Abstract
We investigated the association between lung function and atrial fibrillation (AF) in 21,349 adults without AF aged ≥ 40 years who underwent spirometry. The study participants were enrolled from the Korean National Health and Nutritional Examination Survey between 2008 and 2016. The primary outcome was new-onset non-valvular AF identified from the National Health Insurance Service database. During the median follow-up of 6.5 years, 2.15% of participants developed new-onset AF. The incidence rate of AF per 1000 person-years was inversely related to the forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC quartile. After adjustment for multiple variables, the AF risk in the lowest FEV1 quartile was 1.64-fold higher than that in the highest quartile (hazard ratio (HR) 1.64 (95% confidence interval (CI) 1.26–2.12) for lowest FEV1 quartile). The lowest quartile of FVC had 1.56-fold higher AF risk than the highest quartile (HR 1.56 (95% CI 1.18–2.08) for lowest FVC quartile). Although the lowest FEV1/FVC quartile was associated with an increased risk of AF in the unadjusted model, this increased risk was not statistically significant in the multivariable analysis. Compared to those with normal lung function, participants with restrictive or obstructive lung function had 1.49 and 1.42-fold higher AF risks, respectively. In this large nationwide cohort study, both obstructive and restrictive patterns of reduced lung function were significantly associated with increased AF risk.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in adults worldwide1. AF is associated with increased risks of stroke, heart failure, and death, leading to a high economic burden2. AF occurs in an estimated 2–4% of adults1. And the incidence rate is expected to gradually increase with the increasing age of the population. Therefore, undiagnosed AF should be recognized in the general population to prevent AF-related complications3. The risk of AF is increased by several factors, including age, male sex, obesity, smoking, alcohol consumption, hypertension, diabetes mellitus (DM), chronic kidney disease (CKD), coronary artery disease, valvular heart disease, heart failure, obstructive sleep apnea, and chronic obstructive pulmonary disease (COPD)2.
AF frequently occurs in patients with airway diseases, such as COPD and asthma. Patients with asthma and COPD have approximately 1.5- and twofold higher AF risks than those without asthma and COPD, respectively4,5. Several factors, such as smoking, hypoxia, inflammation, and increased use of beta-2 agonists, contribute to the development of AF in patients with airway diseases6. Reduced lung function, i.e., low forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), is associated with heart failure and ischemic heart disease (IHD), which are risk factors for AF. In addition, reduced lung function is associated with stroke, which is the most common complication of AF7,8,9. Previous studies have reported that lung function is inversely related to AF risk10,11,12. However, these studies focused on FEV1 and FVC instead of lung function categories, and were conducted in Western populations.
We analyzed a nationwide population-based cohort to investigate the association between reduced lung function and the AF risk in an adult population using two linked Korean National Health Databases: Korean National Health and Nutrition Examination Survey (KNHANES) and National Health Insurance Service (NHIS). To the best of our knowledge, this is the first study to investigate the association between restrictive or obstructive lung function impairment and AF in a nationwide population-based cohort with detailed anthropometric and laboratory results.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of the 21,349 participants. Among the study population, 10.2% and 13.1% had restrictive and obstructive lung function impairments, respectively. Compared to those with normal lung function, participants with restrictive or obstructive lung function impairment had higher prevalence of old age, male sex, current smoking, heavy alcohol consumption, low household income, unemployment, low education, DM, hypertension, dyslipidemia, asthma, prior stroke and prior IHD. Obesity and abdominal obesity were significantly more common in participants with restrictive lung function impairment than the other groups.
Among the study population, 2.15% had new-onset AF during a median follow-up of 6.5 years (interquartile range = 4.5–8.5 years) (Table 2). Compared to those without AF, participants with new-onset AF were more likely to exhibit old age, male sex, current smoking, heavy alcohol consumption, unemployment, obesity, and low income and education levels. Participants with AF had significantly worse baseline lung function and were more likely to have DM, hypertension, asthma, stroke, CKD, and IHD than those without AF.
