).
Lozano-Fuentes, S. et al. Susceptibility and vectorial capacity of American Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to American Zika Virus Strains. J. Med. Entomol. 56, 233–240 (2019).
Lwande, O. W. et al. Globe-trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector-Borne Zoonot. 20, 71–81 (2020).
Busula, A. O., Takken, W., de Boer, J. G., Mukabana, W. R. & Verhulst, N. O. Variation in host preferences of malaria mosquitoes is mediated by skin bacterial volatiles. Med. Vet. Entomol. 31, 320–326 (2017).
Díaz-Santiz, E., Rojas, J. C., Casas-Martínez, M., Cruz-López, L. & Malo, E. A. Rat volatiles as an attractant source for the Asian tiger mosquito Aedes albopictus. Sci. Rep. 10, 1–12 (2020).
Gervasi, S. S. et al. Host stress hormones alter vector feeding preferences, success, and productivity. Proc. R. Soc. B Biol. Sci. 283, 20161278 (2016).
Yan, J. et al. Does bird metabolic rate influence mosquito feeding preference?. Parasite Vector 11, 1–9 (2018).
De Jong, R. & Knols, B. Selection of biting sites on man by two malaria mosquito species. Experientia 51, 80–84 (1995).
Verhulst, N. O., Weldegergis, B. T., Menger, D. & Takken, W. Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodorant compounds. Sci. Rep. 6, 1–9 (2016).
Metz, H. C., Zung, J. L. & McBride, C. S. An assay for quantifying Aedes aegypti host odor preference using a two-port olfactometer. Cold Spring Harp Protoc. https://doi.org/10.1101/pdb.prot108089 (2023).
Ross, P. A., Lau, M.-J. & Hoffmann, A. A. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS ONE. 14, e0224268 (2019).
Ross, P. A., Endersby-Harshman, N. M. & Hoffmann, A. A. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol. Appl. 12, 572–586 (2019).
Gillies, M. Selection for host preference in Anopheles gambiae. Nature 203, 852–854 (1964).
Lord, E. et al. Ancient DNA of guinea pigs (Cavia spp.) indicates a probable new center of domestication and pathways of global distribution. Sci. Rep. 10, 1–9 (2020).
League, G. P. et al. The impact of mating and sugar feeding on blood-feeding physiology and behavior in the arbovirus vector mosquito Aedes aegypti. PLoS Negl. Trop. D 15, e0009815 (2021).
Shragai, T., Harrington, L., Alfonso-Parra, C. & Avila, F. Oviposition site attraction of Aedes albopictus to sites with conspecific and heterospecific larvae during an ongoing invasion in Medellín Colombia. Parasite Vector 12, 1–10 (2019).
Magnusson, A. et al. Package ‘glmmtmb’. R Package Version 0.2. 0 (2017).
Lenth, R. et al. emmeans: estimated marginal means, aka least-squares means (R package version 1.1 (2019).
RCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2021).
Wickham, H. ggplot2: elegant graphics for data analysis. (springer, 2016).
Acknowledgements
The help of so many people made this experiment possible! Thank you to Sakina Isadibir and Summer Kotb for handling the guinea pig and rearing the Princeton colony, Sean Lee, Sylvie Pitcher, and Elisabeth Martin for help with rearing and pilot experiments, and Dr. Erika Mudrak for statistical help. Also thank you to everyone in the McBride lab who provided additional help: Dr. Lukas Weiss, Timothy Schwanitz, and Vitor Dos Anjos. We also greatly appreciate the help from groups that assisted with site identification and/or mosquito collections, including Martin County Mosquito Control, Suffolk Mosquito Control District, Dr. Goudarz Molaei, the USAMD-AFRIMS Vector Biology and Control Team, and collection team at Centre for Research in Infectious Diseases, Yaoundé, Cameroon.
Funding
This work was supported in part by cooperative agreement number U01CK000509, funded by the Centers for Disease Control and Prevention to the Northeast Regional Center for Excellence in Vector Borne Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Further support was provided by NIH/NIAID R01-AI095491.
Author information
Authors and Affiliations
Contributions
K.F. and L.C.H. conceived of the study. K.F., N.B.C., B.K., P.T.L., J.M., A.P., S.E.R., and T.S. planned and executed field collections. K.F., B.K., and A.P. established laboratory colonies and K.F. maintained colonies for experiments. K.F. conducted preference assays, with guidance and input from L.C.H., N.H.R., and C.S.M. K.F. conducted statistical analysis with input from N.H.R. K.F. wrote the manuscript with input from L.C.H. and all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fikrig, K., Rose, N., Burkett-Cadena, N. et al. Aedes albopictus host odor preference does not drive observed variation in feeding patterns across field populations. Sci Rep 13, 130 (2023). https://doi.org/10.1038/s41598-022-26591-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26591-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.