Abstract
Artificial photosynthesis, an umbrella term, is a chemical process that biomimetics natural photosynthesis. In natural photosynthesis, electrons from the water-oxidation reaction are used for carbon dioxide reduction. Herein, we report the reducion of aldehydes and ketones to corresponding alcohols in a simple undivided cell. This reaction utilized inexpensive nickel foam electrodes (1 cm2) and LiClO4 (0.05 M) as a commercially accessible electrolyte in an aqueous medium. Under electrochemical conditions, a series of alcohols (21 examples) produces high selectivity in good yields (up to 100%). Usage the current method, 10 mmol (1060 mg) of benzaldehyde is also successfully reduced to benzyl alcohol (757 mg, 70% isolated yield) without any by‑products. This route to alcohols matched several green chemistry principles: (a) atom economy owing to the use of H2O as the solvent and the source of hydrogen, (b) elimination of the homogeneous metal catalyst, (c) use of smooth reaction conditions, (d) waste inhibition due to low volumetric of by-products, and (e) application of safe EtOH co-solvent. Moreover, the ability of the system to operate with alkyne and alkene compounds enhanced the practical efficiency of this process.
Similar content being viewed by others
Introduction
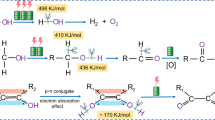
Alcohols find in pharmaceuticals, natural products, agrochemicals, dyes, and polymers1,2,3. The existing methods for carbonyl reduction frequently require metals (e.g. palladium, platinum, ruthenium, osmium, nickel and iron) or stoichiometric reductant reagents (e.g. NaBH4, LiAlH4 and H2) that commonly show limited substrate scope (Fig. 1a)4,5. These systems have demonstrated some drawbacks, such as: using precious, low abundance and toxic metals. On the other hand, hydrogen gas is very flammable and lacks compatibility with a diverse range of functional groups6. Also, the reductants are costly, and metal salts form as by-products during of reaction7.
In recent years, synthetic organic electrochemical protocols have received significant attention since electrochemistry proposes a mild and environmentally-friendly alternative to conventional chemical approaches for organic reactions8,9,10,11. To the best of our knowledge, the electrochemical conversion of carbonyl to corresponding alcohols has a short history of merely several decades, and most of the methodology leads to pinacol products12,13,14,15. Calvo et al. reported the electrochemical reduction of ketones to corresponding alcohols using the palladium wire as a working electrode16. Gutierrez et al. developed an electrochemical system using Pd, Rh and Pt catalysts. The resulting catalyst system was successfully applied to reduce butyraldehyde, furfural, acetophenone, and benzaldehyde17. Mechanistic studies of electrochemical hydrogenation of carbonyl compounds were conducted by Lilga’s group18. Lercher and Gutiérrez's group examined the carbon-supported Pd, Re, Pt and Ni catalysts for benzaldehyde reductio19. Cathodic reduction of 5-hydroxymethylfurfural was performed on the solid electrode in the presence and the absence of glucose by Koper's group20. A similar study in 2016 showed that the reduction of 5-hydroxymethylfurfural on solid electrodes in an acidic solution produced 2,5- Bis(hydroxymethyl) furan, 2,5- Bis(hydroxymethyl) tetrahydrofuran, and 2,5-dimethylfuran21. Li et al. pointed to mechanisms of electrochemical reduction of furfural on metallic Cu electrodes in acidic media. This mechanism may happen through either electrocatalytic hydrogenation or electro-reduction paths22. In a subsequent study, Attard’s group examined the reduction of ketopantolactone (KPL) and ethyl pyruvate (EP) on platinum electrodes23,24. Koper and co-workers reported the hydrogenation of acetophenone, 4-acetylpyridine, and acetone at platinum single-crystal electrodes (Fig. 1b)25,26.
