Abstract
Although there is still no standard treatment for recurrent glioblastoma multiforme (rGBM), re-irradiation could be a therapeutic option. We retrospectively evaluated the efficacy and safety of re-irradiation using helical TomoTherapy (HT) with a simultaneous integrated boost (SIB) technique in patients with rGBM. 24 patients with rGBM underwent HT-SIB. A total dose of 20 Gy was prescribed to the Flair (fluid-attenuated inversion recovery) planning tumor volume (PTV) and 25 Gy to the PTV-boost (T1 MRI contrast enhanced area) in 5 daily fractions to the isodose of 67% (maximum dose within the PTV-boost was 37.5 Gy). Toxicity was evaluated by converting the 3D-dose distribution to the equivalent dose in 2 Gy fractions on a voxel-by-voxel basis. Median follow-up after re-irradiation was 27.8 months (range 1.6–88.5 months). Median progression-free survival (PFS) was 4 months (95% CI 2.0–7.9 months), while 6-month PFS was 41.7% (95% CI 22.2–60.1 months). Median overall survival following re-irradiation was 10.7 months (95% CI 7.4–16.1 months). There were no cases of re-operation due to early or late toxicity. Our preliminary results suggest that helical TomoTherapy with the proposed SIB technique is a safe and feasible treatment option for patients with rGBM, including those large disease volumes, reducing toxicity.
Similar content being viewed by others
Introduction
Glioblastoma (GBM) relapses up to 90% of cases in close proximity to the site of resection or the initially irradiated tumor1. Median survival after progression is around 6 months2. The management of recurrent glioblastoma (rGBM) is challenging and there is still no standard treatment3,4. Radiotherapy (RT) could represent a valid treatment option. Historically, radiation oncologists have been cautious about re-irradiating brain tumors because of the risk of toxicity5. There are over 50 clinical studies in the literature on the re-irradiation of gliomas6,7, the majority retrospective in nature and reporting a variety of techniques, including brachytherapy, single-fraction stereotactic radiosurgery (SRS), fractionated stereotactic RT (FSRT) or hypofractionated stereotactic radiotherapy (HSRT)3,8,9,10,11,12,13,14,15,16,17,18.
Traditionally, the definition of the target volume for re-irradiation of rGBM is based on T1-weighted magnetic resonance imaging (MRI) with gadolinium19. Contrast enhancement is a consequence of the disruption of the blood–brain barrier, which does not necessarily reflect the real tumor extension in gliomas. Gross tumor mass has been detected beyond the margins of contrast enhancement, in the surrounding edema and even in adjacent normal-appearing brain tissue20,21,22. This prompted us to investigate the potential for re-irradiating rGBM using image-guided helical TomoTherapy (HT) with a simultaneous integrated boost (SIB) technique (HT-SIB) to simultaneously treat the tumor and the peritumoral area where there is suspected tumor diffusion. HT is capable of exploiting the biological advantages of SIB, an accelerated form of radiotherapy in which a higher dose can be delivered to the gross tumor volume (GTV), while a lower dose is simultaneously delivered to areas of subclinical disease23,24,25. Other studies have evaluated the feasibility of HT-SIB in the treatment of brain tumor and brain metastases, with good results26,27,28. HT combines fan-beam intensity-modulated radiotherapy with megavoltage computed tomography (MVCT) imaging for accurate patient positioning. Such a combination is a potentially viable alternative to conventional stereotactic frame systems for precision radiotherapy28. The purpose of the present retrospective study was to evaluate the efficacy and safety of this treatment in rGBM.
Patients and methods
Patient eligibility
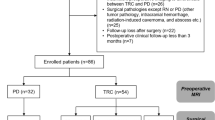
This retrospective analysis included patients with rGBM treated in a single institute. Data on patient characteristics and treatment were retrospectively collected from computerized medical records in accordance with institutional ethical policies. Adult patients (aged ≥ 18 years) with clinical and /or imaging evidence of malignant GBM progression or recurrence as defined by RANO (response assessment for neuro-oncology) criteria29 received salvage re-irradiation (reRT). Main inclusion criteria are shown in Table 1. Exclusion criteria were as follows: (a) radiotherapy < 12 weeks prior to the diagnosis of progression if the lesion was in the radiation field and (b) T1-enhanced lesion and T2/Flair (T2-weighted fluid-attenuated inversion recovery) non-enhanced areas involving the brainstem and/or optic apparatus. At the time of recurrence, patients were evaluated for salvage treatment (including surgery, RT or chemotherapy) based on their clinical conditions, tumor site and volume, and hematologic rescue. A multidisciplinary team including a medical oncologist, radiation oncologist and neurosurgeon evaluated each clinical case to define the appropriate therapeutic strategy.
