Abstract
The current study aimed to evaluate whether cerebrospinal fluid (CSF) neuron-specific enolase (NSE) levels are elevated in amyotrophic lateral sclerosis (ALS) and are effective in distinguishing ALS from cervical spondylotic myelopathy (CSM). We retrospectively evaluated 45 patients with ALS, 23 with CSM, 28 controls, and 10 with Parkinson’s disease (PD) who underwent analysis of CSF NSE levels. The control group comprised patients aged above 45 years who underwent lumbar puncture because of suspected neurological disorders that were ruled out after extensive investigations. CSF NSE levels were evaluated using the electro-chemiluminescent immunoassay. The ALS group had significantly higher CSF NSE levels than the CSM and control groups (P < 0.001 for both comparisons). The CSM, control, and PD groups did not significantly differ in terms of CSF NSE levels. A receiver-operating characteristic curve analysis was performed to assess the diagnostic value of CSF NSE levels in distinguishing ALS from CSM. The area under the curve for CSF NSE levels was 0.86. The optimal cutoff value was 17.7 ng/mL, with a specificity of 87% and a sensitivity of 80%. Hence, CSF NSE levels are elevated in ALS and are effective in distinguishing ALS from CSM.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal disease characterized by the neurodegeneration of both upper and lower motor neurons. The pathogenesis of the condition is unclear, and its diagnosis is made clinically1. As there are no specific tests for ALS, a detailed set of diagnostic criteria has been established2. However, some patients with ALS do not fulfill the clinical criteria on ALS particularly at the early stage, and they are misdiagnosed with different neurological and medical disorders1,3. Importantly, a misdiagnosis of cervical spondylotic myelopathy (CSM) is an important problem. ALS is most commonly misdiagnosed as CSM4,5. Patients with ALS present with focal muscle weakness and atrophy without bulbar symptoms at the early stage of the disease3,6, which is similar to cervical spondylosis (CS). Among CS, ALS characterized by lower limb spasm but without radicular pain might be easy to distinguish from cervical spondylotic radiculopathy, but not from CSM. Further, a misdiagnosis of CSM may lead to unnecessary surgery7,8 and subsequently more rapid deterioration because some patients with ALS experience accelerated disease progression after operation9,10. A novel tool is required to differentiate ALS from CSM.
Neuron-specific enolase (NSE) is a glycolytic enzyme predominantly observed in neurons and endocrine cells11. The intraneuronal NSE is secreted into the extracellular space after substantial neuronal damage. However, NSE is not physically secreted. Therefore, an elevated CSF NSE level mainly reflects neuronal damage12. In fact, this phenomenon is observed in different conditions associated with central nervous system damage, such as traumatic brain injury13, traumatic spinal cord injury14, acute brain infarction15,16, Parkinson’s disease (PD)17, Alzheimer’s disease18 multiple system atrophy19, bacterial meningoencephalitis20, and Creutzfeldt–Jakob disease21. However, thus far, there have been no reports, at least those written in English, about CSF NSE levels in ALS.
Therefore, this study investigated whether CSF NSE levels are elevated in ALS and whether they are a useful biomarker for distinguishing ALS from CSM.
Results
Characteristics of patients
The characteristics of the four groups at time of CSF sampling are shown in Table 1. One-third of patients with ALS did not present with bulbar symptoms, and about one-half had cervical cord compression on magnetic resonance imaging (MRI). All patients finally fulfilled the criteria on definite, probably, or PLS ALS. However, approximately one-half did not meet the criteria at time of CSF sampling. There were no significant differences in terms of age and the proportion of male patients between the ALS, CSM, control, and PD groups. There was no significant difference in terms of disease duration between the ALS, CSM, and PD groups. All patients with CSM had cervical cord compression on MRI according to the inclusion criteria of this study. Further, there were significant differences in the proportion of patients with cervical cord compression between the four groups (P < 0.001).