Artificial photosynthesis is a chemical process that biomimetic natural photosynthesis. In the mentioned process, water and carbon dioxide are transformed into carbohydrates and oxygen using sunlight27. Indeed, in natural photosynthesis, electrons from the water-oxidation reaction are used for carbon dioxide reduction. Oxygen-evolution reaction (OER) by water-oxidation reaction is a fundamental electrode reaction owing to its essential applications in electrochemical energy apparatus such as regenerative fuel cells and rechargeable metal-air batteries28,29,30,31. Ru and Ir oxides have been employed in the OER reactions as catalysts, though low abundance and high cost have limited their widespread use32. Recently, increasing attempts have been made to design Ni, Co, Mn and Fe-based transition metal oxides or hydroxides that replaced Ru- and Ir-based OER catalysts33,34,35,36,37,38,39. Ni and Cu foams have been widely noticed as electrodes in OER reactions due to their 3D conductive frameworks and porous structures40,41,42,43. The high porosity and surface area of 3D nanoporous foams show increased conductivity compared to 2D planar electrodes44. For example, Li et al. thermally synthesized a 3D NiO/Ni foam as OER catalysis. The oxidation of nickel compounds under thermal conditions led to the NiO film45. Also, a simple one-step process for synthesizing NiO on the surface of Ni foam during electrochemical alcohol oxidation, without the need for an external thermal or nickel source, is reported in our previous article46.
While carbonyl compounds are reduced on solid-metal electrodes using expensive homogeneous and heterogeneous metals, we propose an alternative electrochemical methodology without requiring specialized reductants via low-cost OER electrons (Fig. 1c).
Results and discussion
Reduction of carbonyl and other functional groups
Changing several electrodes as anode and cathode seriously affected the reaction efficiency (Table 1). Running the reaction with aluminum, magnesium, iron, and titanium cathode produced 4%, 37%, 32% and 5% yields (entries 1–4, respectively). Testing of other anodes, such as titanium, did not improve the flow of the reaction, while nickel–iron foam showed an improvement process (entries 5 and 6). The iron foam anode improved by 95% product with high selectivity (entry 7). The highest yield was obtained using nickel foam as both the anode and cathode, while the formation of by-products was not observed (entry 8).
Replacing LiClO4 with other electrolytes such as NaCl, LiBr, and KIO3 led to a notable drop in the yield (entries 9–11). Unsurprisingly, no reaction occurred without electrolytes (entry 12). Moreover, by changing the electrolyte to 0.2 mmol, the amount of product decreased to 59% (entry 13). While CH3CN and ethanol were almost equally efficient, the latter choice was for its superior safety profile (entry 14). However, the reaction did not proceed when ethanol was used instead of a mixture of ethanol and H2O (entry 15). Changing the voltage to 2.3 V or 1.3 V decreased the yield of the reaction (entries 16 and 17).
So, the final optimized conditions for reducing benzaldehyde to benzyl alcohol are as follows: LiClO4 as the supporting electrolyte (0.5 mmol) in H2O: EtOH (6 mL) under Constant- potential conditions in an undivided cell.
With the optimized reaction conditions in hand, we probed a wide range of aldehydes and ketones to explore the ability and compatibility of this electrochemical hydroxylation reaction. As shown in Table 2, various benzaldehydes with different substituents offered the corresponding alcohols in moderate to excellent yields.
Simple benzaldehyde could furnish the desired benzyl alcohol in a good yield (2a). Fortunately, o-bromo, o-chloro, and m- chloro benzaldehyde worked well in this system and provided the products (2b, 2c, 2d) in 75–100% yields. Our results indicated that electron-withdrawing groups such as cyano, chloro, and bromo at the para position afforded 21%, 59%, and 90% isolated yields. Electron-donating groups like isopropyl, methyl, and methoxy at the para position were all converted in 30–100% yields (2 h–2j). The reduction of 2, 6-dichlorobenzaldehyde was more challenging than the 2, 4-dichlorobenzaldehyde due to its satirically hindered. 2, 4-Dimethylbenzaldehyde and 2, 4-dichlorobenzaldehyde provided the corresponding alcohols in 100% yield (2 l and 2 m). Notably, 2, 4-dimethoxybenzaldehyde produced the desired alcohol in a trace yield (2n). The 1-naphthaldehyde and 2-naphthaldehyde substrates provided the desired 2o and 2p in 45% and 57% yield, respectively. As examples of ketones, Acetophenone successfully transformed into 1-phenylethanol in 88% yield (2q), while the reduction of benzophenone failed (2r). No product was detected when cyclohexanone and hexanal were utilized as reaction substrates (2 s and 2t). In all cases, the yield obtained from this method has been very successful compared to the results of previous reports.