Imaging and delineation
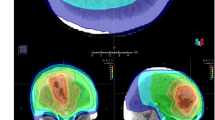
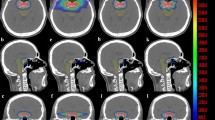
Planning computed tomography (CT) (BrillianceBig Bore CT Philips, Crowley, UK) was obtained with a 1–3-mm slice thickness. Patients were placed in the supine position with arms close to their body and were immobilized with a frameless thermoplastic mask. A co-registration of volumetric CT and MR sequences (1–3-mm slice thickness T1, enhanced T1, Flair and T2 MRI) was performed was used to define the target and organs at risk (OAR). The planning target volume (PTV Flair) was defined on the area of peritumoral edema using T1, T2 and Flair sequences with a 2-mm margin expansion. The PTV-boost was defined as the visible tumor on enhanced T1-MRI with a 1-mm margin expansion (Fig. 1a). OARs were identified as healthy brain, optic chiasm, optic nerves and brainstem.
Treatment planning
A total dose of 20 Gy was prescribed to the PTV Flair (99% isodose line covering 99% of the PTV), 25 Gy was prescribed to the PTV-boost in 5 daily fractions at the isodose of 67% (i.e. maximum dose within the PTV-boost was 37.5 Gy) (Fig. 1b). Planning was performed using Hi-art Helical TomoTherapy inverse planning software (TomoTherapy Inc., Madison, WI, USA).We evaluated the D98%30 (dose to 98% of the PTV = near-minimum dose), D2% (dose to 2% of the PTV = near-maximum dose) and D50% (dose to 50% of the PTV = median absorbed dose) for PTV Flair and PTV-boost.
Treatment toxicity was evaluated by converting the 3D-dose distribution to the equivalent dose in 2 Gy fractions (EQD2) on a voxel-by-voxel basis. EQD2 was defined as the total dose delivered in 2-Gy fractions at an alpha/beta (α/β) ratio of 2 Gy for normal brain tissue, optic pathway and brainstem due to the low repair capacity of these OARs31.
Assessment of response and toxicity
The assessment of radiological and clinical response was based on MRI sequences obtained before and after HT-SIB. Baseline evaluation included volumetric T1 gadolinium-enhanced, fluid-attenuated inversion recovery imaging (FLAIR), axial T2-weighted imaging and DWI (diffusion-weighted imaging) MRI. We also used dynamic susceptibility contrast-enhanced (DSC) to establish progression/pseudoprogression and radiation necrosis. In difficult cases we used O-(2-[18F]fluoroethyl-)-l-tyrosine (18F-FET) PET/CT to support the differential diagnosis of PD or treatment-related changes. The assessment of toxicity was based on clinical examination carried out on the first, third and last day of re-irradiation, then 40 days after the end of re-RT and every 3 months thereafter. Patients were followed until disease progression or death. All toxicities were recorded and graded according to NCI CTCAE (National Cancer Institute Common Toxicity Criteria for Adverse Events), version 4.3.
Statistical analysis
Overall survival (OS) from primary diagnosis was defined as the time from surgery until death from any cause, or until the date of the last follow-up. Progression-free survival (PFS) after salvage therapy was defined as the time from the start of salvage TomoTherapy until disease progression or last follow-up. OS after salvage therapy was defined as the time from the start of salvage tomotherapy until death from any cause or until the date of the last follow-up. Time from the initial RT to salvage RT was defined as the time from date of adjuvant RT until the date of salvage TomoTherapy.
Event-time distributions were estimated using the Kaplan–Meier method, with 95% confidence intervals (95% CI) provided where appropriate. The role of the stratification factor was analyzed with the log-rank test. Median and range were calculated for continuous variables, while numbers and percentages were used for categorical variables. Statistical analyses were carried out with STATA/MP 15.1 for Windows (StataCorp LLC, College Station, TX, US).