CSF NSE levels
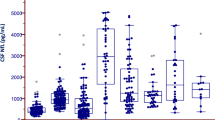
The ALS group (mean ± standard deviation: 21.0 ± 5.1 ng/mL) had significantly higher CSF NSE levels than the CSM (13.7 ± 4.3 ng/mL, P < 0.001) and control (13.6 ± 4.0 ng/mL, P < 0.001) groups (Fig. 1). There was no significant difference in terms of CSF NSE levels between the CSM, control, and PD groups. To control the confounding effects of age and sex, we further performed several subgroup analyses of male and female patients and those aged < 70 and ≥ 70 years, respectively (Fig. 2), although the PD group was excluded from the further analysis because of its small sample size. In the subgroup analyses of men and those aged < 70 and ≥ 70 years, the ALS group had significantly higher CSF NSE levels than the CSM group (P < 0.001 for male patients, P = 0.001 for those aged < 70 years, and P = 0.002 for those aged ≥ 70 years) and the control group (P < 0.001 for male patients, P = 0.017 for those aged < 70 years, and P < 0.001 for those aged ≥ 70 years). The CSF NSE levels did not differ between the CSM and control groups. In a subgroup analysis of women, the ALS group had significantly higher CSF NSE levels than the control group (P = 0.001). Moreover, the ALS group had higher CSF NSE levels than the CSM group (P = 0.133) although the results did not significantly differ possibly due to the small sample size. An ROC curve analysis was performed to assess the diagnostic value of CSF NSE levels in distinguishing ALS from CSM (Fig. 3). The AUC of CSF NSE levels was 0.86. The optimal cutoff value was 17.7 ng/mL, with a specificity of 87% and sensitivity of 80%.
CSF NSE levels of the ALS, CSM, and control groups based on the subgroup analyses of (A) male and (B) female patients, (C) those aged < 70 years, and (D) those aged ≥ 70 years. The solid line represents the mean CSF NSE levels of each group. ALS amyotrophic lateral sclerosis, CSF cerebrospinal fluid, CSM cervical spondylotic myelopathy, NSE neuron-specific enolase.
Figure 4 shows the associations between CSF NSE levels and clinical characteristics in patients with ALS at the time of CSF sampling. Patients with possible or suspected ALS (22.5 ± 5.2 ng/mL) had significantly higher CSF NSE levels than those with definite, probable, or PLS ALS (19.5 ± 4.5 ng/mL, P = 0.046). Moreover, the CSF NSE levels were significantly higher in patients with ALS who had an ALSFRS-R score of > 36 (22.4 ± 5.3 ng/mL) than in those with an ALSFRS-R score of ≤ 36 (19.2 ± 4.3 ng/mL, P = 0.037). There were no significant differences in terms of CSF NSE levels between patients with and without bulbar symptoms; those who had a disease duration of ≤ 12 and > 12 months; and those with and without cervical cord compression on MRI. ROC curve analysis was performed to assess the diagnostic value of CSF NSE levels in distinguishing ALS with several features from CSM. The AUCs of CSF NSE levels were 0.91 in ALS without bulbar symptoms, 0.87 in ALS with cervical cord compression, and 0.91 in ALS that do not fulfill the criteria on definite, probable, or PLS.
Associations between CSF NSE levels and clinical characteristics in patients with ALS at the time of CSF sampling. The CSF NSE levels of patients (A) with a disease duration of ≤ 12 and > 12 months, (B) those with and without bulbar symptoms, (C) those with an ALSFRS-R score of > 36 and ≤ 36, (D) those with and without cervical cord compression on MRI, and (E) those with possible or suspected ALS and definite, probable, or PLS ALS. The solid line represents the mean CSF NSE levels of each group. ALS amyotrophic lateral sclerosis, ALSFRS-R revised ALS functional rating scale score, CSF cerebrospinal fluid, CSM cervical spondylotic myelopathy, NSE neuron-specific enolase, PLS probable laboratory-supported.