Lung function quartile and AF
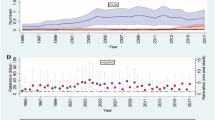
The incidence of AF was inversely related to FEV1, FVC, and FEV1/FVC quartiles. From the highest to lowest quartiles, the incidence rates were 2.82, 2.55, 3.10, and 5.08 for FEV1; 2.11, 2.64, 3.52, and 5.32 for FVC; 1.87, 2.60, 3.51, and 5.71 for FEV1/FVC (Table 3). Therefore, lung function quartiles were inversely associated with AF risk. After adjustment for multiple variables, the AF risks were 1.64-fold higher for the lowest compared to the highest FEV1 quartile (HR 1.64 (95% CI 1.26–2.12) for the lowest FEV1 quartile), and 1.56-fold higher for the lowest compared to the highest FVC quartile (HR 1.56 (95% CI 1.18–2.08) for the lowest FVC quartile), respectively. Although the lowest FEV1/FVC quartile was associated with increased AF risk in the unadjusted model, this inverse association was not statistically significant in the multivariable analysis. Figure 1 shows the AF incidence rates and HRs by lung function deciles.
Incidence rate and hazard ratio of atrial fibrillation according to the decile of lung function measurements. (A) FEV1, (B) FVC, and (C) FEV1/FVC. All HRs adjusted for covariates including age, sex, BMI, household income, education, exercise, alcohol consumption, smoking, hypertension, DM, dyslipidemia, white blood cell count, eGFR, stroke, IHD, and asthma.
Restrictive or obstructive lung function impairment and AF
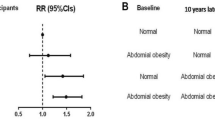
Among participants with restrictive and obstructive lung function impairment, the incidence rates of newly diagnosed AF were 5.92 and 6.96 per 1000 person-years, respectively (Table 4). The age- and sex-adjusted HR for AF according to lung function impairment was 1.64 (95% CI 1.27–2.11) for restrictive lung function impairment and 1.42 (95% CI 1.11–1.81) for obstructive lung function impairment. After adjusting for age, sex, BMI, household income, education, exercise, alcohol consumption, smoking, hypertension, DM, dyslipidemia, stroke, IHD, asthma, white blood cell and eGFR, AF was significantly associated with reduced lung function (HR 1.49 (95% CI 1.15–1.92) for restrictive lung function impairment, HR 1.42 (95% CI 1.11–1.82) for obstructive lung function impairment, respectively)) (Table 4, Fig. 2).
Subgroup analysis
Figure 3 shows the results of the subgroup analysis of reduced lung function and AF risk according to age, sex, smoking, alcohol consumption, DM, hypertension, CKD, stroke, and IHD. The association between reduced lung function and AF risk did not vary according to any of these factors. There were no significant interactions observed in any subgroup.
Subgroup analysis for atrial fibrillation risk. All HRs adjusted for covariates including age, sex, BMI, household income, education, exercise, alcohol consumption, smoking, hypertension, DM, dyslipidemia, white blood cell count, eGFR, stroke, IHD, and asthma. CKD chronic kidney disease, IHD ischemic heart disease.
Discussion
In this large national cohort study, we found that reduced lung function was associated with increased AF risk, with similar risks for obstructive and restrictive lung function impairments. Lung function quartiles were inversely related to the AF risk. The main finding of the present study was that individuals with obstructive or restrictive lung function impairments had approximately 1.4-fold higher AF risk than those with normal lung function.
Most studies of pulmonary function and AF risk have been multicenter cohort studies that mainly focused on the FEV1 and FVC values, rather than categories of lung function impairment11,12,13,14. Moreover, previous studies had significant differences in the FEV1 and FVC categories, follow-up duration and study design. A large Swedish population-based cohort study with a long follow-up duration of 24.8 years showed an inverse relationship between lung function and AF incidence by gender, but many patients were treated in an outpatient rather than an inpatient setting, hospitalized patients were excluded from the study10. The Atherosclerosis Risk In Communities (ARIC) study also reported an inverse relationship between lung function and AF, with a relatively high rate of AF development (11%) during the follow-up of 11 years. However, in the ARIC study, 73.8% of participants were White, who have a higher AF risk than Asians12,13. In addition, the ARIC investigators reported that obstructive lung function impairment was associated with increased AF incidence. However, they did not analyze restrictive lung function impairment in the study. In the Multi-Ethnic Study of Atherosclerosis (MESA) study, the AF incidence varied by race and was significantly lower in non-White patients compared to White patients15. In a cohort study conducted in specific regions of Korea, new-onset AF was identified in 1.7% of the population, and individuals in the lowest FEV1 quartile had 1.59-fold higher AF risk than those in the highest quartile, which is in line with the results of our study. However, in the previous study, AF was not defined using the ICD-10 code14. In the present nationwide population cohort study, including data from the baseline physical examination, laboratory findings, outpatient visits, hospitalization, and medication claims, the AF risk was significantly inversely associated with FEV1 and FVC quartiles after multivariable adjustment. Interestingly, patients with obstructive or restrictive lung function impairment had a 1.4-fold higher AF incidence than those with normal lung function.