Ethics approval and consent to participate
This study was approved by the Ethics Committee IRST IRCCS AVR (approval number L2P1912 of 15/05/2019) and carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. In accordance with Italian legislation, written informed consent for observational retrospective studies carried out in Scientific Institutes for Research, Hospitalization and Healthcare (IRCCS) was not required.
Results
Patients
Twenty-four consecutive patients (10 females and 14 males) with rGBM were treated at our institute between August 2008 and December 2017. Median age was 57 years (range 27–70 years). All patients had a Karnofsky Performance status (KPS) of ≥ 60. The entire cohort underwent initial post-operative fractionated RT with a standard dose schedule of 60 Gy in 30 fractions. Specific patient characteristics are reported in Table 2 and Supplementary Table S1. The median interval between primary RT and salvage RT was 18.7 months (range 3.6–64.8 months). Ten (41.66%) patients underwent HT-SIB as first salvage treatment at relapse, 14 (58.33%) as second treatment after salvage chemotherapy (5 patients) or salvage surgery (9 patients). These last 9 patients progressed and were re-irradiated a median of 4.7 months after re-surgery (95% CI 2.1–19.0).
Treatment characteristics
The median volume of PTV Flair was 107 cc (range 9.8–395.0 cc) and the median PTV-boost was 33 cc (range 6.7–196.4 cc). In patients who were borderline for re-irradiation, especially those with large tumor volumes, the final decision to re-irradiate was based on the following: KPS of the patient, age at time of HT-SIB, interval between the first and second radiotherapy course, and the proximity of critical organs to the targets. The median values of D98%, D2% and D50% for the PTV Flair and PTV- boost are shown in Table 3. We tailored the treatment for each patient, analyzing case by case. The dose to the OARs and the EQD2 are reported in Table 4. We considered a cumulative EQD2 Gy2 value of < 55 Gy2 for optic nerves and chiasm and < 60 Gy2 for brainstem as dose constraints for planning. No dose constraint violations were registered.
Outcomes
Median follow-up from re-irradiation was 27.8 months (range 1.6–88.5 months). During treatment dexamethasone ≥ 2 mg was administered to all patients. No acute or late neurologic toxicity > grade 2 (CTCAE version 4)32 was observed. All 24 patients completed the prescribed radiation dose without interruption. All but 3 required daily doses of dexamethasone ≥ 4 mg for > 8 weeks after HT-SIB. Median PFS (mPFS) for all patients was 4 months (95% CI 2.0–7.9 months), 3-month PFS was 66.7% (95% CI 44.3–81.7 months) and 6-month PFS was 41.7% (95% CI 22.2–60.1 months). Median OS (mOS) of re-irradiated patients was 10.7 months (95% CI 7.4–16.1 months) (Fig. 2). No significant differences were observed on the basis of age at the time of HT-SIB or on the basis of re-surgery before re-RT. Patients aged < 56 years had a mOS of 7.8 months (95% CI 4.4–18.3) vs. 10.7 months (95% CI 3.5–23.0) for those ≥ 56 years (p = 0.881). Patients who underwent re-surgery before re-irradiation showed a mOS of 10.7 months (95% CI 4.4–23.0) vs. 8.1 months (95% CI 3.8–18.3) for those who did not require re-surgery (p = 0.621).
At the time of the last observation, 6 (25%) patients were still alive, while 18 (75%) had died. Among the former group, 4 patients progressed according to RANO criteria after a median of 4 months (range 4–5 months), while 2 had a complete response (CR) to HT-SIB lasting 24 months and 27 months. The latter patient relapsed (2.5-cm lesion) within 2 cm of the re-irradiated lesion and was able to undergo second surgery because of good performance status (KPS 90%), the small size of the new lesion and the long DFS. Histology showed methylated MGMT GBM (34%), negative IDH1 and IDH2, Ki-67 18%, and no radionecrosis. mOS for the entire group calculated from the primary diagnosis was 44.4 months (95% CI 25.1–50.4 months).