Discussion
The primary finding of this study is that CSF NSE levels are elevated in ALS. Based on a previous study, they are influenced by age and sex22. Moreover, this research showed elevated CSF NSE levels in ALS via the subgroup analyses of male and female patients and those aged < 70 and ≥ 70 years, which were performed to control the confounding effects of age and sex. To the best of our knowledge, this study, at least among those written in English, first showed elevated CSF NSE levels in ALS. Another main finding is that CSF NSE levels are higher in ALS than in CSM; therefore, they are useful in distinguishing ALS from CSM. In relation to the finding, the CSF NSE levels were not elevated in CSM. In the literature, the CSF NSE levels in CSM are controversial. That is, a previous report showed high CSF NSE levels in CSM23. Meanwhile, another revealed normal levels24. Taken together, CSF NSE levels in CSM may not be as elevated as those in ALS. Hence, they can be used to distinguish ALS from CSM.
There are several explanations why the ALS group had higher CSF NSE levels than not only the control but also CSM groups, even though NSE is generally a non-specific marker of neural damage12. First, the difference in CSF NSE levels may reflect different degrees of neural damage. A widespread and aggressive neural damage in ALS can result in significantly elevated CSF NSE levels. However, a limited and non-aggressive neural damage in CSM may not. Second, differences in CSF NSE levels can reflect varying affected areas. CSM patients have degenerations in the anterior horn cells of the spinal cord. On the other hand, ALS patients have degenerations not only in the anterior horn cells of the spinal cord but also the Betz cells of the brain cortex and the motor nuclei of the brainstem. Notably, the brain cortex, which could be involved in ALS but not in CSM, has been reported to have high NSE levels25. Third, differences in CSF NSE levels may reflect varying pathologic processes. Although NSE is expressed in neuronal cells and is secreted into the extracellular space after substantial damage of neuronal cells as mentioned previously, NSE has been reported to be included in not only neuronal cells but also microglia26, astrocytes27, and oligodendrocytes28. Moreover, NSE expression and activity are markedly increased in neuronal and glial cells under several pathologic processes. That is, NSE may not be a non-specific marker of neural damage, but may play a role in several pathologic processes in which not only neuronal cells but only glial cells may be involved, and the mechanism might occur in ALS. In fact, NSE has been increased and involved in pathologic processes such as neuroinflammation, particularly in the expression of pro-inflammatory cytokines and the proliferation of inflammatory glial cells25,29. In addition, the importance of neuroinflammation in ALS has been reported30. However, specific pathologic processes related to elevated CSF NSE levels in ALS is not addressed. Moreover, the pathologic process related to the elevation of CSF NSE levels might not be triggered by toxic factors in the CSF of patients with ALS. Askanas et al. treated cultured rat motor neurons with CSF from patients with ALS and measured the NSE levels in the neurons primarily as an index of neuronal health31. The authors found that treatment with CSF from patients with ALS did not alter NSE levels in cultured rat motor neurons; thus, they concluded that the study failed to demonstrate the presence of toxic factors in the CSF from patients with ALS that would influence rat motor neurons. We further speculate that they also failed to demonstrate the presence of toxic factors in the CSF from patients with ALS which would trigger pathologic changes related to increased NSE expression in rat motor neurons.
Spinal MRI is a useful but insufficient tool for distinguishing ALS from CSM. The reason is that ALS patients must frequently exhibit degenerative disk disease and spondylosis of the cervical spine in MRI imaging regardless of symptomatic or asymptomatic, because both ALS and CSM preferentially affect individuals of middle of old age. In fact, approximately half of the patients with ALS were reported to have concomitant CS32. Moreover, asymptomatic disk disease and spondylosis of the cervical spine in MRI imaging were reported to be frequent in individuals of middle of old age33. The findings are also consistent with our results that one-half of patients with ALS had cervical cord compression on MRI regardless of symptomatic or asymptomatic. Subsequently, the misdiagnosis of CSM has been reported to be frequent among patients with ALS4,5, and concomitant ALS might be missed in those with CSM. In terms of treatment approaches, a misdiagnosis of CSM in patients with ALS is crucial because it may lead to unnecessary surgery and subsequent more rapid deterioration as mentioned in introduction. Notably, overlooking concomitant ALS in patients with CSM might be also crucial. Surgical treatment should be carefully considered in patients with CSM and concomitant ALS, even in those with symptomatic CSM and concomitant ALS. Although surgery for CS results in temporary alleviation, the major cause of motor symptoms is usually attributed to ALS in those patients and the motor symptoms might deteriorate more rapidly in some cases32. Therefore, CSF NSE level is a useful marker to determine the presence of ALS without the potential influence of CSM.