The mechanisms underlying the association between decreased lung function and AF are unclear. However, previous studies have suggested several mechanisms, including hypoxia, systemic inflammation, increased sympathetic nerve activation, and the use of COPD treatments, such as beta-2 agonists and oral steroids6. Hypoxia is common in individuals with decreased lung function and causes pulmonary vasoconstriction, which leads to pulmonary hypertension and increased afterload to the right side of the heart16,17. Moreover, chronic hypoxia regulates the expression of hypoxia-inducible factors 1 and 2, the production of reactive oxygen species, blood pressure, and vascular inflammation18. Atrial structural remodeling plays a key role in the development of AF in patients with impaired lung function. In addition, elevated white blood cell count and C-reactive protein levels are associated with increased AF incidence19. Fogarty et al.20 reported that the C-reactive protein level is inversely related to lung function. The present study also showed that the white blood cell count was elevated in individuals with restrictive and obstructive lung function impairments, in line with previous studies. Sympathetic nerve activation, which is related to AF progression, is often found in patients with reduced lung function21. Inhaled beta-2 agonists and anticholinergics, the main treatments for COPD, are highly associated with tachyarrhythmia22. In addition, oral corticosteroids and theophylline increase the risk of AF23.
The present study of nationwide population showed increased AF incidence in patients with reduced lung function. Therefore, individuals with reduced lung function may be potential candidates for more meticulous screening of AF. To the best of our knowledge, this was the first study to investigate the association between obstructive or restrictive lung function impairment and AF risk using detailed anthropometric and laboratory results in a nationwide cohort.
This study has several limitations. First, clinical outcomes relied only on claims data and there might be missed diagnoses of asymptomatic and paroxysmal AF. Second, the pulmonary function tests used in this study could not assess the severity of COPD because of the pre-bronchodilator results. Third, this study was conducted with a single Asian ethnic group. Fourth, we measured lung function using only one method, spirometry without chest computed tomography, which indicates the presence or absence of bronchiectasis or emphysema. However, spirometry is a simple and easy method to check lung function, and trained examiners measured lung function with a predefined protocol. Fifth, medications that could increase the incidence of AF, such as beta-2 agonists and oral steroids were not considered. Sixth, compared to population without lung function impairment, those with obstructive or restrictive lung function impairment may have higher detection rate of AF, due to this group tends to get more medical attention and examination. Last, in this study, more subjects had minor reduced lung function than severely reduced lung function, because KNHANES data were obtained from subjects participating in a national survey. However, the KNHANES data can represent the public health of the entire population of Korea, which has significant implications in many respects24.
Conclusions
We found that reduced lung function was an independent risk factor for AF in a nationwide population cohort study. Subjects with impaired lung function have a high risk of AF, and both obstructive and restrictive lung function impairment have similar AF risks. Future prospective research is needed to clarify the mechanism of association between reduced lung function and AF.
Methods
Source of the database and study population
We derived the data by cross-referencing KNHANES and NHIS. In this study, we used KNHANES data to collect study population and NHIS data to determine clinical outcomes. The KNHANES has been performed by the Korean Centers for Disease Control and Prevention at 3-year intervals since 1998 to monitor the general health and nutritional status of the civilian, noninstitutionalized Korean population25. Sampling units were households selected via a stratified, multistage probability-sampling design that considered geographic area, sex, and age group, by reference to household registries. NHIS is a social insurance payment system that covers approximately 97% of the Korean population. The Korean NHIS data include all national health checkup data and claim data, including drug prescriptions, diagnostic codes for the International Classification of Disease-10 (ICD-10) disease coding system, and claimed treatment details26. All KNHANES participants provided written informed consent for participation.