Discussion
Several authors have investigated the feasibility of the SIB technique for the treatment of primary and metastatic brain lesions23,24,26,28,33,34,35,36,37,38. Rodrigues et al.24 delivered 60 Gy in 10 fractions to 1–3 brain metastases synchronously with 30 Gy whole brain irradiation (WBRT) using HT. The doses were delivered without dose-limiting central nervous system toxicity, as assessed 3 months after treatment. Bauman et al.28 confirmed the feasibility of SIB for individual brain metastases during a course of WBRT using HT. All of the studies confirmed that HT-SIB is capable of delivering a homogeneous brain dose and reasonably conformal dose to metastases, with surgical precision. Baisden et al.36 treated primary brain tumors with the HT-SIB technique, with a significant sparing of normal brain parenchyma compared to conventional HT with sequential boost. A dose of 50 Gy was prescribed to the larger PTV1, while the PTV-boost received a total of 60 Gy. The authors reported that HT-SIB plans resulted in a reduction in the mean brain dose, the volume of normal brain receiving 45 Gy (V45) and the volume of normal brain receiving 5 Gy compared with sequential boost plans. HT proved capable of treating multiple targets in the same region simultaneously at varying dose levels, lending itself naturally to SIB methods. The main advantages in using this technique to treat rGBM are (1) reduction in treatment time, with subsequent decrease in patient discomfort; (2) delivery of a higher biologically equivalent dose to the PTV-boost, which may improve tumor control; (3) delivery of 2 dose levels to PTVs without increasing brain toxicity; (4) possibility of treating large tumor volumes, with a low risk of acute and sub-acute toxicity.
In the present study the HT-SIB plans were created to deliver a total dose of 20 Gy to the PTV Flair (99% isodose line covering 99% of the PTV) and 25 Gy to the PTV-boost in 5 fractions. The prescribed dose isoline to the PTV-boost was 67% (i.e. maximum dose within the PTV-boost was 37.5 Gy). By using a 67% isodose prescription, the PTV-boost received a much higher and inhomogeneous dose than that of the PTV Flair (Fig. 1c). The inhomogeneous dose inside the PTV-boost, in delivering a higher dose to the hypoxic tumor cells, theoretically results in improved local control. Recently, Lucia et al.39 established that stereotactic radiotherapy delivery with inhomogeneous dose distribution led to better local control than homogeneous distribution in brain metastases, especially higher D2% values. In fact, our D2% values of the PTV-boost ranged from 3160 to 4008 cGy, while those of the PTV Flair ranged from 2270 to 3789 cGy. The ICRU (International Commission on Radiation Units) previously recommended that dose values in the PTV be confined within the range of 95–107% of the prescribed dose40. These constraints are not used in stereotactic radiotherapy (SRT) as some clinicians prefer to deliver a high dose to the middle of the target. However, as highlighted in the ICRU Report 9141 on Stereotactic Radiotherapy, the dose is often prescribed to the 60–80% isodose (relative to maximum dose) line which is located on the outline of the PTV.
In the re-irradiation setting42,43, a smaller irradiated volume is preferable in terms of toxicity, while limiting treatment to contrast-enhancing lesions may lead to lower local control given the invasiveness of gliomas14. Accurately determining tumor spread is especially difficult in diffuse tumors, thus increasing the risk of local failure. Contouring recurrent high-grade glioma on the basis of gadolinium-enhanced T1-weighted gadolinium MRI alone guarantees a specificity of only 50%44. Other imaging modalities have been used to delineate the GTV, including MR-spectroscopy, perfusion-weighted imaging and diffusion weighted imaging10,45, 11C-methionine positron emission tomography (MET-PET)46, and18 F-dihydroxyphenylalanine (DOPA) PET47. In the majority of studies12,17,48,49,50, the authors delineate only contrast-enhancing lesion on T1-weighted images GTV, while the clinical target volume (CTV) is equal to the GTV. A further millimetric margin is usually added to the CTV to create a planning target volume (PTV). As reported by some authors11,15,51, we also included the area of peritumoral edema, defined on the Flair sequences with a 2-mm margin expansion, in the PTV Flair, whereas the PTV-boost was defined as the visible tumor on enhanced T1-MRI with a 1-mm margin expansion.