Distinguishing ALS from CSM is challenging, particularly when patients with ALS present with cervical cord compression on MRI and they do not experience bulbar symptoms and do not fulfill the criteria on ALS. In this study, patients with ALS and such features had significantly higher CSF NSE levels than those with ALS without such features, or the CSF NSE levels of the former group was as high as those of the latter group. In detail, patients with ALS who do not fulfil the criteria on definite, probable, or PLS ALS had significantly higher CSF NSE levels than those who fulfilled the criteria. The CSF NSE levels of patients with ALS with cervical cord compression was as high as those of patients with ALS without compression. Moreover, the CSF NSE levels of patients with ALS without bulbar symptoms was as high as those of patients with ALS with the symptoms. Consequently, the diagnostic values of CSF NSE levels in distinguishing ALS with such features from CSM were higher or as high as those of CSF NSE levels in distinguishing whole ALS from CSM. In cases in which patients with ALS are challenging to distinguish from those with CSM, CSF NSE can be used. Hence, it may be an effective biomarker.
The reason why patients with ALS who do not fulfil the criteria had significantly higher CSF NSE levels than those who fulfilled the criteria is uncertain. However, it could be explained by a hypothesis that CSF NSE levels might decrease with disease progression at a certain stage because it could be accompanied by a decreased number of motor neurons, which might be the source of CSF NSE. Notably, the hypothesis could also explain our findings that patients with mild ALS had higher CSF NSE levels than other patients.
CSF NSE levels have been reported to be elevated in PD17. In this study, while mean CSF NSE levels were higher in PD group than in CSM and control groups and lower in PD groups than in ALS group, these differences did not reach statistical significance. Because these findings may be influenced by small sample size of the patients especially with PD, further studies are needed.
Our study had several limitations. First, it had a small sample size and was retrospective in nature. Hence, further large prospective studies should be conducted. Second, the control group only comprised unhealthy patients who underwent lumbar puncture, which is an invasive test, because of suspected neurological disorders that were ruled out after extensive investigations.
CSF NSE levels are elevated in ALS. Further, they can effectively distinguish ALS from CSM and prevent the misdiagnosis of CSM in patients with ALS. Thus, unnecessary surgery and subsequent rapid deterioration may be prevented. Notably, numerous physicians including those in general medical institutions can benefit from the use of this biomarker in daily clinical practice because NSE is a common tumor marker for diseases including small lung cancer and can be measured in general medical institutions. In addition, because elevated CSF NSE levels in ALS may reflect a specific pathologic process, this finding could provide new perspectives regarding the understanding of ALS pathogenesis and could facilitate the development of appropriate treatments.
Materials and methods
Patients
We retrospectively evaluated 45 patients with ALS, 23 with CSM, 10 with PD, and 28 controls who were admitted to Osaka Medical and Pharmaceutical University Hospital and who underwent lumbar puncture and subsequent analysis of CSF NSE levels from January 2014 to January 2021. Patients were diagnosed with definite, probable, or probable laboratory-supported (PLS) ALS according to the revised El Escorial criteria2. Since the criteria include EMG findings of fibrillation potentials, positive sharp waves, large motor unit potentials, reduced interference pattern, and unstable motor unit potentials, we performed EMG in all ALS patients and carefully check the presence or absence of these findings. The diagnosis of ALS based on the criteria requires the absence of electrophysiological or pathological evidence of other disease processes that might explain the signs of lower and/or upper motor neuron degeneration. Therefore, we performed nerve conduction study in all ALS patients. The diagnosis also requires the absence of neuroimaging evidence of other disease processes that might explain the observed clinical and electrophysiological signs, we performed brain MRI in all but one ALS patient in whom we performed brain CT at least, and cervical MRI in 21 of the 41 ALS patients. Those with ALS and concomitant CSM were classified under the ALS group. CSM was diagnosed based on the presence of myelopathic symptoms, such as limb numbness, problems with fine motor skills, and gait disturbance, and radiologic cervical cord compression in the stenotic canal, which is correlated with the patients’ symptoms. The control group comprised patients aged above 45 years who underwent lumbar puncture due to suspected neurological disorders that were ruled out after extensive investigations. As NSE was reported to be a biomarker of PD17, we included a group of 10 PD patients. Not only controls and patients diagnosed with ALS, CSM, and PD at the time of CSF sampling but also those diagnosed at a later time (up to February 2021) were included. Patients with other concomitant neurological or neuromuscular disorders were excluded.