In this study, data from the KNHANES between 2008 and 2016 were used. We excluded participants among the 40,279 sample individuals as follow, aged < 40 years (n = 4772), participants without spirometry results (n = 10,705), participants who were already diagnosed with AF during wash-out period for 1 year (n = 458), or missing data (n = 2995) (Fig. 4).
The institutional review board of the Catholic University of Korea (IRB no: VC21ZISI0041) approved this study. The study was conducted in compliance with the Declaration of Helsinki. Informed consent was obtained from all participants at the time of survey collection.
Clinical and laboratory measurements
Details of the KNHANES regarding health surveys, standardized physical examinations, laboratory tests, and definitions of risk factors have been described in a previous paper25. Specially trained examiners performed the physical examination by a standardized method. Body mass index (BMI) was calculated as participant body weight in kilograms divided by the square of height in meters. Obesity was defined as subjects with BMI more than 25 kg/m227. Waist circumference (WC) was measured at the midpoint between the lowest rib and the anterior iliac crest in the standing position. Abdominal obesity was defined as subjects with WC more than 90 cm in men and more than 80 cm in women. The definition of abdominal obesity was a WC ≥ 90 cm in men and ≥ 85 cm28. However, our data were provided by masking in 10 cm increments, and the standard of 80 cm in women has been applied. The health-related behavior surveys included well-established questionnaires to determine the demographic and socioeconomic characteristics of the population. Smoking status was divided into three categories: nonsmoker, ex-smoker, or current smoker. Alcohol consumption was assessed based on the average number of alcoholic beverages and frequency of drinking. Heavy drinkers were defined as subjects who drank more than 30 g/day, and subjects drinking less than 30 g/day were classified as mild to moderate drinkers29. Moderate physical activity was defined as walking at least 150 min per week29. Household income was divided into quartile groups: lowest, lower middle, higher middle, and highest. Occupation status was divided into yes or no. High education level was defined as subjects who finished high school education or more.
DM was defined as a fasting glucose level ≥ 126 mg/dL, current use of antidiabetic medications, or a self-reported physician diagnosis of DM30. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, the current use of antihypertensive medications, or a self-reported physician diagnosis of hypertension31. Dyslipidemia was defined as total cholesterol ≥ 240 mg/dL, use of cholesterol-lowering medications, or a self-reported physician diagnosis of dyslipidemia. Asthma, prior stroke, and prior IHD were defined as a self-reported diagnosis by a physician in the health interview surveys31.
After overnight fasting, blood samples were collected from participants’ antecubital veins. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Renal Diet equation from baseline serum creatinine32. CKD was defined as eGFR < 60 mL/min/1.73 m2 for 3 months or more.
Spirometry
Spirometry was performed in individuals aged ≥ 40 years, except those for whom accurate results could not be obtained, such as those with history of laparotomy, ophthalmic surgery, open heart surgery, stroke, myocardial infarction, or cardiac arrest within the past 3 months; aortic aneurysm, retinal detachment, pneumothorax, urinary incontinence, or pulmonary tuberculosis; hemoptysis within the past 1 month; chest or abdominal pain; pain in the mouth or face when biting the mouthpiece; systolic blood pressure > 200 mmHg; diastolic blood pressure > 140 mmHg; dementia; reduced consciousness. Spirometry was performed by four technicians, each of whom underwent two education sessions on lung function tests and quality control. Trained technicians measured the FEV1, FVC and FEV1/FVC ratio using a dry rolling-seal spirometer (model 2130; Sensor Medics, Yorba Linda, CA, USA) and the American Thoracic Society/European Respiratory Society criteria for the standardization of lung function tests33. All of the spirometry values were described as prebronchodilator results34. Normal predictive values were derived taking into account age, sex, height, and ethnicity in large population studies of healthy subjects35. Analyses were performed only on data that met the following criteria: (i) 2 acceptable spirometry curves showing correct test initiation and expiration for at least 6 s and (ii) a greatest difference between two measurements of FEV1 or FVC of < 150 mL. The spirometry results were classified into three groups: normal and restrictive and obstructive lung function impairment. Participants with FEV1/FVC ≥ 0.7 and FVC ≥ 80% of the normal predicted value were considered normal. A restrictive pattern was defined as FEV1/FVC ≥ 0.7 and FVC < 80% predicted. An obstructive pattern was defined as FEV1/FVC < 0.736,37.