The median PTV Flair value was 107 cc (range 9.8–395 cc) while that of the PTV-boost was 33 cc (range 6.7 cc to 196.4 cc). Despite the volume of treatment, mPFS was 4 months (95% CI 2.0–7.9 months), 3-month PFS was 66.7% (95% CI 44.3–81.7 months) and 6-month PFS was 41.7% (95% CI 22.2–60.1 months). mOS was 10.7 months (95% CI 7.4–16.1 months). In the literature, studies on the re-irradiation of rGBM rarely report the mPFS, while mOS ranges from 6.7 to 12 months10,11,12,13,14,15,16,17,18. Diverse radiotherapy regimens are used, doses ranging from 22 to 35 Gy in 5–10 sessions, with treatment volumes of 24 cm3 to 69.5cm3. Vodemark et al.17 treated recurrent high grade glioma (median volume 15 ml) with a median dose of 30 Gy in 6 fractions of 5 Gy/die, reporting a mPFS of 4.6 months and a mOS of 7.9 months but no severe toxicity. Fokas et al.48 re-irradiated 53 rGBM patients (median volume of 35 ml) with HSRT, delivering a median total dose of 30 Gy in a median of 10 fractions. Twelve-month actuarial PFS was 22%, with a mOS of 9 months. Re-irradiation was well tolerated (no acute or late toxicity > grade 2). Ernst-Stecken et al.52 evaluated the efficacy and side-effects of HSRT of 35 Gy in 5 fractions (3 times/week), median volume 22.4 ml (0.77–21.94 ml), observing no severe toxicity and a 12-month PFS of 53%. Toxicity reported in the literature is highly variable, some authors observing no severe toxicity, others reporting 12.5% of pathologically proven radionecrosis15. In our series there were no cases of acute neurologic toxicity > grade 2 (CTCAE vers. 4.03) during treatment and no re-operations were needed due to early or late toxicity.
Mayer et al.53,54 published a review on radiation tolerance of the human brain, concluding that radiation-induced necrosis of normal brain tissue occurred with a cumulative equivalent dose of 2 Gy fractions > 100 Gy2. Smaller volumes and more conformal techniques such as FSRT and SRS allow safe delivery of higher EQD2 cumulative doses (90–133.9 Gy and 11.6–137.2 Gy, respectively).The authors hypothesized that the re-irradiation and EQD2 cumulative doses increased when techniques such as FSRT and SRS were used, without, however, increasing the risk of normal brain necrosis. In a review on cranial re-irradiation, Nieder et al.6 reported that a fraction size of 3–5 Gy was well tolerated in limited-volume recurrences (< 75 ml) as long as the total dose was limited to 30 Gy-35 Gy.
In our study, the EQD2 value of 25 Gy to the isodose line of 67% was 87.58 Gy2, while the EQD2 of 20 Gy was 74.8 Gy2, based on the median value of D2% (37.15 Gy and 34.02 Gy, respectively).Taking into account the uniformity of the initial radiation treatment in which the healthy brain received a uniform dose of 60 Gy (2 Gy/die for 30 sessions) and the EQD2 calculated for both prescription doses, we determined a cumulative EQD2 of 147.58 Gy2 and 134.8 Gy2. Such high values carry the risk of severe toxicity, which, on the contrary, we did not observe. HT-SIB probably delivers a marginal low dose that inhomogeneously increases inside the target. It enables large tumor volumes to be treated that previously would not have been contemplated due to the risk of toxicity. The HT-SIB technique, in creating highly conformal dose distribution, potentially reduces the dose to surrounding critical structures (Table 4). The data for our case series are modest, with mOS percentages similar to those of other re-irradiation studies. It is clear, however, that greater efforts are needed to improve the prognosis of patients with recurrent glioblastoma.
The limitations of this study include its retrospective nature, selection bias, lack of biological information and various treatment factors, including chemotherapy and surgery before re-irradiation, and evaluation of quality of life, all of which made it difficult to interpret outcome.