Information about age, sex, disease duration, neurological symptoms, disability, spinal MRI findings, and ALS categories according to the El Escorial criteria were collected at the time of CSF sampling. Based on the presence of a concave defect in the cervical cord caused by the impingement of the disc or osseous material on MRI regardless of defect degree and its symptoms, cervical cord compression was considered (Fig. 5). Disability associated with ALS was determined using the Revised ALS Functional Rating Scale Score (ALSFRS-R), which has a maximum of 48 points. Lower scores represent a more severe disease stage34.
Cervical cord compression on MRI. (A) T1- and (B) T2-weighted sagittal images of 50-year-old man with CSM show posterolateral disk protrusion at C3/4 levels producing concave defect in cervical cord (arrow). (C) T1-weighed axial image at C3/4 level shows posterolateral disk protrusion (arrow) compressing spinal cord (arrowhead). CSM cervical spondylotic myelopathy.
This study was conducted according to the 2013 Helsinki Declaration, and the Osaka Medical and Pharmaceutical University Ethics Committee approved the study protocol and the need for informed consent was waived because this was a retrospective study and the data were collected without individual patient identifiers (Approval number # 2020-189).
CSF NSE analysis
CSF samples were collected via lumbar puncture. Then, they were immediately brought to the laboratory for analysis. CSF NSE levels were evaluated using the electro-chemiluminescent immunoassay performed by SRL (Tokyo, Japan). The detection limit was 0.1 ng/mL.
Statistical analysis
The Mann–Whitney U test was used to assess differences in continuous variables between two groups. The Kruskal–Wallis test, followed by the Dunn’s multiple comparison test, was utilized to evaluate differences between three or four groups. Meanwhile, the chi-square test was applied to examine categorical variables. To investigate the accuracy of biomarkers in differentiating ALS from CSM, a receiver operating characteristic (ROC) curve analysis was performed by calculating the area under the ROC curve (AUC). The optimal cutoff value was chosen using the maximized Youden index. The values were expressed as mean ± standard deviation, and a P value of < 0.05 was considered statistically significant. All analyses were performed using the JMP software version 15.0 (SAS Institute Inc., Cary, NC, the USA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hardiman, O., van den Berg, L. H. & Kiernan, M. C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 639–649. https://doi.org/10.1038/nrneurol.2011.153 (2011).
Brooks, B. R., Miller, R. G., Swash, M., Munsat, T. L. & World Federation of Neurology Research Group on Motor Neuron, D. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Later. Scler Other Motor Neuron Disord. 1, 293–299. https://doi.org/10.1080/146608200300079536 (2000).
Jacobson, R. D., Goutman, S. A. & Callaghan, B. C. Pearls & Oy-sters: The importance of atypical features and tracking progression in patients misdiagnosed with ALS. Neurology 86, e136-139. https://doi.org/10.1212/WNL.0000000000002522 (2016).
Cellura, E., Spataro, R., Taiello, A. C. & La Bella, V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin. Neurol. Neurosurg. 114, 550–554. https://doi.org/10.1016/j.clineuro.2011.11.026 (2012).