To find further applications for the procedure, we attempted to extend the reaction scope to challenging substrates such as C=C and C≡C bond reductions. The hydrogenation of alkene and alkyne has also been an essential tool in synthesizing medicines and pharmaceutical industries48,49. However, these reactions often require the use of noble transition-metal catalysts (e.g. Pd and Pt) or Renay-Ni, which is less expensive albeit hard to handle50,51,52. The summarized -electrochemical hydrogenation of unsaturated organic compounds reports were collected in a review article by Ye in 202153. Under the present system, styrene and phenylacetylene afforded ethylbenzene in a 99% yield. Further investigation indicates a low level of selectivity found under our method for the C=O bond reduction over C=C hydrogenation. To verify this claim, we investigated the reaction of cinnamaldehyde and 3-phenylpropiolaldehyde. The GC results showed that hydrogenation of C=C was faster than C=O reduction (Fig. 2a,b). To demonstrate the scalability of this new technology, a 1.061 g scale reduction of benzaldehyde was performed using inexpensive nickel foam as anode and cathode in a breaker, and the corresponding product 2a resulted in a near quantitative 70% isolated yield (Fig. 2c).
We observed a featureless cyclic voltammogram without any cathodic peak for a mixture of EtOH/ benzaldehyde and EtOH/ H2O/ benzaldehyde, whereas cyclic voltammetry recorded from benzaldehyde displayed a new cathodic peak near − 2.2 V (Fig. 3a). Likewise, Fig. 3b exhibits linear sweep voltammograms (LSV) prepared using electrolyte solutions containing benzaldehyde, EtOH, EtOH/ H2O, and a mixture of EtOH/ H2O/ benzaldehyde, suggesting that benzaldehyde reduction takes place at near − 2.0 V (see more details in supporting information)18.
(a) Cyclic voltammograms of benzaldehyde (black), benzaldehyde in EtOH (green), and benzaldehyde in EtOH: H2O (red) on nickel foam. (b) Linear sweep voltammetry scans (LSV) with LiClO4 and NH4PF6 as the supporting electrolyte when containing benzaldehyde (black), benzaldehyde in EtOH (green), EtOH: H2O (blue), and benzaldehyde in EtOH and H2O (red). Scan rate set at 20 mV/s with a nickel foam working electrode. An Ag/AgCl electrode was used as the reference and a nickel foam as the counter electrode.
Although the mechanism of carbonyl reduction was not discussed herein, the experimental and theoretical study of benzaldehyde reduction by Rousseau and Lilga’s group strongly implied that in protic solvents (H2O or EtOH), alcohol is the favorite product.
Unlike protic solvents in aprotic conditions, dimerization was preferred, and the pinacol product was obtained18. In addition, the current electrochemical methodology failed to produce stable C–N products for the reduction of imines such as N-benzylideneaniline. However, aldehyde reduction afforded only trace quantities of benzyl alcohol (Fig. 4).
A comparison of direct cathodic reduction of benzaldehyde with other chemical and electrochemical methods is summarized in Table 3.
Conclusions
In summary, an innovative electrochemical protocol for reducing carbonyl groups to the corresponding alcohols was developed. The reaction was performed under external reductant–free conditions to provide a broad range of alcohol and alkane compounds in good yields. Furthermore, the scalability of our procedure was confirmed with the reduction of benzaldehyde on a 1.0 g scale. We believe this green and mild approach to preparing alcohols and alkanes will apply in academic and industrial modern fields.
Methods
Materials
All chemical materials were used without further purification. Lithium perchlorate (LiClO4), benzaldehyde, 4-methoxybenzaldehyde, 4-isopropyl benzaldehyde, 4-chlorobenzaldehyde, 3-chlorobenzaldehyde, 2,4-dichlorobenzaldehyde, 2,6-dichlorobenzaldehyde, 2, 4-dimethylbenzaldehyde, 2, 4-dimethoxybenzaldehyde, cyclohexanone, hexanal, 2-chlorobenzaldehyde, 3-chlorobenzaldehyde, 4-cyanobenzaldehyde, 4-methylbenzaldehyde, 1-naphthaldehyde, 2-naphthaldehyde, acetophenone, benzophenone, cinnamaldehyde, and solvents were purchased from Sigma-Aldrich or Merck Company. 3-phenylpropiolaldehyde was synthesized according to the procedure followed by Kim47. The products were characterized by gas chromatography (GC) and thin-layer chromatography (TLC). Preparative thin layer chromatography (PTLC) separations were carried out on 0.25 or 0.5 mm E. Merck silica gel plates (60F-254).