Conclusions
The preliminary results from the present study suggest that HT with the proposed SIB technique is a safe and feasible, albeit not curative, treatment option for patients with rGBM, including those with large tumors. Further studies in primary and recurrent settings are needed to confirm our findings.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- EQD2:
-
Equivalent dose in 2 Gy fractions
- FSRT:
-
Fractionated stereotactic radiotherapy
- GBM:
-
Glioblastoma
- GTV:
-
Gross tumor volume
- HSRT:
-
Hypofractionated stereotactic radiotherapy
- HT:
-
Helical tomotherapy
- HT-SIB:
-
Helical tomotherapy with a simultaneous integrated boost technique
- MRI:
-
Magnetic resonance imaging
- MVCT:
-
Megavoltage computed tomography
- OAR:
-
Organs at risk
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PTV:
-
Planning target volume
- reRT:
-
Re-irradiation
- rGBM:
-
Recurrent glioblastoma multiforme
- RT:
-
Radiotherapy
- SIB:
-
Simultaneous integrated boost
- SRS:
-
Stereotactic radiosurgery
- WBRT:
-
Whole brain irradiation
References
Jansen, E. P., Dewit, L. G., van Herk, M. & Bartelink, H. Target volumes in radiotherapy for high-grade malignant glioma of the brain. Radiother. Oncol. 56(2), 151–156. https://doi.org/10.1016/S0167-8140(00)00216-4 (2000).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10(5), 459–466. https://doi.org/10.1016/S1470-2045(09)70025-7 (2009).
Niyazi, M. et al. Therapeutic options for recurrent malignant glioma. Radiother. Oncol. 98(1), 1–14. https://doi.org/10.1016/j.radonc.2010.11.006 (2011).
Seystahl, K., Wick, W. & Weller, M. Therapeutic options in recurrent glioblastoma: An update. Crit. Rev. Oncol. Hematol. 99, 389–408. https://doi.org/10.1016/j.critrevonc.2016.01.018 (2016).
Shaw, E. et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases. Int. J. Radiat. Oncol. 47(2), 291–298. https://doi.org/10.1016/S0360-3016(99)00507-6 (2000).
Nieder, C., Andratschke, N. H. & Grosu, A. L. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 36(10), 4985–4996. https://doi.org/10.21873/anticanres.11067 (2016).
Scoccianti, S. et al. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit. Rev. Oncol. Hematol. 126, 80–91. https://doi.org/10.1016/j.critrevonc.2018.03.024 (2018).
Combs, S. E., Debus, J. & Schulz-Ertner, D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer 7, 167. https://doi.org/10.1186/1471-2407-7-167 (2007).
Patel, M. et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: Radiographic response and improved survival. J. Neurooncol. 92(2), 185–191. https://doi.org/10.1007/s11060-008-9752-9 (2009).
Conti, A. et al. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. (Wien) 154, 203–209. https://doi.org/10.1007/s00701-011-1184-1 (2012).
Ogura, K. et al. Efficacy of salvage stereotactic radiotherapy for recurrent glioma: Impact of tumor morphology and method of target delineation on local control. Cancer Med. 2(6), 942–949. https://doi.org/10.1002/cam4.154 (2013).
Combs, S. E., Thilmann, C., Edler, L., Debus, J. & Schulz-Ertner, D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J. Clin. Oncol. 23(34), 8863–8869. https://doi.org/10.1200/JCO.2005.03.4157 (2005).
Ernst-Stecken, A., Ganslandt, O., Lambrecht, U., Sauer, R. & Grabenbauer, G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother. Oncol. 81(1), 18–24. https://doi.org/10.1016/j.radonc.2006.08.024 (2006).
Fogh, S. E. et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 28(18), 3048–3053. https://doi.org/10.1200/JCO.2009.25.6941 (2010).
Kim, B. et al. Treatment of recurrent high grade gliomas with hypofractionated stereotactic image-guided helical tomotherapy. Clin. Neurol. Neurosurg. 113(6), 509–512. https://doi.org/10.1016/j.clineuro.2011.02.001 (2011).
Shepherd, S. F. et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int. J. Radiat. Oncol. Biol. Phys. 37(2), 393–398. https://doi.org/10.1016/S0360-3016(96)00455-5 (1997).
Vordermark, D. et al. Hypofractionated stereotactic re-irradiation: Treatment option in recurrent malignant glioma. BMC Cancer 5, 1–7. https://doi.org/10.1186/1471-2407-5-55 (2005).
Kim, H. R. et al. Outcome of salvage treatment for recurrent glioblastoma. J. Clin. Neurosci. 22(3), 468–473. https://doi.org/10.1016/j.jocn.2014.09.018 (2015).