Rowland, L. P. Diagnosis of amyotrophic lateral sclerosis. J. Neurol. Sci. 160(Suppl 1), S6-24. https://doi.org/10.1016/s0022-510x(98)00193-2 (1998).
Williams, J. R., Fitzhenry, D., Grant, L., Martyn, D. & Kerr, D. A. Diagnosis pathway for patients with amyotrophic lateral sclerosis: Retrospective analysis of the US Medicare longitudinal claims database. BMC Neurol. 13, 160. https://doi.org/10.1186/1471-2377-13-160 (2013).
Kraemer, M., Buerger, M. & Berlit, P. Diagnostic problems and delay of diagnosis in amyotrophic lateral sclerosis. Clin. Neurol. Neurosurg. 112, 103–105. https://doi.org/10.1016/j.clineuro.2009.10.014 (2010).
Yoshor, D., Klugh, A. 3rd., Appel, S. H. & Haverkamp, L. J. Incidence and characteristics of spinal decompression surgery after the onset of symptoms of amyotrophic lateral sclerosis. Neurosurgery 57, 984–989. https://doi.org/10.1227/01.neu.0000180028.64385.d3 (2005) (discussion 984–989).
Sostarko, M., Vranjes, D., Brinar, V. & Brzovic, Z. Severe progression of ALS/MND after intervertebral discectomy. J. Neurol. Sci. 160(Suppl 1), S42-46. https://doi.org/10.1016/s0022-510x(98)00197-x (1998).
Pinto, S., Swash, M. & de Carvalho, M. Does surgery accelerate progression of amyotrophic lateral sclerosis?. J. Neurol. Neurosurg. Psychiatry 85, 643–646. https://doi.org/10.1136/jnnp-2013-305770 (2014).
Kato, K., Ishiguro, Y., Suzuki, F., Ito, A. & Semba, R. Distribution of nervous system-specific forms of enolase in peripheral tissues. Brain Res 237, 441–448. https://doi.org/10.1016/0006-8993(82)90455-3 (1982).
Isgro, M. A., Bottoni, P. & Scatena, R. Neuron-specific enolase as a biomarker: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 867, 125–143. https://doi.org/10.1007/978-94-017-7215-0_9 (2015).
Herrmann, M. et al. Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor. Neurol. Neurosci. 14, 109–114 (1999).
Pouw, M. H. et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: A descriptive analysis of 16 subjects. Spinal Cord. 52, 428–433. https://doi.org/10.1038/sc.2014.26 (2014).
Selakovic, V., Raicevic, R. & Radenovic, L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J. Clin. Neurosci. 12, 542–547. https://doi.org/10.1016/j.jocn.2004.07.019 (2005).
Hay, E. et al. Cerebrospinal fluid enolase in stroke. J. Neurol. Neurosurg. Psychiatry 47, 724–729. https://doi.org/10.1136/jnnp.47.7.724 (1984).
Papuc, E. & Rejdak, K. Increased cerebrospinal fluid S100B and NSE reflect neuronal and glial damage in Parkinson’s disease. Front. Aging Neurosci. 12, 156. https://doi.org/10.3389/fnagi.2020.00156 (2020).
Christl, J. et al. Association of cerebrospinal fluid S100B protein with core biomarkers and cognitive deficits in prodromal and mild Alzheimer’s disease. J. Alzheimers Dis. 72, 1119–1127. https://doi.org/10.3233/JAD-190550 (2019).
Abdo, W. F. et al. Cerebrospinal fluid analysis differentiates multiple system atrophy from Parkinson’s disease. Mov. Disord. 19, 571–579. https://doi.org/10.1002/mds.10714 (2004).
Kepa, L. Evaluation of cerebrospinal fluid and plasma neuron-specific enolase (NSE) concentration in patients with purulent, bacterial meningoencephalitis. Przegl. Epidemiol. 63, 23–28 (2009).
Zerr, I. et al. Cerebrospinal fluid concentration of neuron-specific enolase in diagnosis of Creutzfeldt–Jakob disease. Lancet 345, 1609–1610. https://doi.org/10.1016/s0140-6736(95)90118-3 (1995).