Clinical outcomes
The primary outcome was newly diagnosed non-valvular AF, either one diagnosis (ICD-10 code of I48.0-I48.4, I48.9) during hospitalization or more than two diagnoses in the outpatient clinics, until censoring or death38. A washout period of 1 year was set to ensure AF was newly diagnosed. To evaluate newly diagnosed AF, we used data derived from a 2007 cohort of the NHIS with clinical follow-up for the primary outcome through December 31, 2018.
Statistical analysis
Summary statistics are expressed as the means and standard deviations for continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared using Student’s t-test or analysis of variance, as appropriate. Categorical variables were compared using the chi-square test. Multiple comparisons were assessed using the Bonferonni correction. The IR of AF was calculated by dividing the number of AF cases by the sum of the follow-up duration and is presented as the rate per 1000 person-years. Participants were followed until the first diagnosis of AF, censoring by death, or December 31, 2018. Clinical outcomes were determined using the Kaplan–Meier method and compared using the log-rank test. Cox-proportional-hazard models were performed to analyze the impact of lung function on AF risk. The hazard ratio (HR) and 95% confidence interval (CI) were also calculated. A p value < 0.05 was considered statistically significant. To account for potentially confounding clinical covariates and to adjust the established risk factors for AF, multivariable Cox regression models were adjusted for age and sex (model 1); age, sex, BMI, household income, education, exercise, alcohol consumption, smoking, hypertension, DM, and dyslipidemia (model 2); and the variables in model 2 plus white blood cell count, eGFR, stroke, IHD, and asthma (model 3). Time-scale of the models was time on study. Statisticians confirmed the proportional hazard assumption through statistical tests based on Schoenfeld residuals and graphical diagnosis through log–log plots. In Fig. 1, p for trend was calculated through cox proportional regression analysis by considering the decile as a continuous variable. We also conducted subgroup analyses stratified by the confounding factors for the sensitivity analysis. The potential effect of modifications by the subgroups was evaluated using stratified analysis and interaction testing with a likelihood ratio test. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Abbreviations
- AF:
-
Atrial fibrillation
- DM:
-
Diabetes mellitus
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- IHD:
-
Ischemic heart disease
- KNHANES:
-
Korean National Health and Nutrition Examination Survey
- NHIS:
-
National Health Insurance Service
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- ICD:
-
International classification of disease
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- ARIC:
-
Atherosclerosis risk in communities
- MESA:
-
Multi-ethnic study of atherosclerosis study
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation 139, e56–e528 (2019).
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 42, 373–498 (2021).
Krijthe, B. P. et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 34, 2746–2751 (2013).
Ganga, H. V., Nair, S. U., Puppala, V. K. & Miller, W. L. Risk of new-onset atrial fibrillation in elderly patients with the overlap syndrome: a retrospective cohort study. J. Geriatric Cardiol. JGC 10, 129–134 (2013).
Tattersall, M. C. et al. Persistent asthma is associated with increased risk for incident atrial fibrillation in the MESA. Circul. Arrhyth. Electrophysiol. 13, e007685 (2020).
Simons, S. O. et al. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur. Heart J. 42, 532–540 (2021).
Sin, D. D., Wu, L. & Man, S. F. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127, 1952–1959 (2005).
Agarwal, S. K. et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur. J. Heart Fail. 14, 414–422 (2012).
Truelsen, T., Prescott, E., Lange, P., Schnohr, P. & Boysen, G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int. J. Epidemiol. 30, 145–151 (2001).
Johnson, L. S., Juhlin, T., Engström, G. & Nilsson, P. M. Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: the Malmo Preventive Project. Europace : Eur. Pacing Arrhyth. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhyth. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 16, 182–188 (2014).
Buch, P., Friberg, J., Scharling, H., Lange, P. & Prescott, E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur. Respir. J. 21, 1012–1016 (2003).
Li, J. et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 129, 971–980 (2014).
Chahal, H. et al. Ability of reduced lung function to predict development of atrial fibrillation in persons aged 45 to 84 Years (from the Multi-Ethnic Study of Atherosclerosis-Lung Study). Am. J. Cardiol. 115, 1700–1704 (2015).