Niyazi, M. et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother. Oncol. 118(1), 35–42. https://doi.org/10.1016/j.radonc.2015.12.003 (2016).
Watanabe, M., Tanaka, R. & Takeda, N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34(6), 463–469. https://doi.org/10.1007/BF00598951 (1992).
Li, Y., Rey-Dios, R., Roberts, D. W., Valdés, P. A. & Cohen-Gadol, A. A. Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg. 82(1–2), 175–185. https://doi.org/10.1016/j.wneu.2013.06.014 (2014).
Chang, P. D., Chow, D. S., Yang, P. H., Filippi, C. G. & Lignelli, A. Predicting glioblastoma recurrence by early changes in the apparent diffusion coefficient value and signal intensity on flair images. Am. J. Roentgenol. 208(1), 57–65. https://doi.org/10.2214/AJR.16.16234 (2017).
Rodrigues, G. et al. A pooled analysis of arc-based image-guided simultaneous integrated boost radiation therapy for oligometastatic brain metastases. Radiother. Oncol. 102(2), 180–186. https://doi.org/10.1016/j.radonc.2011.05.032 (2012).
Rodrigues, G. et al. Phase I trial of simultaneous in-field boost with helical tomotherapy for patients with one to three brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 80(4), 1128–1133. https://doi.org/10.1016/j.ijrobp.2010.03.047 (2011).
Nahum, A. E. The radiobiology of hypofractionation. Clin. Oncol. 27(5), 260–269. https://doi.org/10.1016/j.clon.2015.02.001 (2015).
Ni, L. & Liang, X. Feasibility of simultaneous integrated boost with forward intensity-modulated radiation therapy for multiple brain metastases. Contemp. Oncol. (Pozn) 18(3), 187–191. https://doi.org/10.5114/wo.2014.43156 (2014).
Tomita, N. et al. Helical tomotherapy for brain metastases: Dosimetric evaluation of treatment plans and early clinical results. Technol. Cancer. Res. Treat. 7(6), 417–424. https://doi.org/10.1177/153303460800700602 (2008).
Bauman, G. et al. Simultaneous infield boost with helical tomotherapy for patients with 1 to 3 brain metastases. Am. J. Clin. Oncol. 30(1), 38–44. https://doi.org/10.1097/01.coc.0000245473.41035.c4 (2007).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972. https://doi.org/10.1200/JCO.2009.26.3541 (2010).
Report 83. J ICRU 2010;10:1–93. https://doi.org/10.1093/jicru/10.1.
Withers, H. R. Biologic basis for altered fractionation schemes. Cancer 55(9 Suppl), 2086–2895. https://doi.org/10.1002/1097-0142(19850501)55:9+%3c2086::aid-cncr2820551409%3e3.0.co;2-1 (1985).
U.S. Department of Health and Human Services, U.S. National Institutes of Health, U.S. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009, https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf (2009).
Oh, E. S. et al. Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost- intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer. Radiat. Oncol. J. 36(3), 200–209. https://doi.org/10.3857/roj.2018.00073 (2018).
Ferro, M. et al. Intensity modulated radiation therapy with simultaneous integrated boost in patients with brain oligometastases: A phase 1 study (ISIDE-BM-1). Int. J. Radiat. Oncol. 97(1), 82–90. https://doi.org/10.1016/j.ijrobp.2016.09.020 (2017).
Gupta, T. et al. Planning and delivery of whole brain radiation therapy with simultaneous integrated boost to brain metastases and synchronous limited-field thoracic radiotherapy using helical tomotherapy: A preliminary experience. Technol. Cancer Res. Treat. 8(1), 15–22. https://doi.org/10.1177/153303460900800103 (2009).
Baisden, J. M. et al. Helical tomotherapy simultaneous integrated boost provides a dosimetric advantage in the treatment of primary intracranial tumors. Neurol. Res. 33(8), 820–824. https://doi.org/10.1179/1743132811Y.0000000035 (2011).
Suzuki, M. et al. Feasibility study of the simultaneous integrated boost (SIB) method for malignant gliomas using intensity-modulated radiotherapy (IMRT). Jpn. J. Clin. Oncol. 33(6), 271–277. https://doi.org/10.1093/jjco/hyg053 (2003).