The nickel foam (Nanobazar), iron foam (Suzhou JSD foam metal Co., Ltd), iron-nickel foam (Suzhou JSD foam metal Co., Ltd), aluminum foil (Suzhou JSD foam metal Co., Ltd), and titanium foil (Suzhou JSD foam metal Co., Ltd) purchased from commercial sources. The electrochemical reactions were conducted through a Model JPS-302D tracking dual DC power supply. Cyclic voltammetry analysis was carried out on a Palmsens (Emstat3 +) electrochemical workstation, using a nickel foam as the working electrode, a Pt electrode as a counter electrode, and an Ag/AgCl as a reference electrode. The cyclic voltammogram was recorded at a scan rate of 20 mV/s. GC yield was performed on a Model TOF LC/MS gas chromatograph spectrometer. 1H NMR spectra were measured in CDCl3 using a 400 MHz spectrometer (Bruker, Switzerland). 13C NMR spectra were measured in CDCl3 at 101 MHz. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiple.
General experimental procedure
In a 10 ml beaker with a stirring bar, the starting material (0.5 mmol), LiClO4 (0.5 mmol) in 6.0 mL H2O, and EtOH (5:1) were added successively. The electrolytic cell was equipped with nickel foam (1 cm × 1 cm) as an anode and a cathode. The solution was electrolyzed at ambient temperature under a constant voltage (3.4 V). After completing the reaction, the mixture was extracted with ethyl acetate. The obtained organic layer dried over Na2SO4 and was used for GC and NMR spectroscopy.
Procedure for gram-scale synthesis of 2a
In a 100 ml beaker with a stirring bar, the starting material (10.0 mmol), LiClO4 (5.0 mmol) in 60 mL H2O, and EtOH (5:1) were added successively. The electrolytic cell was equipped with nickel foam (5 cm × 5 cm) as an anode and a cathode. The solution was electrolyzed at ambient temperature under a constant voltage (6.8 V). After completing the reaction, the mixture was extracted with ethyl acetate. The obtained organic layer dried over Na2SO4 and was used for GC and NMR spectroscopy.
Data availability
All data generated or analyzed during this study are included in this published article [and its Supplementary Information files].
References
Fokin, I. & Siewert, I. Chemoselective electrochemical hydrogenation of ketones and aldehydes with a well-defined base-metal catalyst chem. Eur. J. 26, 14137. https://doi.org/10.1002/chem.202002075 (2020).
Yang, Z. et al. Iridium-catalyzed highly efficient chemoselective reduction of aldehydes in water using formic acid as the hydrogen source. Green Chem. 19, 3296–3301. https://doi.org/10.1039/C7GC01289F (2017).
Isoni, V., Mendoza, K., Lim, E. & Teoh, S. K. Screwing NaBH4 through a barrel without a bang: A kneaded alternative to fed-batch carbonyl reductions. Org. Process Res. Dev. 21, 992–1002. https://doi.org/10.1021/acs.oprd.7b00107 (2017).
Bullock, R. M. Abundant metals give precious hydrogenation performance. Science 342, 1054–1055. https://doi.org/10.1126/science.1247240 (2013).
Chakraborty, S., Bhattacharya, P., Dai, H. & Guan, H. Nickel and iron pincer complexes as catalysts for reducing carbonyl compounds. Acc. Chem. Res. 48, 1995–2003. https://doi.org/10.1021/acs.accounts.5b00055 (2015).
Magano, J. & Dunetz, J. R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 16, 1156–1184. https://doi.org/10.1021/op2003826 (2012).
O’Brien, K. E. & Wicht, D. K. A greener organic chemistry experiment: Reduction of citronellal to citronellol using poly (methylhydro) siloxane. Green Chem. Lett. Rev. 1, 149–154. https://doi.org/10.1080/17518250802512475 (2008).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81. https://doi.org/10.1038/nature17431 (2016).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: Scope, mechanism and applications. J. Am. Chem. Soc. 141, 6392–6402. https://doi.org/10.1021/jacs.9b01886 (2019).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579. https://doi.org/10.1126/science.aan6206 (2017).