Nygaard, O., Langbakk, B. & Romner, B. Neuron-specific enolase concentrations in serum and cerebrospinal fluid in patients with no previous history of neurological disorder. Scand. J. Clin. Lab. Investig. 58, 183–186. https://doi.org/10.1080/00365519850186562 (1998).
Yasuda, T., Yanagi, T., Mokuno, K. & Kato, K. Neuron-specific enolase (NSE) and S-100b protein in cerebrospinal fluid of patients with cervical spondylosis–the relations with MRI findings and the changes of NSE and S-100b protein levels through Glisson’s traction. Rinsho Shinkeigaku 32, 1299–1301 (1992).
Takahashi, H. et al. Axonal damage is remarkable in patients with acutely worsening symptoms of compression myelopathy: Biomarkers in cerebrospinal fluid samples. Eur. Spine J. 27, 1824–1830. https://doi.org/10.1007/s00586-018-5549-5 (2018).
Haque, A., Polcyn, R., Matzelle, D. & Banik, N. L. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. https://doi.org/10.3390/brainsci8020033 (2018).
Pislar, A., Bozic, B., Zidar, N. & Kos, J. Inhibition of cathepsin X reduces the strength of microglial-mediated neuroinflammation. Neuropharmacology 114, 88–100. https://doi.org/10.1016/j.neuropharm.2016.11.019 (2017).
Vinores, S. A. & Rubinstein, L. J. Simultaneous expression of glial fibrillary acidic (GFA) protein and neuron-specific enolase (NSE) by the same reactive or neoplastic astrocytes. Neuropathol. Appl. Neurobiol. 11, 349–359. https://doi.org/10.1111/j.1365-2990.1985.tb00031.x (1985).
Deloulme, J. C. et al. Expression of the neuron-specific enolase gene by rat oligodendroglial cells during their differentiation. J. Neurochem. 66, 936–945. https://doi.org/10.1046/j.1471-4159.1996.66030936.x (1996).
Haque, A., Ray, S. K., Cox, A. & Banik, N. L. Neuron specific enolase: A promising therapeutic target in acute spinal cord injury. Metab. Brain Dis. 31, 487–495. https://doi.org/10.1007/s11011-016-9801-6 (2016).
Chiot, A., Lobsiger, C. S. & Boillee, S. New insights on the disease contribution of neuroinflammation in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 32, 764–770. https://doi.org/10.1097/WCO.0000000000000729 (2019).
Askanas, V., Marangos, P. J. & Engel, W. K. CSF from amyotrophic lateral sclerosis patients applied to motor neurons in culture fails to alter neuron-specific enolase. Neurology 31, 1196–1197. https://doi.org/10.1212/wnl.31.9.1196 (1981).
Yamada, M., Furukawa, Y. & Hirohata, M. Amyotrophic lateral sclerosis: Frequent complications by cervical spondylosis. J. Orthop Sci. 8, 878–881. https://doi.org/10.1007/s00776-003-0712-0 (2003).
Teresi, L. M. et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology 164, 83–88. https://doi.org/10.1148/radiology.164.1.3588931 (1987).
Cedarbaum, J. M. et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 169, 13–21. https://doi.org/10.1016/s0022-510x(99)00210-5 (1999).
Author information
Authors and Affiliations
Contributions
A.T. designed the study, carried out the acquisition of data, and wrote the manuscript. T.H. designed the study, carried out the acquisition of data, analyzed the data, and wrote the manuscript. D.N. analyzed the data. T.K. supervised the manuscript. S.I. supervised the manuscript. T.T. supervised the manuscript. F.K. supervised the manuscript. S.A. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsukahara, A., Hosokawa, T., Nishioka, D. et al. Neuron-specific enolase level is a useful biomarker for distinguishing amyotrophic lateral sclerosis from cervical spondylotic myelopathy. Sci Rep 11, 22827 (2021). https://doi.org/10.1038/s41598-021-02310-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02310-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.