Kim, B. S. et al. The relationship between decreased pulmonary function and atrial fibrillation in general population: findings from Ansung-Ansan cohort of the Korean Genome and Epidemiology Study. J. Cardiol. 74, 488–493 (2019).
Rodriguez, C. J. et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann. Epidemiol. 25, 71–76, 76.e71 (2015).
Elia, D. et al. Pulmonary hypertension and chronic lung disease: where are we headed? Eur. Resp. Rev. Off. J. Eur. Resp. Soc. 28 (2019).
Walters, T. E. et al. Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: insights into the mechanism of pulmonary vein arrhythmogenesis. Circul. Arrhyth. Electrophysiol. 7, 1189–1197 (2014).
Linz, D. et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 3, 532–540 (2018).
Rienstra, M. et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am. J. Cardiol. 109, 533–537 (2012).
Fogarty, A. W., Jones, S., Britton, J. R., Lewis, S. A. & McKeever, T. M. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax 62, 515–520 (2007).
Linz, D. et al. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 287, 181–188 (2019).
Lahousse, L., Verhamme, K. M., Stricker, B. H. & Brusselle, G. G. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Resp. Med. 4, 149–164 (2016).
Huerta, C., Lanes, S. F. & García Rodríguez, L. A. Respiratory medications and the risk of cardiac arrhythmias. Epidemiology (Cambridge, Mass.) 16, 360–366 (2005).
Kim, Y. The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol. Health 36, e2014002 (2014).
Kweon, S. et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 43, 69–77 (2014).
Lee, Y. H., Han, K., Ko, S. H., Ko, K. S. & Lee, K. U. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diab. Metabol. J. 40, 79–82 (2016).
Seo, M. H. et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obes. Metabol. Synd. 28, 40–45 (2019).
Seo, M. H. et al. Prevalence of obesity and incidence of obesity-related comorbidities in Koreans Based on National Health Insurance Service Health Checkup Data 2006–2015. J. Obes. Metabol. Synd. 27, 46–52 (2018).
Park, S. et al. Dose-response relationship between cigarette smoking and risk of ulcerative colitis: a nationwide population-based study. J. Gastroenterol. 54, 881–890 (2019).
Choi, S. I. et al. Relationship between regional body fat distribution and diabetes mellitus: 2008 to 2010 Korean National Health and Nutrition Examination Surveys. Diab. Metabol. J. 41, 51–59 (2017).
Park, S. E. et al. Dose-dependent effect of smoking on risk of diabetes remains after smoking cessation: a nationwide population-based cohort study in Korea. Diab. Metabol. J. (2021).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 53, 982–992 (2009).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338 (2005).
Kim, S. W. et al. The relationship between the number of natural teeth and airflow obstruction: a cross-sectional study using data from the Korean National Health and Nutrition Examination Survey. Int. J. Chronic Obstr. Pulm. Dis. 11, 13–21 (2016).
Ranu, H., Wilde, M. & Madden, B. Pulmonary function tests. Ulster Med. J. 80, 84–90 (2011).
Park, H. J. et al. Comorbidities in obstructive lung disease in Korea: data from the fourth and fifth Korean National Health and Nutrition Examination Survey. Int. J. Chronic Obst. Pulm. Dis. 10, 1571–1582 (2015).
Godfrey, M. S. & Jankowich, M. D. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest 149, 238–251 (2016).
Kang, S. H. et al. Underweight is a risk factor for atrial fibrillation: A nationwide population-based study. Int. J. Cardiol. 215, 449–456 (2016).
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number: HI18C0275).
Author information
Authors and Affiliations
Contributions
S.N.L. and S.H.K. take full responsibility for the content of this manuscript, including the data and analyses. S.H.H. made substantial contributions to the concept and design of the study. K.D.H. developed the design of the methodology. D.G.M., S.K.K., K.D.Y., and Y.B.A. made substantial contributions to the analysis and interpretation of data. S.N.L., S.H.K. and S.K.K. drafted the initial manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.N., Ko, SH., Her, SH. et al. Association between lung function and the risk of atrial fibrillation in a nationwide population cohort study. Sci Rep 12, 4007 (2022). https://doi.org/10.1038/s41598-022-07534-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07534-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.