Li, T., Cao, Y., He, J. & Berlinguette, C. P. Electrolytic CO2 reduction in tandem with oxidative organic chemistry. ACS Cent. Sci. 3, 778–783. https://doi.org/10.1021/acscentsci.7b00207 (2017).
Kashimura, S. & Ishifune, M. Reduction of oxygen-containing compounds. Encycl. Electrochem. https://doi.org/10.1002/9783527610426.bard080007 (2007).
Hammerich, O. & Speiser, B. Organic Electrochemistry (FL: CRC Press, 2016). https://doi.org/10.1201/b19122.
Nakahara, K. et al. Electrochemical pinacol coupling of acetophenone using boron-doped diamond electrode. ChemElectroChem 6, 4153–4157. https://doi.org/10.1002/celc.201900202 (2019).
Kronenwetter, H., Husek, J., Etz, B., Jones, A. & Manchanayakage, R. Electrochemical pinacol coupling of aromatic carbonyl compounds in a [BMIM][BF4]–H2 O mixture. Green Chem. 16, 1489–1495. https://doi.org/10.1039/C3GC41641K (2014).
Villalba, M., del Pozo, M. & Calvo, E. J. Electrocatalytic hydrogenation of acetophenone and benzophenone using palladium electrodes. Electrochim. Acta 164, 125–131. https://doi.org/10.1016/j.electacta.2015.02.113 (2015).
Sanyal, U., Lopez-Ruiz, J., Padmaperuma, A. B., Holladay, J. & Gutiérrez, O. Y. Electrocatalytic hydrogenation of oxygenated compounds in aqueous phase. Org. Process Res. Dev. 22, 1590–1598. https://doi.org/10.1021/acs.oprd.8b00236 (2018).
Cantu, D. C. et al. A combined experimental and theoretical study on the activity and selectivity of the electrocatalytic hydrogenation of aldehydes. ACS Catal. 8, 7645–7658. https://doi.org/10.1021/acscatal.8b00858 (2018).
Song, Y. et al. Hydrogenation of benzaldehyde via electrocatalysis and thermal catalysis on carbon-supported metals. J. Catal. 359, 68–75. https://doi.org/10.1016/j.jcat.2017.12.026 (2018).
Kwon, Y., de Jong, E., Raoufmoghaddam, S. & Koper, M. T. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in the absence and presence of glucose. Chemsuschem 6(9), 1659–1667. https://doi.org/10.1002/cssc.201300443 (2013).
Kwon, Y., Birdja, Y. Y., Raoufmoghaddam, S. & Koper, M. T. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in acidic solution. Chemsuschem 8(10), 1745–1751. https://doi.org/10.1002/cssc.201500176 (2015).
Chadderdon, X. H. et al. Mechanisms of furfural reduction on metal electrodes: Distinguishing pathways for selective hydrogenation of bioderived oxygenates. J. Am. Chem. Soc. 139, 14120–14128. https://doi.org/10.1021/jacs.7b06331 (2017).
Rees, N. V. et al. In situ surface-enhanced Raman spectroscopic studies and electrochemical reduction of α-ketoesters and self condensation products at platinum surfaces. J. Phys. Chem. C 115(4), 1163–1170. https://doi.org/10.1021/jp106495b (2011).
Hazzazi, O. A. et al. Electrochemical studies of irreversibly adsorbed ethyl pyruvate on Pt {h k l} and epitaxial Pd/Pt {h k l} adlayers. J. Electroanal. Chem. 640, 8–16. https://doi.org/10.1016/j.jelechem.2009.12.026 (2010).
Bondue, C. J., Calle-Vallejo, F., Figueiredo, M. C. & Koper, M. Structural principles to steer the selectivity of the electrocatalytic reduction of aliphatic ketones on platinum. Nat. Catal. 2, 243–250. https://doi.org/10.1038/s41929-019-0229-3 (2019).