Levegrün, S. et al. Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: Evaluation of dose distribution characteristics and comparison with alternative techniques. Int. J. Radiat. Oncol. 86(4), 734–742. https://doi.org/10.1016/j.ijrobp.2013.03.031 (2013).
Lucia, F. et al. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother. Oncol. 130, 132–138. https://doi.org/10.1016/j.radonc.2018.06.039 (2019).
Landberg, T. et al. Report 62. J. Int. Comm. Radiat. Units Meas. https://doi.org/10.1093/jicru/os32.1.Report62 (1999).
Wilke, L. et al. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams. Strahlenther. Onkol. 195(3), 193–198. https://doi.org/10.1007/s00066-018-1416-x (2019).
Krauze, A. V. et al. Re-irradiation for recurrent glioma- the NCI experience in tumor control, OAR toxicity and proposal of a novel prognostic scoring system. Radiat. Oncol. 12(1), 191. https://doi.org/10.1186/s13014-017-0930-9 (2017).
Krauze, A. V. et al. Expert consensus on re-irradiation for recurrent glioma. Radiat. Oncol. 12(1), 194. https://doi.org/10.1186/s13014-017-0928-3 (2017).
Rachinger, W. et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57(3), 505–511. https://doi.org/10.1227/01.neu.0000171642.49553.b0 (2005).
Popp, I. et al. Diffusion-weighted MRI and ADC versus FET-PET and GdT1w-MRI for gross tumor volume (GTV) delineation in re-irradiation of recurrent glioblastoma. Radiother. Oncol. 130, 121–131. https://doi.org/10.1016/j.radonc.2018.08.019 (2019).
Grosu, A. L. et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 63(2), 511–519. https://doi.org/10.1016/j.ijrobp.2005.01.056 (2005).
Minniti, G. et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J. Neurooncol. 111(2), 187–194. https://doi.org/10.1007/s11060-012-0999-9 (2013).
Fokas, E. et al. Hypofractionated stereotactic reirradiation of recurrent glioblastomas. Strahlenther. Onkol. 185(4), 235–240. https://doi.org/10.1007/s00066-009-1753-x (2009).
Hudes, R. S. et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 43(2), 293–298. https://doi.org/10.1016/S0360-3016(98)00416-7 (1999).
Navarria, P. et al. Hypofractionated stereotactic radiation therapy in recurrent high-grade glioma: A new challenge. Cancer Res. Treat. 48(1), 37–44. https://doi.org/10.4143/crt.2014.259 (2016).
Hundsberger, T. et al. Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J. Neurooncol. 112(1), 133–139. https://doi.org/10.1007/s11060-013-1044-3 (2013).
Ernst-Stecken, A., Ganslandt, O., Lambrecht, U., Sauer, R. & Grabenbauer, G. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J. Neurooncol. 81(3), 287–294. https://doi.org/10.1007/s11060-006-9231-0 (2007).
Mayer, R. & Sminia, P. Reirradiation tolerance of the human brain. Int. J. Radiat. Oncol. Biol. Phis. 70(5), 1350–1360. https://doi.org/10.1016/j.ijrobp.2007.08.015 (2008).
Sminia, P. & Mayer, R. External beam radiotherapy of recurrent glioma: Radiation tolerance of the human brain. Cancers (Basel). 4(2), 379–399. https://doi.org/10.3390/cancers4020379 (2012).
Acknowledgements
The authors wish to thank Gráinne Tierney for editorial assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
D.A. conceived and designed the study, collected and assembled the study data, analyzed and interpreted the data, drafted the manuscript. E.P. and G.G. conceived and designed the study, analyzed and interpreted the data. A.S. analyzed interpreted the data. S.P.C., L.T. and M.P. collected and assembled the study data. F.F. statistical analysis. A.T., A.S. and R.P. analyzed and interpreted the data. A.R. revised the manuscript for important intellectual content. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arpa, D., Parisi, E., Ghigi, G. et al. Re-irradiation of recurrent glioblastoma using helical TomoTherapy with simultaneous integrated boost: preliminary considerations of treatment efficacy. Sci Rep 10, 19321 (2020). https://doi.org/10.1038/s41598-020-75671-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75671-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.