Bondue, C. J. & Koper, M. T. Electrochemical reduction of the carbonyl functional group: The importance of adsorption geometry, molecular structure and electrode surface structure. J. Am. Chem. Soc. 141, 12071–12078. https://doi.org/10.1021/jacs.9b05397 (2019).
Listorti, A., Durrant, J. & Barber, J. Solar to fuel. Nat. Mater. 8, 929–930. https://doi.org/10.1038/nmat2578 (2009).
Suen, N. T. et al. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 46, 337–365. https://doi.org/10.1039/C6CS00328A (2017).
McCrory, C. C., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135(45), 16977–16987. https://doi.org/10.1021/ja407115p (2013).
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co (oxyhydr) oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 1, 711–719. https://doi.org/10.1038/s41929-018-0141-2 (2018).
Mamaca, N. et al. Electrochemical activity of ruthenium and iridium based catalysts for oxygen evolution reaction. Appl. Catal. B 111, 376–380. https://doi.org/10.1016/j.apcatb.2011.10.020 (2012).
Gust, D., Moore, T. A. & Moore, A. L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 42, 1890–1898. https://doi.org/10.1021/ar900209b (2009).
Gao, M. et al. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 136, 7077–7084. https://doi.org/10.1021/ja502128j (2014).
Huang, J. et al. CoOOH nanosheets with high mass activity for water oxidation. Angew. Chem. Int. Ed. 127(30), 8846–8851. https://doi.org/10.1002/ange.201502836 (2015).
Wang, Y. et al. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 4, 1–7. https://doi.org/10.1002/aenm.201400696 (2014).
Sun, Y. et al. Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci. 5, 3976–3982. https://doi.org/10.1039/C4SC00565A (2014).
Fominykh, K. et al. Iron-doped nickel oxide nanocrystals as highly efficient electrocatalysts for alkaline water splitting. ACS Nano 9, 5180–5188. https://doi.org/10.1021/acsnano.5b00520 (2015).
Meng, Y. et al. Structure–property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 136, 11452–11464. https://doi.org/10.1021/ja505186m (2014).
Gong, M. et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 135, 8452–8455. https://doi.org/10.1021/ja4027715 (2013).
Zhu, W., Zhang, R., Qu, F., Asiri, A. M. & Sun, X. Design and application of foams for electrocatalysis. ChemCatChem 9, 1721–1743. https://doi.org/10.1002/cctc.201601607 (2017).
Lewis, N. S. & Nocera, D. G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. U.S.A. 103, 15729–15735. https://doi.org/10.1073/pnas.0603395103 (2006).
Najafpour, M. M. & Moghaddam, N. J. Iron oxide deposited on metallic nickel for water oxidation. Sustain. Energy Fuels 1, 658–663. https://doi.org/10.1039/C7SE00064B (2017).
Najafpour, M. M. & Moghaddam, N. J. An efficient nickel oxides/nickel structure for water oxidation: A new strategy. New J. Chem. 41, 1909–1913. https://doi.org/10.1039/C6NJ02842J (2017).
Lu, Z. et al. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 50, 6479–6482. https://doi.org/10.1039/C4CC01625D (2014).
Liang, J., Wang, Y. Z., Wang, C. C. & Lu, S. Y. In situ formation of NiO on Ni foam prepared with a novel leaven dough method as an outstanding electrocatalyst for oxygen evolution reactions. J. Mater. Chem. A 4, 9797–9806. https://doi.org/10.1039/C6TA03729A (2016).
Behrouzi, L. et al. Electrochemical alcohols oxidation mediated by N-hydroxyphthalimide on nickel foam surface. Sci. Rep. 10, 1–11. https://doi.org/10.1038/s41598-020-75397-8 (2020).
Lee, K. Y., Lee, M. J., GowriSankar, S. & Kim, J. N. Convenient synthesis of arylpropargyl aldehydes and 4-aryl-3-butyn-2-ones from arylacetylenes and amide acetals. Tetrahedron Lett. 45, 5043–5046. https://doi.org/10.1016/j.tetlet.2004.04.193 (2004).
Scharnagl, F. K. et al. Hydrogenation of terminal and internal olefins using a biowaste-derived heterogeneous cobalt catalyst. Sci. Adv. 4, 1248. https://doi.org/10.1126/sciadv.aau1248 (2018).
Qin, Y. et al. Metal-free chemoselective hydrogenation of unsaturated carbon–carbon bonds via cathodic reduction. Org. Chem. Front. 7, 1817–1822. https://doi.org/10.1039/D0QO00547A (2020).
Li, X. et al. Mott-Schottky effect leads to alkyne semihydrogenation over Pd-nanocube@ N-doped carbon. ACS Catal. 9, 4632–4641. https://doi.org/10.1021/acscatal.9b01001 (2019).
Steffan, M. et al. Carbon-carbon double bond versus carbonyl group hydrogenation: Controlling the intramolecular selectivity with polyaniline-supported platinum catalysts. Adv. Synth. Catal. 350, 1337–1348. https://doi.org/10.1002/adsc.200800035 (2008).
Wang, W., Niu, J. & Yang, Z. An efficient reduction of unsaturated bonds and halogen-containing groups by nascent hydrogen over Raney Ni catalyst. J. Hazard. Mater. 389, 121912. https://doi.org/10.1016/j.jhazmat.2019.121912 (2020).
Shi, Z. et al. Recent advances in the electrochemical hydrogenation of unsaturated hydrocarbons. Curr. Opin. Electrochem. 28, 100713. https://doi.org/10.1016/j.coelec.2021.100713 (2021).
Vilar, M., Oliveira, J. L. & Navarro, M. Investigation of the hydrogenation reactivity of some organic substrates using an electrocatalytic method. Appl. Catal. A 372, 1–7. https://doi.org/10.1016/j.apcata.2009.09.041 (2010).
Vilar, M. & Navarro, M. β-cyclodextrin as inverse phase transfer catalyst on the electrocatalytic hydrogenation of organic compounds in water. Electrochim. Acta 59, 270–278. https://doi.org/10.1016/j.electacta.2011.10.094 (2012).
Casadei, M. A. & Pletcher, D. The influence of conditions on the electrocatalytic hydrogenation of organic molecules. Electrochim. Acta 33, 117–120. https://doi.org/10.1016/0013-4686(88)80042-2 (1988).
Santana, D. S., Lima, M. V., Daniel, J. R. & Navarro, M. Electrocatalytic hydrogenation of organic compounds using current density gradient and sacrificial anode of nickel. Tetrahedron Lett. 44, 4725–4727. https://doi.org/10.1016/S0040-4039(03)01035-9 (2003).
Flinker, M. et al. Efficient water reduction with sp3-sp3 diboron (4) compounds: Application to hydrogenations, H–D exchange reactions and carbonyl reductions. Angew. Chem. Int. Ed. 56, 15910–15915. https://doi.org/10.1002/anie.201709685 (2017).
Chakraborty, S., Bhattacharya, P., Dai, H. & Guan, H. Nickel and iron pincer complexes as catalysts for the reduction of carbonyl compounds. Acc. Chem. Res. 48, 1995–2003. https://doi.org/10.1021/acs.accounts.5b00055 (2015).
Armstrong, K. C. & Waymouth, R. M. Electroreduction of benzaldehyde with a metal–ligand bifunctional hydroxycyclopentadienyl molybdenum (II) hydride. Organometallics 39, 4415–4419. https://doi.org/10.1021/acs.organomet.0c00630 (2020).
Khalilzadeh, M. A., Hosseini, A., Tajbakhsh, M. & Mohannazadeh, F. LiAlH4/silica chloride as a new chemoselective system for reduction of carbonyl compounds and phosphine oxides. J. Iran. Chem. Soc. 5, 699–705. https://doi.org/10.1007/BF03246152 (2008).
Acknowledgements
The authors would like to thank the Institute for Advanced Studies in Basic Sciences for supporting this work.
Author information
Authors and Affiliations
Contributions
The research project was proposed by L.B., B.K. and M.M.N. The experiments were performed by L.B., Z.Z. and M.F. The manuscript was written by L.B., B.K. and M.M.N with contributions from all authors. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behrouzi, L., Zand, Z., Fotuhi, M. et al. Water oxidation couples to electrocatalytic hydrogenation of carbonyl compounds and unsaturated carbon–carbon bonds by nickel. Sci Rep 12, 19968 (2022). https://doi.org/10.1038/s41598-022-23777-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23777-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.